Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Curcumin Treatment of Mice
2.4. Cells
2.5. Curcumin Treatment of Lipopolysaccharide (LPS)-Challenged Neural Cells
2.6. Reverse-Transcription Polymerase Chain Reaction and Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. RNA Interference and Transfection
2.8. Immunoblotting of CISD2
2.9. Chemicals and Fluorescent Dyes for the Study of Mitochondrial Function
2.10. Cell Viability
2.11. Statistical Analysis
3. Results
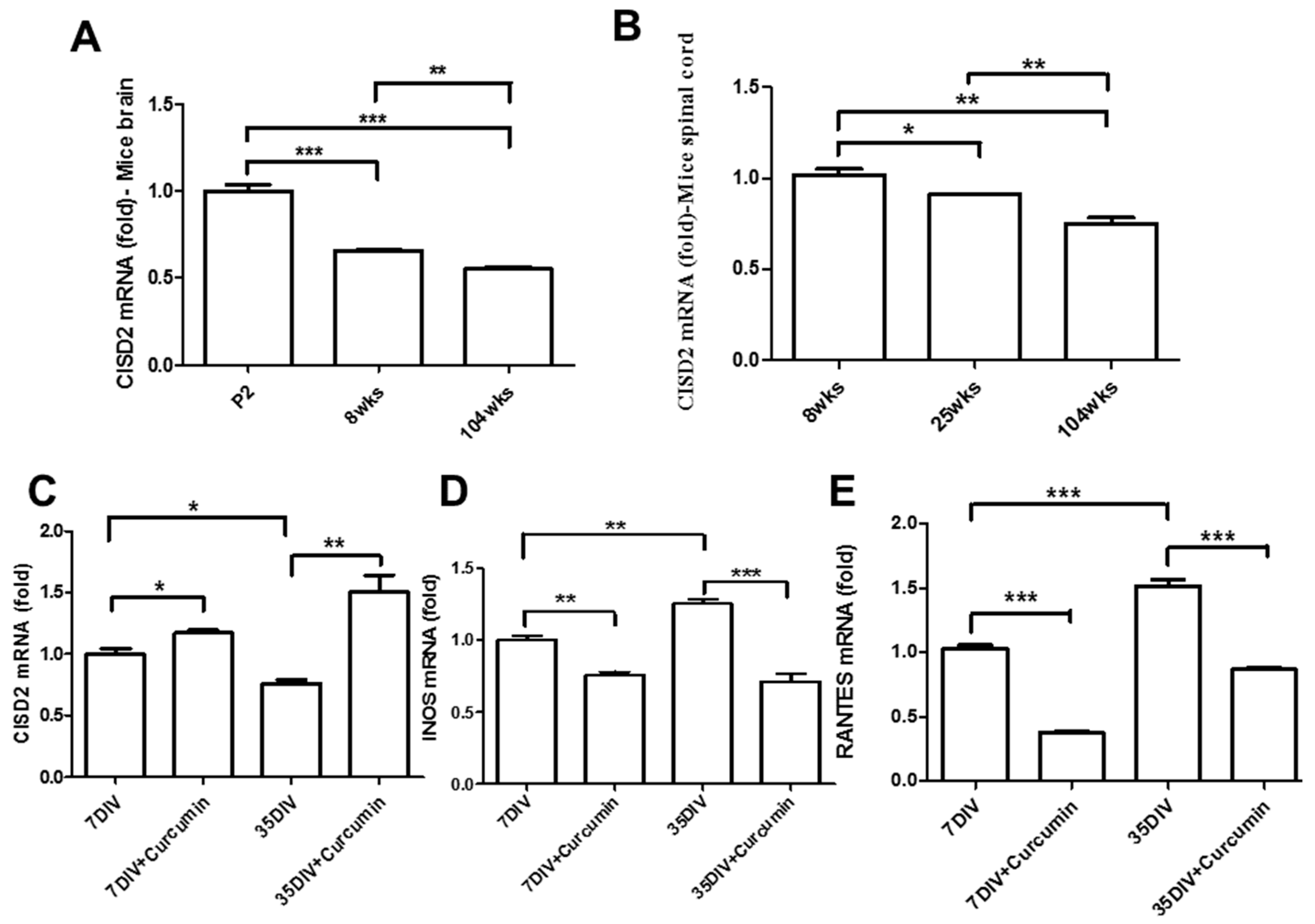
3.1. Aging-Driven Decline in CISD2 Expression in Naturally Aged Mice and Curcumin Protected against Aging-Driven Decline in CISD2 Expression as Well as Aging-Augmented Inflammatory Responses in Long-Term Cultures of Neural Cells
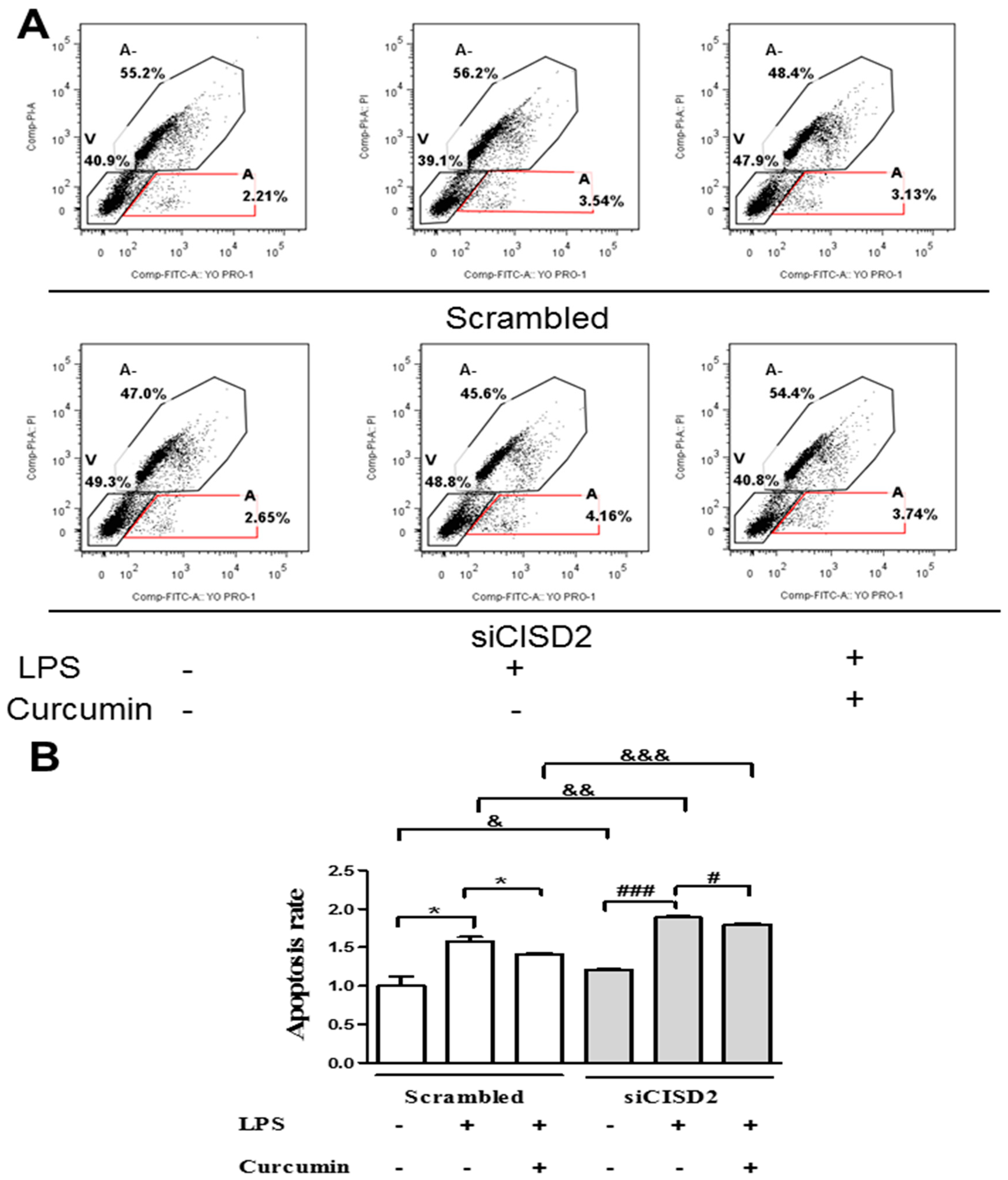
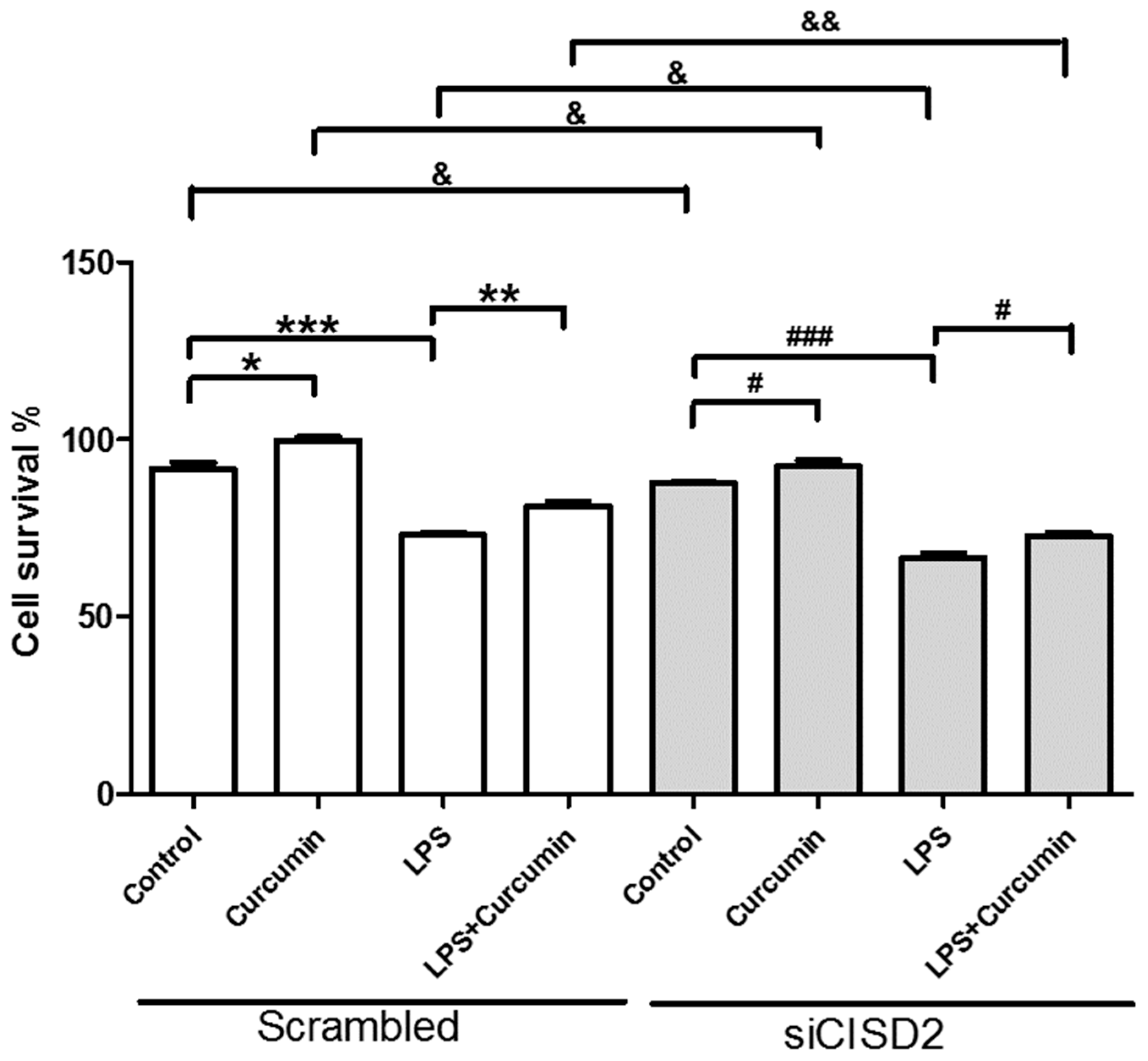
3.2. Knockdown of CISD2 Expression in Neural Cells Led to High No Production with a Strong Inflammatory Reaction, Enhanced Apoptosis, and Attenuated Cell Survival
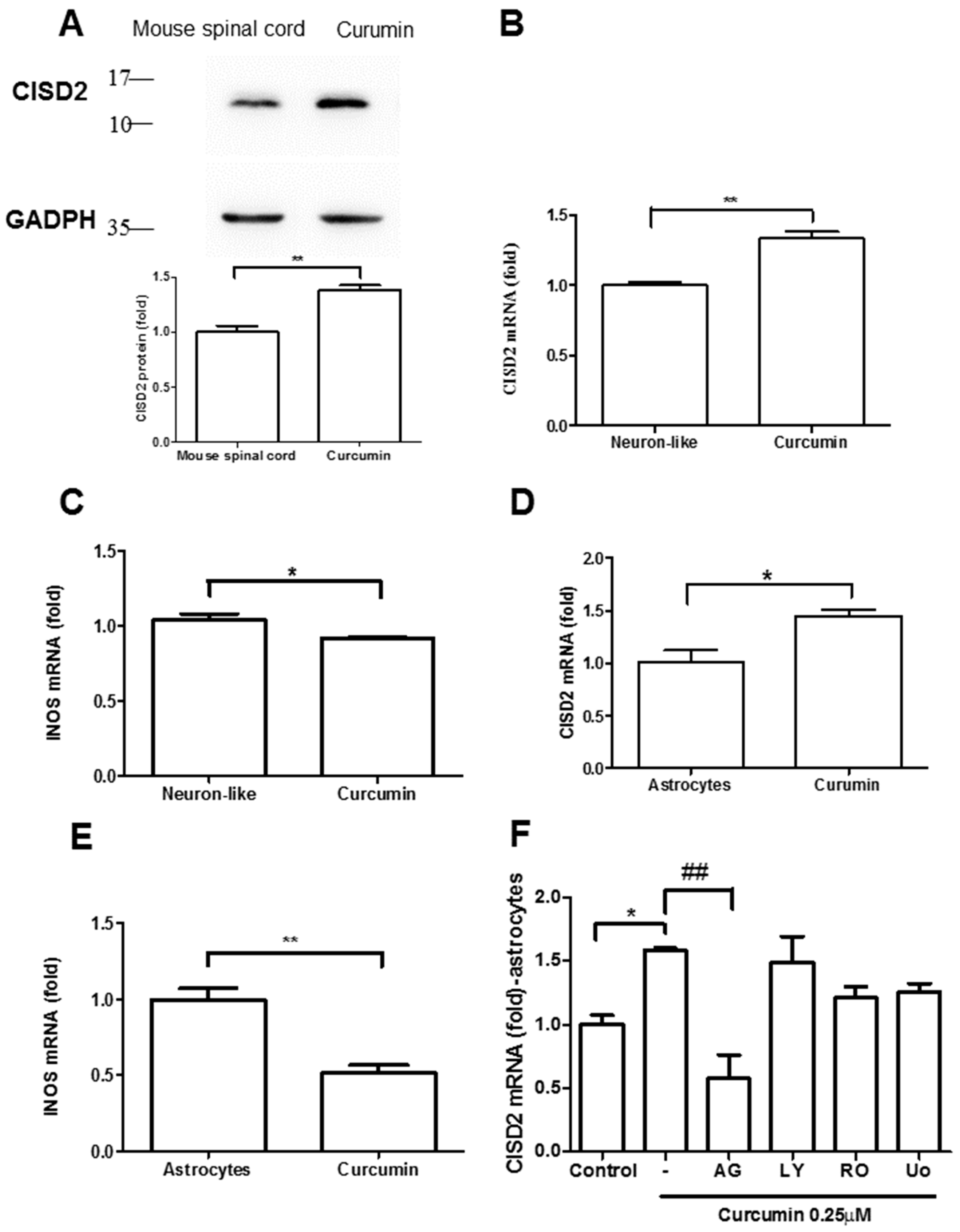
3.3. Curcumin Enhanced CISD2 mRNA and Protein Expression In Vivo and In Vitro via JAK/STAT Signaling Pathways
