Self-Reported Non-Celiac Gluten Sensitivity in Brazil: Translation, Cultural Adaptation, and Validation of Italian Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire
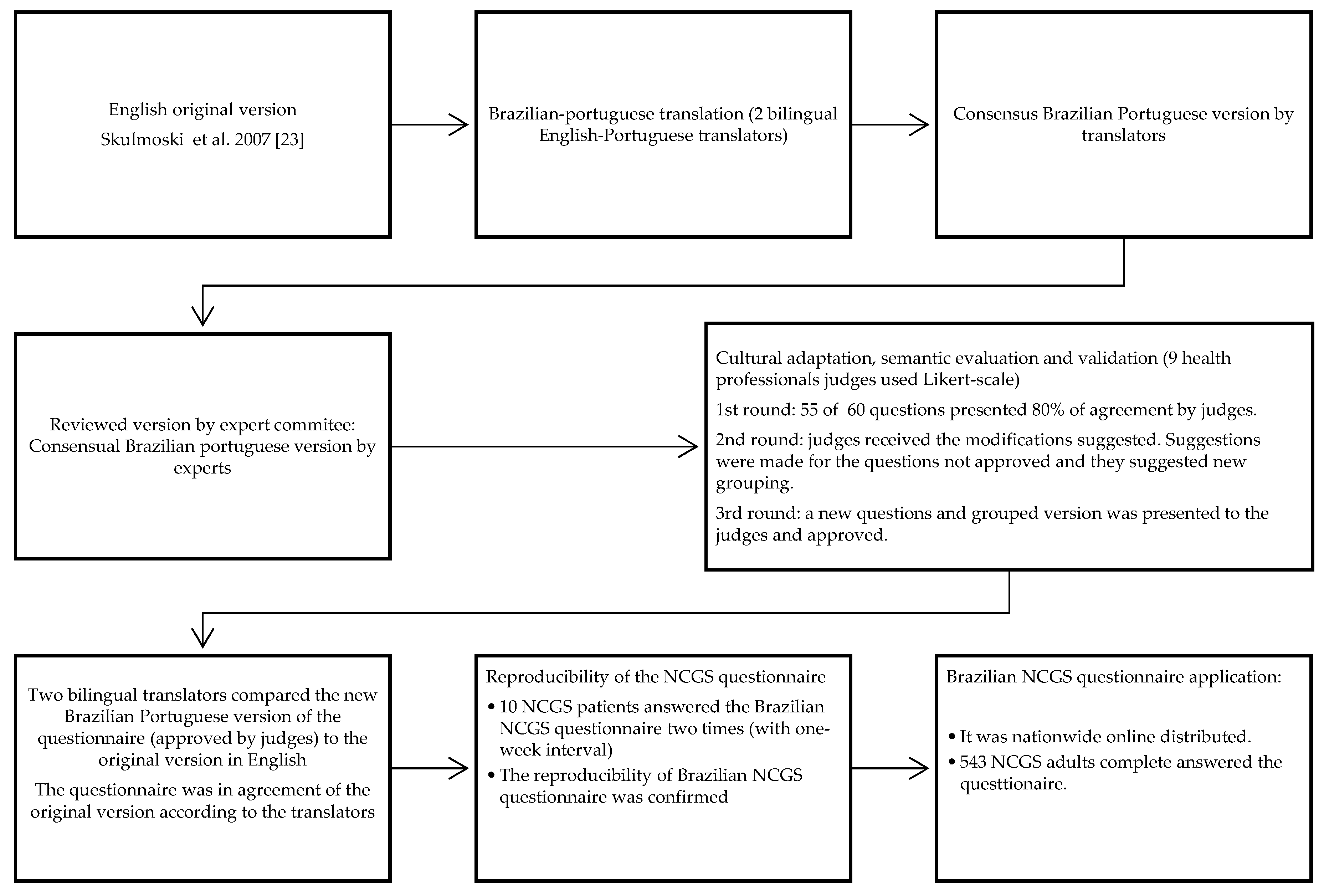
2.2. Translation, Cultural Adaptation, and Validation
2.2.1. Translation and Retranslation
2.2.2. Cultural Adaptation, Semantic Evaluation, and Validation
2.2.3. Reproducibility Analysis
2.3. Brazilian Questionnaire Application
3. Results
3.1. Translation, Cultural Adaptation, Semantic Evaluation, and Content Validation
- A total of three rounds were necessary to obtain an agreement among the experts for content validation and semantic evaluation. In the first round of the questionnaire, questions were considered adequate regarding reliability, clarity and easy comprehension. The questionnaire was sent to 17 experts, and only nine completed the evaluation in the first round. In the first round, almost 92% (N = 55) of the questions were approved. The experts suggested changes in the questions that were not approved and only those questions were sent in the second round for evaluation. In the second round, we obtained answers from 11 experts, and 89% of the questions were approved. Therefore, the third round of expert evaluation was necessary for the remainning questions. The experts suggested grouping the questions as previously described, and all questions were approved in the third round.
