Metabolic Abnormalities in Normal Weight Children Are Associated with Increased Visceral Fat Accumulation, Elevated Plasma Endotoxin Levels and a Higher Monosaccharide Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment of Dietary Intake, Leisure Time Physical Activities and Anthropometric Measurements
2.3. Blood Pressure, Abdominal Ultrasound and Glucose Metabolism
2.4. Laboratory Measurements
2.5. Endotoxin Concentrations and Small Intestinal Bacterial Overgrowth
2.6. Statistical Analysis
3. Results
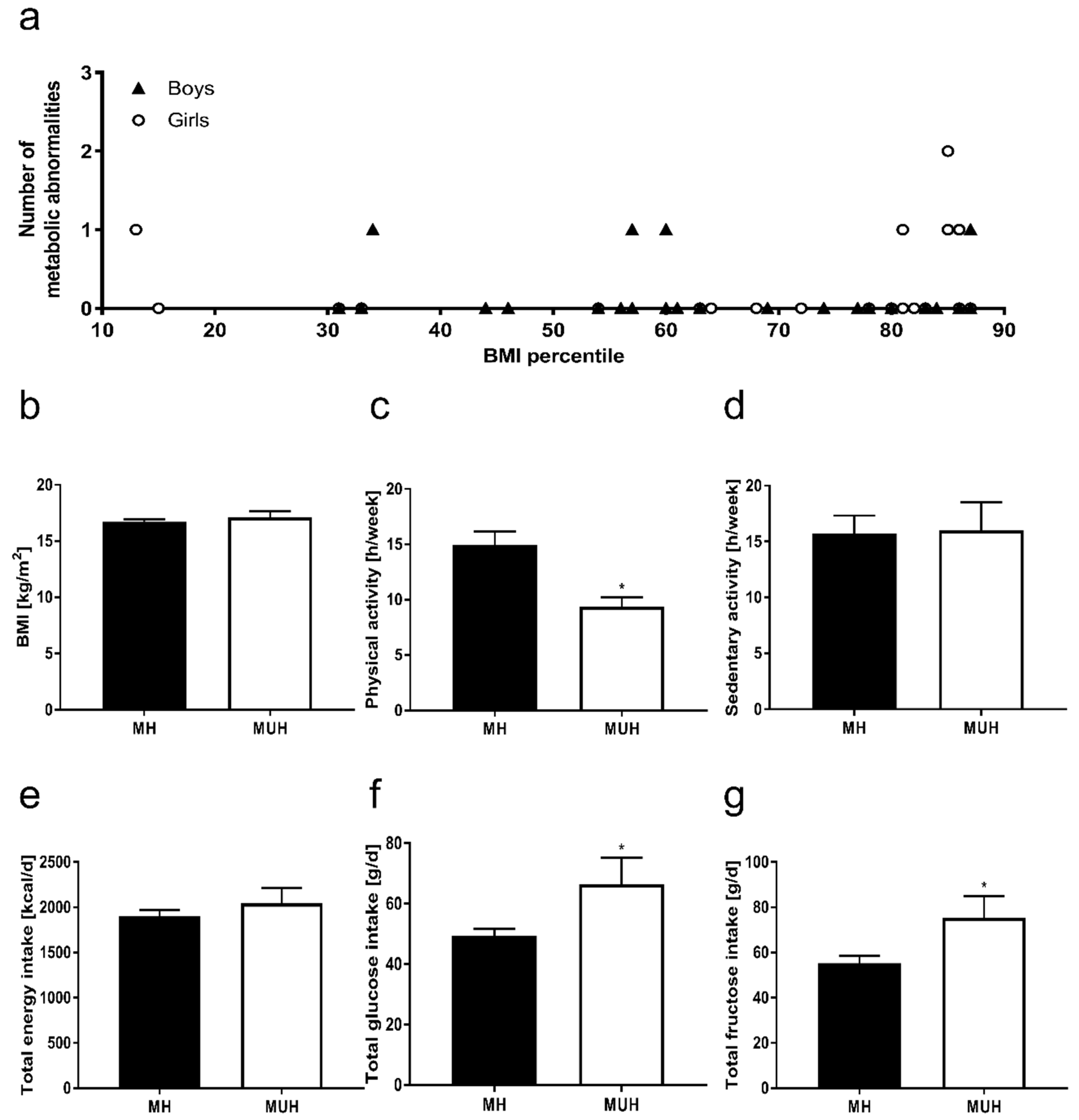
3.1. Anthropometry and Metabolic Characteristics of Participants
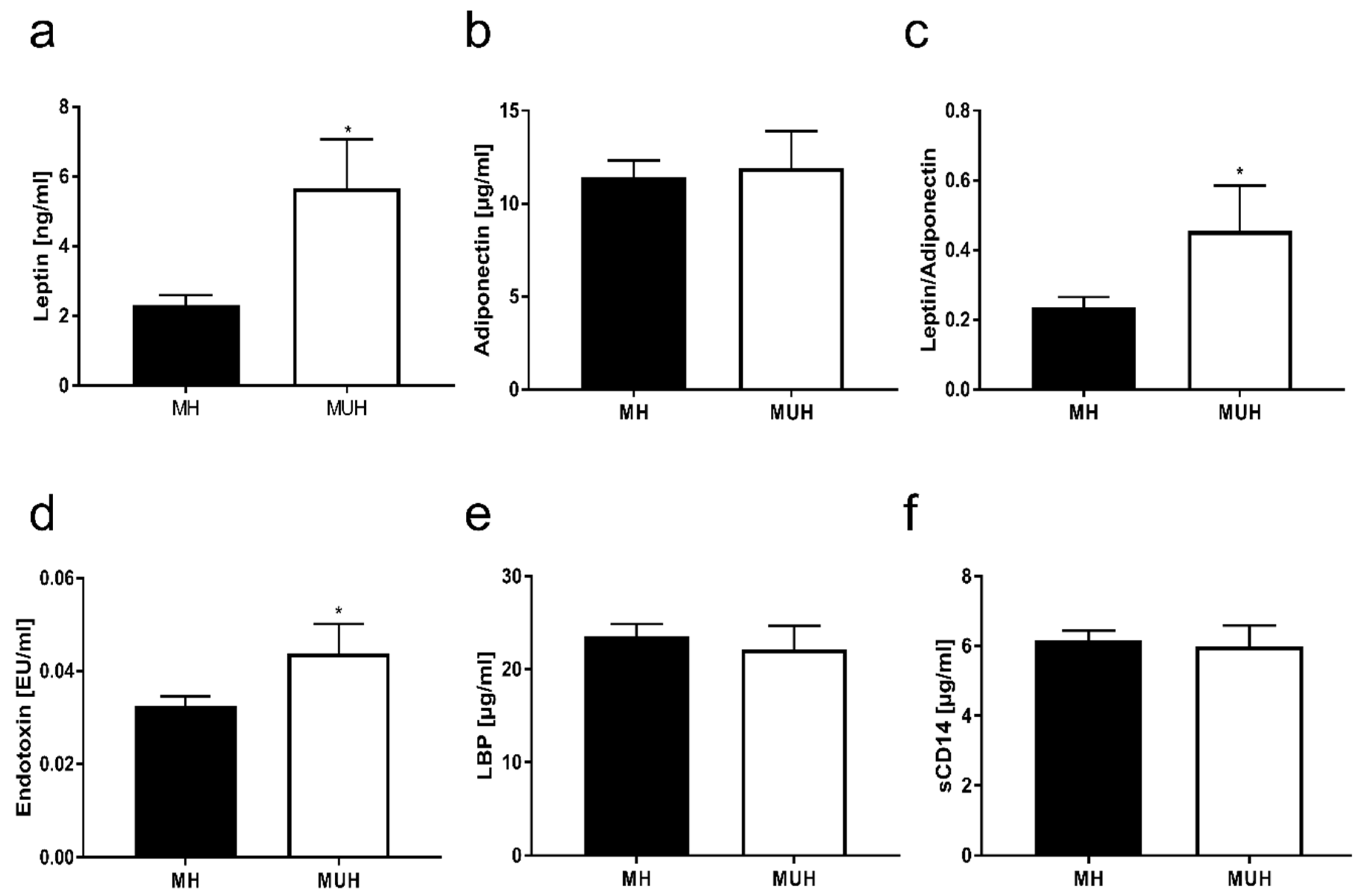
3.2. Pro-Inflammatory Markers and Adipokines in Blood of Normal Weight Children with and without Metabolic Abnormalities
3.3. Markers of Intestinal Permeability in Blood of Normal Weight Children with and without Metabolic Abnormalities
3.4. Nutritional Intake and Physical Activity of Normal Weight Children with and without Metabolic Abnormalities
4. Discussion
4.1. Metabolic Abnormalities in Normal Weight Children Are Associated with a Monosaccharide-Rich Diet and Lower Physical Activity
4.2. Metabolic Abnormalities in Normal Weight Children Are Associated with Altered Intestinal Barrier Function
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Saydah, S.; Bullard, K.M.; Cheng, Y.; Ali, M.K.; Gregg, E.W.; Geiss, L.; Imperatore, G. Trends in cardiovascular disease risk factors by obesity level in adults in the united states, nhanes 1999–2010. Obesity 2014, 22, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third national health and nutrition examination survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.B.; Ozel, Y.; Engstler, A.J.; Puchinger, S.; Wagnerberger, S.; Hulpke-Wette, M.; Bischoff, S.C.; Bergheim, I. Differences in the prevalence of metabolic disorders between prepubertal boys and girls from 5 to 8 years of age. Acta Paediatr. 2014, 103, e154–e160. [Google Scholar] [CrossRef]
- Ding, W.Q.; Yan, Y.K.; Zhang, M.X.; Cheng, H.; Zhao, X.Y.; Hou, D.Q.; Mi, J. Hypertension outcomes in metabolically unhealthy normal-weight and metabolically healthy obese children and adolescents. J. Hum. Hypertens. 2015, 29, 548–554. [Google Scholar] [CrossRef]
- Kuzik, N.; Carson, V.; Andersen, L.B.; Sardinha, L.B.; Grontved, A.; Hansen, B.H.; Ekelund, U.; International Children’s Accelerometry Database, C. Physical activity and sedentary time associations with metabolic health across weight statuses in children and adolescents. Obesity 2017, 25, 1762–1769. [Google Scholar] [CrossRef]