3.4. Curcumin Enhanced an LPS-Triggered Reduction in Mitochondrial Membrane Potential In Vitro
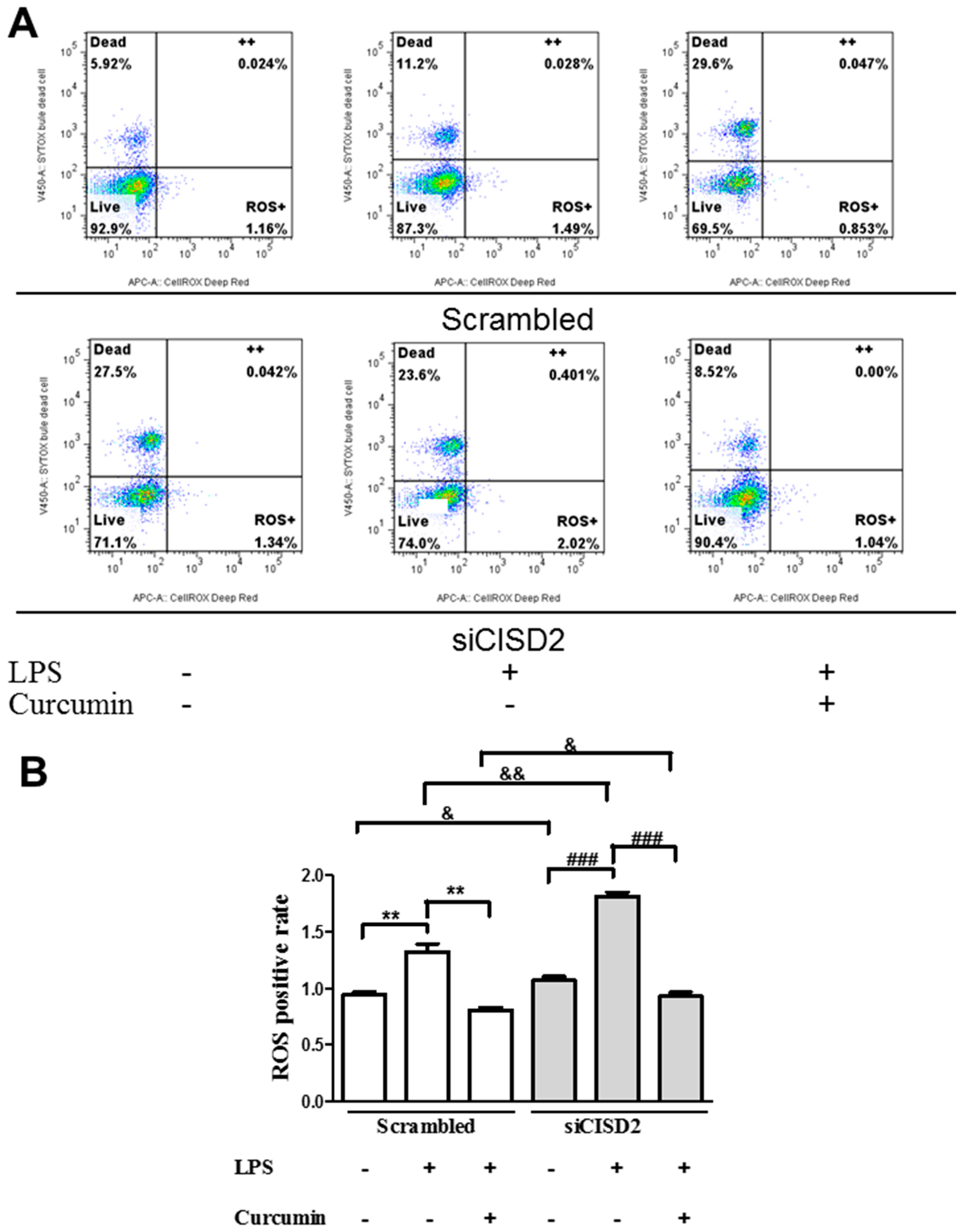
3.5. Curcumin Protected against LPS-Augmented Reactive Oxygen Species (ROS) Formation In Vitro
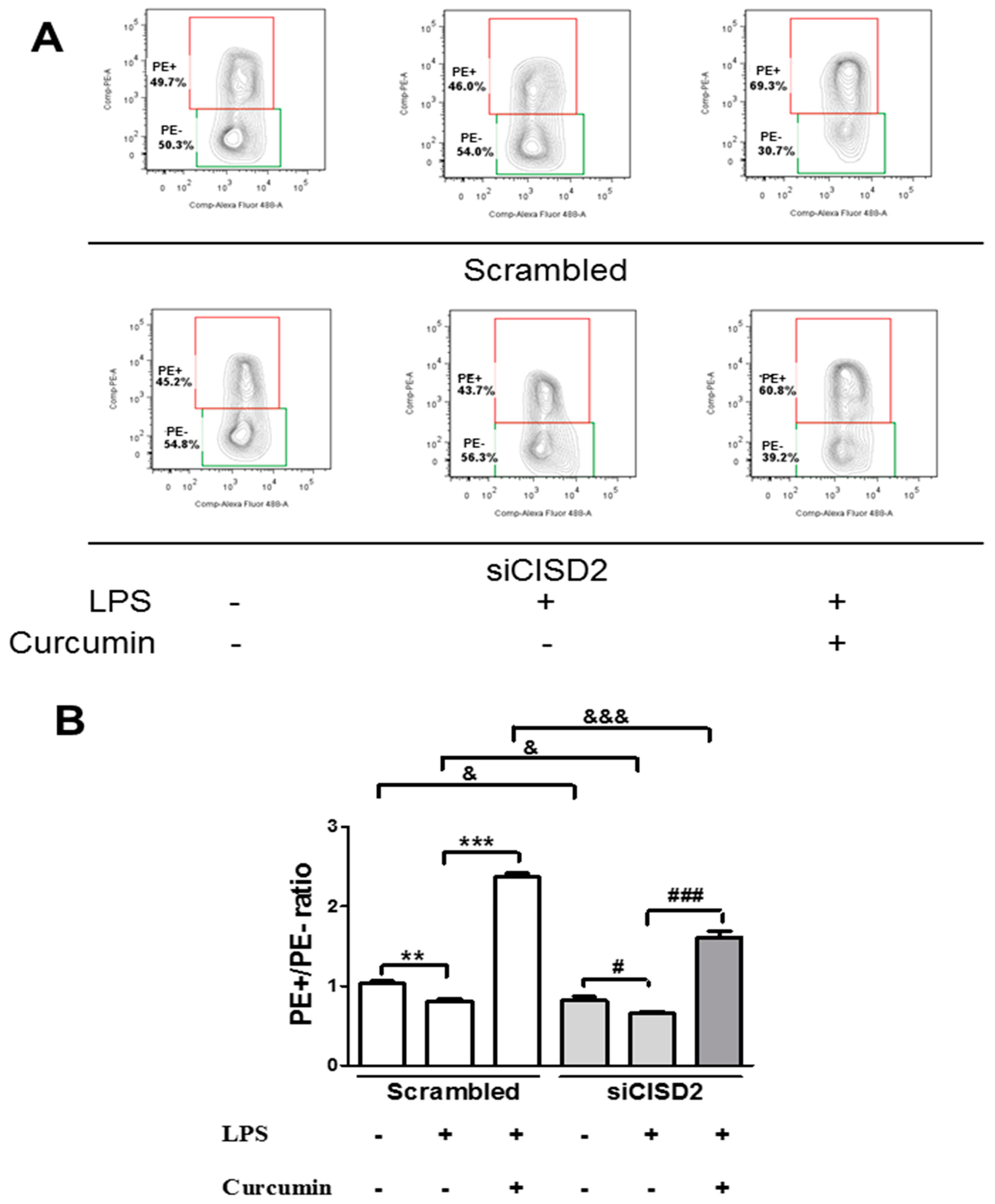
3.6. Curcumin Inhibited LPS-Induced Apoptosis In Vitro
3.7. Curcumin Enhanced Cell Survival in Non-Stressed and Injury-Challenged Neural Cells
3.8. Attenuated Survival Enhancing Effect of Curcumin in Non-Stressed as Well as LPS-Challenged Neural Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kline, K.A.; Bowdish, D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef]
- Maragakis, N.J.; Rothstein, J.D. Mechanisms of Disease: Astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006, 2, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El, K.J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Ravari, A.; Mirzaei, T.; Kennedy, D.; Kazemi, A.M. Chronoinflammaging in Alzheimer; A systematic review on the roles of toll like receptor 2. Life Sci. 2017, 171, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Boveris, A. Brain mitochondrial dysfunction in aging, neurodegeneration, and Parkinson’s disease. Front. Aging Neurosci. 