Reproducibility of the Brazilian NCGS Questionnaire
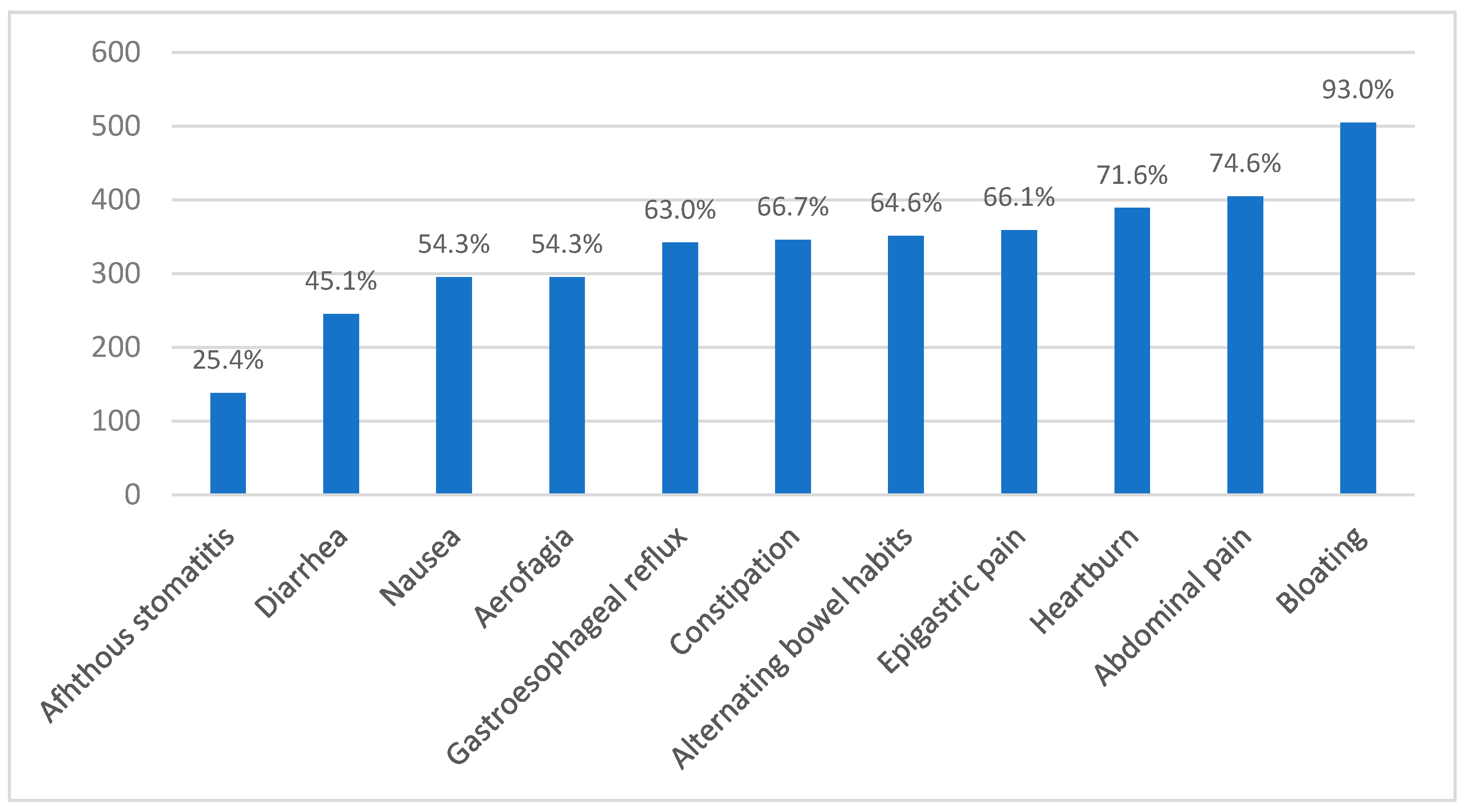
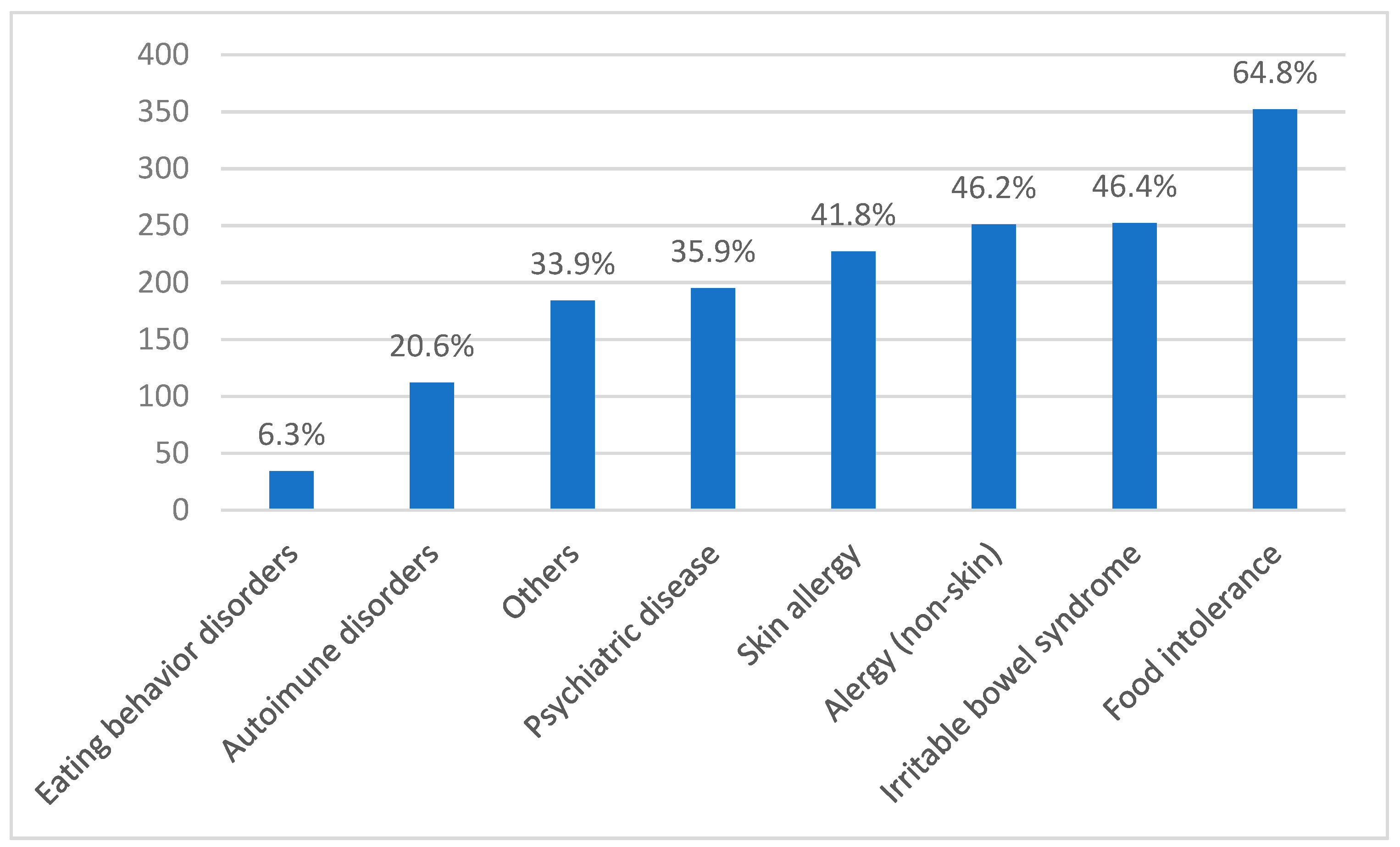
3.2. Brazilian NCGS Questionnaire Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molina-Infante, J.; Santolaria, S.; Sanders, D.S.; Fernandez-Bañares, F. Systematic review: Noncoeliac gluten sensitivity: EBSCOhost. Aliment. Pharmacol. Ther. 2015, 41, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; De Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Bulsa, G. Non coeliac gluten sensitivity—A new disease with gluten intolerance. Clin. Nutr. 2015, 34, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Bascuñán, K.; Vecchi, M.; Doneda, L.; Bardella, M.; Lombardo, V.; Scricciolo, A.; Branchi, F.; Elli, L. Exposure to Different Amounts of Dietary Gluten in Patients with Non-Celiac Gluten Sensitivity (NCGS): An Exploratory Study. Nutrients 2019, 11, 136. [Google Scholar] [CrossRef]
- Beck, M. Clues to Gluten Sensitivity. Wall Str. J. 2011. Available online: https://www.wsj.com/articles/SB10001424052748704893604576200393522456636 (accessed on 14 January 2019).
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinšek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Schäfer, C.; Kleine-Tebbe, J.; Ahrens, B.; Bachmann, O.; Ballmer-Weber, B.; Beyer, K.; Bischoff, S.C.; Blümchen, K.; Dölle, S.; et al. Non-celiac gluten/wheat sensitivity (NCGS)—A currently undefined disorder without validated diagnostic criteria and of unknown prevalence. Allergo J. Int. 2018, 27, 147–151. [Google Scholar] [CrossRef]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- DiGiacomo, D.V.; Tennyson, C.A.; Green, P.H.; Demmer, R.T. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: Results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand. J. Gastroenterol. 