- Magnussen, C.G.; Koskinen, J.; Chen, W.; Thomson, R.; Schmidt, M.D.; Srinivasan, S.R.; Kivimaki, M.; Mattsson, N.; Kahonen, M.; Laitinen, T.; et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: The bogalusa heart study and the cardiovascular risk in young finns study. Circulation 2010, 122, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Gray-McGuire, C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The princeton lipid research clinics follow-up study. Pediatrics 2007, 120, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.; Van Hulst, A.; von Oettingen, J.E.; Benedetti, A.; Paradis, G. Normal weight metabolically unhealthy phenotype in youth: Do definitions matter? Pediatr. Diabetes 2019, 20, 143–151. [Google Scholar] [CrossRef]
- Ruderman, N.; Chisholm, D.; Pi-Sunyer, X.; Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 1998, 47, 699–713. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Berchtold, P.; Schneider, S. Obesity-associated disorders in normal-weight individuals: Some speculations. Int. J. Obes. 1982, 6, 151–157. [Google Scholar] [PubMed]
- Ruderman, N.B.; Schneider, S.H.; Berchtold, P. The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 1981, 34, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Rodriguez-Moran, M. Metabolically obese normal-weight children. World J. Clin. Pediatr. 2012, 1, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Cook, S.R.; Motlagh, M.E.; Gouya, M.M.; Ardalan, G.; Motaghian, M.; Majdzadeh, R.; Ramezani, M.A. Metabolically obese normal weight and phenotypically obese metabolically normal youths: The caspian study. J. Am. Diet. Assoc. 2008, 108, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yaghootkar, H.; Scott, R.A.; White, C.C.; Zhang, W.; Speliotes, E.; Munroe, P.B.; Ehret, G.B.; Bis, J.C.; Fox, C.S.; Walker, M.; et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes 2014, 63, 4369–4377. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, K.; Kimura, I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Manco, M.; Devito, R.; Piemonte, F.; Nobili, V. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 645–649. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (tlr) activation and tlr ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Nier, A.; Engstler, A.J.; Maier, I.B.; Bergheim, I. Markers of intestinal permeability are already altered in early stages of non-alcoholic fatty liver disease: Studies in children. PLoS ONE 2017, 12, e0183282. [Google Scholar] [CrossRef]