2010, 2, 34. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- McGeer, P.L.; Rogers, J.; McGeer, E.G. Inflammation, anti-inflammatory agents and Alzheimer disease: The last 12 years. J. Alzheimers Dis. 2006, 9, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S232–S240. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L.; Dragicevic, N.; Seifert, K.; Choi, D.Y.; Liu, M.; Kim, H.C.; Cass, W.A.; Sullivan, P.G.; Bing, G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007, 100, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. Biomed. Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel-Ares, M.N.; Riveiro-Naveira, R.R.; Vaamonde-Garcia, C.; Loureiro, J.; Hermida-Carballo, L.; Blanco, F.J.; Lopez-Armada, M.J. Mitochondrial dysfunction promotes and aggravates the inflammatory response in normal human synoviocytes. Rheumatology (Oxford) 2014, 53, 1332–1343. [Google Scholar] [CrossRef]
- Lin, C.C.; Chiang, T.H.; Chen, W.J.; Sun, Y.Y.; Lee, Y.H.; Lin, M.S. CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury 2015, 46, 2341–2350. [Google Scholar] [CrossRef]
- Chen, Y.F.; Kao, C.H.; Chen, Y.T.; Wang, C.H.; Wu, C.Y.; Tsai, C.Y.; Liu, F.C.; Yang, C.W.; Wei, Y.H.; Hsu, M.T.; et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009, 23, 1183–1194. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Free radical reactions of curcumin in membrane models. Free Radic. Biol. Med. 1997, 23, 838–843. [Google Scholar] [CrossRef]
- Weber, W.M.; Hunsaker, L.A.; Gonzales, A.M.; Heynekamp, J.J.; Orlando, R.A.; Deck, L.M.; Vander Jagt, D.L. TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem. Pharmacol. 2006, 72, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.S.; Lee, Y.H.; Chiu, W.T.; Hung, K.S. Curcumin provides neuroprotection after spinal cord injury. J. Surg. Res. 2011, 166, 280–289. [Google Scholar] [CrossRef]
- Lin, M.S.; Sun, Y.Y.; Chiu, W.T.; Hung, C.C.; Chang, C.Y.; Shie, F.S.; Tsai, S.H.; Lin, J.W.; Hung, K.S.; Lee, Y.H. Curcumin Attenuates the Expression and Secretion of RANTES after Spinal Cord Injury In Vivo and Lipopolysaccharide-Induced Astrocyte Reactivation In