2013, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Tovoli, F.; Granito, A.; Negrini, G.; Guidetti, E.; Faggiano, C.; Bolondi, L. Long term effects of gluten-free diet in non-celiac wheat sensitivity. Clin. Nutr. 2019, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Giambalvo, O.; Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 2017, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- van Gils, T.; Nijeboer, P.; IJssennagger, C.E.; Sanders, D.S.; Mulder, C.J.J.; Bouma, G. Prevalence and Characterization of Self-Reported Gluten Sensitivity in The Netherlands. Nutrients 2016, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; López-Gallardo, J.; Vergara-Jiménez, M.; Cabrera-Chávez, F. Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population. Nutrients 2015, 7, 6000–6015. [Google Scholar] [CrossRef]
- Zylberberg, H.M.; Yates, S.; Borsoi, C.; Green, P.H.R.; Lebwohl, B. Regional and National Variations in Reasons for Gluten Avoidance. J. Clin. Gastroenterol. 2018, 52, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, A.; Borghini, R.; Di Tola, M.; Marino, M.; Urciuoli, C.; Isonne, C.; Puzzono, M.; Porowska, B.; Rumi, G.; Lonardi, S.; et al. Intestinal, Systemic, and Oral Gluten-related Alterations in Patients With Nonceliac Gluten Sensitivity. J. Clin. Gastroenterol. 2016, 50, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, D.; Bajor, J.; Papp, M.; Miheller, P.; Herszényi, L.; Tulassay, Z.; Juhász, M. Questionnaire survey of celiac disease and non-celiac gluten sensitivity in outpatients’ cohorts of three Hungarian gastroenterology referral centres. Z. Gastroenterol. 2015, 53, A25. [Google Scholar] [CrossRef]
- Ribeiro, P.V.D.M.; Santos, A.D.P.; Andreoli, C.S.; Ribeiro, S.M.R.; Jorge, M.D.P.; Moreira, A.V.B. Nutritional status variation and intestinal and extra intestinal symptomatology in patients with celiac disease and non-celiac gluten sensitivity given specialized dietary advice. Rev. Nutr. 2017, 30, 57–67. [Google Scholar] [CrossRef]