- Thuy, S.; Ladurner, R.; Volynets, V.; Wagner, S.; Strahl, S.; Konigsrainer, A.; Maier, K.P.; Bischoff, S.C.; Bergheim, I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J. Nutr. 2008, 138, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Kuper, M.A.; Strahl, S.; Maier, I.B.; Spruss, A.; Wagnerberger, S.; Konigsrainer, A.; Bischoff, S.C.; Bergheim, I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (nafld). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Fornari, E.; Opri, F.; Corradi, M.; Tommasi, M.; Bonadonna, R.; Maffeis, C. High-fat meal, systemic inflammation and glucose homeostasis in obese children and adolescents. Int. J. Obes. (Lond.) 2017, 41, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Nier, A.; Brandt, A.; Conzelmann, I.B.; Ozel, Y.; Bergheim, I. Non-alcoholic fatty liver disease in overweight children: Role of fructose intake and dietary pattern. Nutrients 2018, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Toshimitsu, K.; Matsuura, B.; Ohkubo, I.; Niiya, T.; Furukawa, S.; Hiasa, Y.; Kawamura, M.; Ebihara, K.; Onji, M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 2007, 23, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.C.; Kusminski, C.M.; Azharian, S.; Gilardini, L.; Kumar, S.; Invitti, C.; McTernan, P.G. Metabolic endotoxaemia in childhood obesity. BMC Obes. 2015, 3, 3. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Engstler, A.J.; Aumiller, T.; Degen, C.; Durr, M.; Weiss, E.; Maier, I.B.; Schattenberg, J.M.; Jin, C.J.; Sellmann, C.; Bergheim, I. Insulin resistance alters hepatic ethanol metabolism: Studies in mice and children with non-alcoholic fatty liver disease. Gut 2016, 65, 1564–1571. [Google Scholar] [CrossRef]
- Maier, I.B.; Ozel, Y.; Wagnerberger, S.; Bischoff, S.C.; Bergheim, I. Dietary pattern and leisure time activity of overweight and normal weight children in germany: Sex-specific differences. Nutr. J. 2013, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Holling, H.; Kamtsiuris, P.; Lange, M.; Thierfelder, W.; Thamm, M.; Schlack, R. [the german health interview and examination survey for children and adolescents (kiggs): Study management and conduct of fieldwork]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, D.; Geiss, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Percentiles of body mass index in children and adolescents evaluated from different regional german studies. Monatsschrift Kinderheilkunde 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Angin, Y.; Arslan, N.; Kuralay, F. Leptin-to-adiponectin ratio in obese adolescents with nonalcoholic fatty liver disease. Turk. J. Pediatr. 2014, 56, 259–266. [Google Scholar] [PubMed]