Vitro. J. Neurotrauma 2011, 28, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Sundar Dhilip, K.S.; Houreld, N.N.; Abrahamse, H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23, 835. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; bdel-Kader, R.; Muller, W.E.; Rimbach, G.; Frank, J. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.S.; Hung, K.S.; Chiu, W.T.; Sun, Y.Y.; Tsai, S.H.; Lin, J.W.; Lee, Y.H. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 931–938. [Google Scholar] [CrossRef]
- Pertusa, M.; Garcia-Matas, S.; Rodriguez-Farre, E.; Sanfeliu, C.; Cristofol, R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 2007, 101, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Currle, D.S.; Hu, J.S.; Kolski-Andreaco, A.; Monuki, E.S. Culture of mouse neural stem cell precursors. J. Vis. Exp. 2007, 2, 152. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Niu, X.; Hu, J.; Yuan, Y.; Sun, S.; Wang, J.; Yu, W.; Wang, C.; Sun, D.; Wang, H. Lin28a protects against hypoxia/reoxygenation induced cardiomyocytes apoptosis by alleviating mitochondrial dysfunction under high glucose/high fat conditions. PLoS ONE 2014, 9, e110580. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Valek, L.; Heidler, J.; Scheving, R.; Wittig, I.; Tegeder, I. Nitric oxide contributes to protein homeostasis by S-nitrosylations of the chaperone HSPA8 and the ubiquitin ligase UBE2D. Redox Biol. 2018, 20, 217–235. [Google Scholar] [CrossRef]
- Rahman, I. Antioxidant therapies in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2006, 1, 15–29. [Google Scholar] [CrossRef]
- Chen, B.; Shen, S.; Wu, J.; Hua, Y.; Kuang, M.; Li, S.; Peng, B. CISD2 associated with proliferation indicates negative prognosis in patients with hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 13725–13738. [Google Scholar]
- Chang, N.C.; Nguyen, M.; Shore, G.C. BCL2-CISD2: An ER complex at the nexus of autophagy and calcium homeostasis? Autophagy 2012, 8, 856–857. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kauppinen, A.; Kaarniranta, K. Context-Dependent Regulation of Autophagy by IKK-NF-kappaB Signaling: Impact on the Aging Process. Int. J. Cell Biol. 2012, 2012, 849541. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.R.; Parnell, L.D.; Ordovas, J.M.; Lai, C.Q. Curcumin and aging. Biofactors 2013, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhuang, Y.; Ying, Z.; Wu, A.; Gomez-Pinilla, F. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience 2009, 161, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. (Lond.) 2007, 4, 8. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting back to the roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Negrette-Guzman, M.; Garcia-Nino, W.R.; Tapia, E.; Zazueta, C.; Huerta-Yepez, S.; Leon-Contreras, J.C.; Hernandez-Pando, R.; paricio-Trejo, O.E.; Madero, M.; Pedraza-Chaverri, J. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evid. Based. Complement. Alternat. Med. 2015, 2015, 917435. [Google Scholar] [CrossRef] [PubMed]
- Sanivarapu, R.; Vallabhaneni, V.; Verma, V. The Potential of Curcumin in Treatment of Spinal Cord Injury. Neurol. Res. Int. 2016, 2016, 9468193. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]

| Gene | Orientation | Sequence |
|---|---|---|
| CISD2 | forward | 5′-AAAATCCCAAGGTGGTGAATGA-3′ |
| reverse | 5′-GGACCGCCAGCACCTACA-3′ | |
| Cyclophilin | forward | 5′-ATGGTCAACCCCACCGTGT-3′ |
| reverse | 5′-CGTGTGAAGTCACCACCCT-3′ | |
| BCL2 | forward | 5′-TGGGATGCCTTTGTGGAACT-3′ |
| reverse | 5′-CAGCCAGGAGAAATCAAACAGA-3′ | |
| iNOS | forward | 5′-CCTCAGTTCTGCGCCTTTG-3′ |
| reverse | 5′-GTTCGTCCCCTTCTCCTGTTG-3′ | |
| RANTES | forward | 5′-TGCCCACGTCAAGGAGTATTT-3′ |
| reverse | 5′-GGCGGTTCCTTCGAGTGA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-C.; Chiang, T.-H.; Sun, Y.-Y.; Lin, M.-S. Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model. Nutrients 2019, 11, 700. https://doi.org/10.3390/nu11030700
Lin C-C, Chiang T-H, Sun Y-Y, Lin M-S. Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model. Nutrients. 2019; 11(3):700. https://doi.org/10.3390/nu11030700
Chicago/Turabian StyleLin, Chai-Ching, Tien-Huang Chiang, Yu-Yo Sun, and Muh-Shi Lin. 2019. "Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model" Nutrients 11, no. 3: 700. https://doi.org/10.3390/nu11030700
APA StyleLin, C.-C., Chiang, T.-H., Sun, Y.-Y., & Lin, M.-S. (2019). Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model. Nutrients, 11(3), 700. https://doi.org/10.3390/nu11030700