- Cabrera-Chávez, F.; Dezar, G.V.A.; Islas-Zamorano, A.P.; Espinoza-Alderete, J.G.; Vergara-Jiménez, M.J.; Magaña-Ordorica, D.; Ontiveros, N. Prevalence of Self-Reported Gluten Sensitivity and Adherence to a Gluten-Free Diet in Argentinian Adult Population. Nutrients 2017, 9, 81. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Rivera-Gutierrez, X.; Cobos-Quevedo, O.D.J.; Grube-Pagola, P.; Meixueiro-Daza, A.; Hernandez-Flores, K.; Cabrera-Jorge, F.J.; Vivanco-Cid, H.; Dowd, S.E.; Remes-Troche, J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients 2018, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Chávez, F.; Granda-Restrepo, D.M.; Arámburo-Gálvez, J.G.; Franco-Aguilar, A.; Magaña-Ordorica, D.; Vergara-Jiménez, M.D.J.; Ontiveros, N. Self-Reported Prevalence of Gluten-Related Disorders and Adherence to Gluten-Free Diet in Colombian Adult Population. Gastroenterol. Res. Pract. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Skulmoski, G.J.; Hartman, F.T.; Krahn, J. The Delphi Method for Graduate Research. J. Inf. Technol. Educ. Res. 2007, 6, 1–21. [Google Scholar] [CrossRef]
- Pratesi, C.; Häuser, W.; Uenishi, R.; Selleski, N.; Nakano, E.; Gandolfi, L.; Pratesi, R.; Zandonadi, R.P. Quality of Life of Celiac Patients in Brazil: Questionnaire Translation, Cultural Adaptation and Validation. Nutrients 2018, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Farage, P.; Zandonadi, R.P.; Ginani, V.C.; Gandolfi, L.; Pratesi, R.; de Medeiros Nóbrega, Y.K. Content validation and semantic evaluation of a check-list elaborated for the prevention of gluten cross-contamination in food services. Nutrients 2017, 9, 36. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S. Navigating the gluten-free boom. J. Am. Acad. Phys. Assist. 2015, 28, 1–7. [Google Scholar] [CrossRef]
- Cooper, B.T.; Holmes, G.K.; Ferguson, R.; Thompson, R.A.; Allan, R.N.; Cooke, W.T. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 1980, 79, 801–806. [Google Scholar] [PubMed]