- Aung, K.; Lorenzo, C.; Hinojosa, M.A.; Haffner, S.M. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J. Clin. Endocrinol. Metab. 2014, 99, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Schick, F.; Haring, H.U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017, 26, 292–300. [Google Scholar] [CrossRef]
- Bizeau, M.E.; Pagliassotti, M.J. Hepatic adaptations to sucrose and fructose. Metabolism 2005, 54, 1189–1201. [Google Scholar] [CrossRef]
- Le, K.A.; Faeh, D.; Stettler, R.; Ith, M.; Kreis, R.; Vermathen, P.; Boesch, C.; Ravussin, E.; Tappy, L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006, 84, 1374–1379. [Google Scholar] [CrossRef]
- Le, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef]
- Bursac, B.N.; Vasiljevic, A.D.; Nestorovic, N.M.; Velickovic, N.A.; Vojnovic Milutinovic, D.D.; Matic, G.M.; Djordjevic, A.D. High-fructose diet leads to visceral adiposity and hypothalamic leptin resistance in male rats—Do glucocorticoids play a role? J. Nutr. Biochem. 2014, 25, 446–455. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1049. [Google Scholar] [CrossRef]
- Katsuki, A.; Sumida, Y.; Urakawa, H.; Gabazza, E.C.; Murashima, S.; Maruyama, N.; Morioka, K.; Nakatani, K.; Yano, Y.; Adachi, Y. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care 2003, 26, 2341–2344. [Google Scholar] [CrossRef] [PubMed]
- Trout, K.K.; Homko, C.; Tkacs, N.C. Methods of measuring insulin sensitivity. Biol. Res. Nurs. 2007, 8, 305–318. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Lumeng, C.N. The initiation of metabolic inflammation in childhood obesity. J. Clin. Investig. 2017, 127, 65–73. [Google Scholar] [CrossRef]
- Ruperez, A.I.; Olza, J.; Gil-Campos, M.; Leis, R.; Bueno, G.; Aguilera, C.M.; Gil, A.; Moreno, L.A. Cardiovascular risk biomarkers and metabolically unhealthy status in prepubertal children: Comparison of definitions. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Gil-Campos, M.; Leis, R.; Bueno, G.; Aguilera, C.M.; Valle, M.; Canete, R.; Tojo, R.; Moreno, L.A.; Gil, A. Presence of the metabolic syndrome in obese children at prepubertal age. Ann. Nutr. Metab. 2011, 58, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Bautista-Castano, I. Etiology of obesity: Two “key issues” and other emerging factors. Nutr. Hosp. 2013, 28 (Suppl. 5), 32–43. [Google Scholar] [CrossRef]