- Fagerdahl, A.-M.; Boström, L.; Ulfvarson, J.; Bergström, G.; Ottosson, C. Translation and validation of the wound-specific quality of life instrument Cardiff Wound Impact Schedule in a Swedish population. Scand. J. Caring Sci. 2014, 28, 398–404. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Conti, M.A.; Scagliusi, F.; Kawamura De Oliveira Queiroz, G.; Hearst, N.; Cordás, T.A. Cross-cultural adaptation: Translation and Portuguese language content validation of the Tripartite Infl uence Scale for body dissatisfaction. Cadernos de Saúde Pública 2010, 26, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Freudenburg, W.R. Gender and Environmental Risk Concerns. Environ. Behav. 1996, 28, 302–339. [Google Scholar] [CrossRef]
- Chen, M. Consumers’ health and taste attitude in Taiwan. Br. Food J. 2013, 115, 526–540. [Google Scholar] [CrossRef]
- Lee, A.R.; Wolf, R.; Contento, I.; Verdeli, H.; Green, P.H.R. Coeliac disease: The association between quality of life and social support network participation. J. Hum. Nutr. Diet. 2016, 29, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Castilhos, A.C.; Gonçalves, B.C.; Macedo e Silva, M.; Lanzoni, L.A.; Metzger, L.R.; Kotze, L.M.S.; Nisihara, R.M. Quality of life evaluation in celiac patients from Southern Brazil quality of life evaluation in celiac patients from Southern Brazil. Arq. Gastroenterol. 2015, 52, 171–175. [Google Scholar] [CrossRef]
- Lillestøl, K.; Berstad, A.; Lind, R.; Florvaag, E.; Arslan Lied, G.; Tangen, T. Anxiety and depression in patients with self-reported food hypersensitivity. Gen. Hosp. Psychiatry 2010, 32, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Usai-Satta, P.; Oppia, F.; Lai, M.; Cabras, F. Motility Disorders in Celiac Disease and Non-Celiac Gluten Sensitivity: The Impact of a Gluten-Free Diet. Nutrients 2018, 10, 1705. [Google Scholar] [CrossRef]
- Araújo, H.M.C.; Araújo, W.M.C. Coeliac disease. Following the diet and eating habits of participating individuals in the Federal District, Brazil. Appetite 2011, 57, 105–109. [Google Scholar] [CrossRef]
- Araújo, H.M.C.; Araújo, W.M.C.; Botelho, R.B.A.; Zandonadi, R.P. Doença celíaca, hábitos e práticas alimentares e qualidade de vida. Rev. Nutr. 2010, 23, 467–474. [Google Scholar] [CrossRef]
- Busby, E.; Bold, J.; Fellows, L.; Rostami, K. Mood Disorders and Gluten: It’s Not All in Your Mind! A Systematic Review with Meta-Analysis. Nutrients 2018, 10, 1708. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Sepulveda, R.; Hing, T.; Shah, N.D.; Choe, M.; Limsui, D.; Shah, S. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Seidita, A.; D’Alcamo, A.; Carroccio, A. Non-Celiac Gluten Sensitivity: Literature Review. J. Am. Coll. Nutr. 2014, 33, 39–54. [Google Scholar] [CrossRef] [PubMed]

| Frequency (N) | Prevalence (%) | |
|---|---|---|
| Pharmacist | 2 | 0.4% |
| Homeopath | 8 | 1.5% |
| Friends | 23 | 4.2% |
| General Practitioner | 26 | 4.8% |
| Gastroenterologist | 86 | 15.8% |
| Others | 116 | 21.4% |
| Patient | 282 | 51.9% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadelha de Mattos, Y.A.; Puppin Zandonadi, R.; Gandolfi, L.; Pratesi, R.; Yoshio Nakano, E.; Pratesi, C.B. Self-Reported Non-Celiac Gluten Sensitivity in Brazil: Translation, Cultural Adaptation, and Validation of Italian Questionnaire. Nutrients 2019, 11, 781. https://doi.org/10.3390/nu11040781
Gadelha de Mattos YA, Puppin Zandonadi R, Gandolfi L, Pratesi R, Yoshio Nakano E, Pratesi CB. Self-Reported Non-Celiac Gluten Sensitivity in Brazil: Translation, Cultural Adaptation, and Validation of Italian Questionnaire. Nutrients. 2019; 11(4):781. https://doi.org/10.3390/nu11040781
Chicago/Turabian StyleGadelha de Mattos, Yanna A., Renata Puppin Zandonadi, Lenora Gandolfi, Riccardo Pratesi, Eduardo Yoshio Nakano, and Claudia B. Pratesi. 2019. "Self-Reported Non-Celiac Gluten Sensitivity in Brazil: Translation, Cultural Adaptation, and Validation of Italian Questionnaire" Nutrients 11, no. 4: 781. https://doi.org/10.3390/nu11040781
APA StyleGadelha de Mattos, Y. A., Puppin Zandonadi, R., Gandolfi, L., Pratesi, R., Yoshio Nakano, E., & Pratesi, C. B. (2019). Self-Reported Non-Celiac Gluten Sensitivity in Brazil: Translation, Cultural Adaptation, and Validation of Italian Questionnaire. Nutrients, 11(4), 781. https://doi.org/10.3390/nu11040781