- Mager, D.R.; Patterson, C.; So, S.; Rogenstein, C.D.; Wykes, L.J.; Roberts, E.A. Dietary and physical activity patterns in children with fatty liver. Eur. J. Clin. Nutr. 2010, 64, 628–635. [Google Scholar] [CrossRef]
- Bailey, D.P.; Charman, S.J.; Ploetz, T.; Savory, L.A.; Kerr, C.J. Associations between prolonged sedentary time and breaks in sedentary time with cardiometabolic risk in 10–14-year-old children: The happy study. J. Sports Sci. 2017, 35, 2164–2171. [Google Scholar] [CrossRef]
- Carson, V.; Janssen, I. Volume, patterns, and types of sedentary behavior and cardio-metabolic health in children and adolescents: A cross-sectional study. BMC Public Health 2011, 11, 274. [Google Scholar] [CrossRef]
- Holman, R.M.; Carson, V.; Janssen, I. Does the fractionalization of daily physical activity (sporadic vs. Bouts) impact cardiometabolic risk factors in children and youth? PLoS ONE 2011, 6, e25733. [Google Scholar] [CrossRef]
- Bergheim, I.; Weber, S.; Vos, M.; Kramer, S.; Volynets, V.; Kaserouni, S.; McClain, C.J.; Bischoff, S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008, 48, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Jin, C.J.; Nolte, K.; Sellmann, C.; Engstler, A.J.; Bergheim, I. Short-term intake of a fructose-, fat- and cholesterol-rich diet causes hepatic steatosis in mice: Effect of antibiotic treatment. Nutrients 2017, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Wylie, A.T.; Tucker, K.L.; Hamp, T.J.; Gharaibeh, R.Z.; Fodor, A.A.; Cullen, J.M. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am. J. Clin. Nutr. 2013, 98, 349–357. [Google Scholar] [CrossRef]
- Seki, K.; Kitade, M.; Nishimura, N.; Kaji, K.; Asada, K.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Okura, Y.; Takaya, H.; et al. Oral administration of fructose exacerbates liver fibrosis and hepatocarcinogenesis via increased intestinal permeability in a rat steatohepatitis model. Oncotarget 2018, 9, 28638–28651. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Ono, M.; Saibara, T. Is impaired kupffer cell function really important to the pathogenesis of nonalcoholic steatohepatitis? J. Gastroenterol. Hepatol. 2012, 27, 622–624. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serino, M.; Luche, E.; Waget, A.; Pardo, G.; Salvador, J.; Ricart, W.; Fruhbeck, G.; Burcelin, R.; et al. Circulating lipopolysaccharide-binding protein (lbp) as a marker of obesity-related insulin resistance. Int. J. Obes. (Lond.) 2012, 36, 1442–1449. [Google Scholar] [CrossRef]
- Jin, C.J.; Engstler, A.J.; Ziegenhardt, D.; Bischoff, S.C.; Trautwein, C.; Bergheim, I. Loss of lipopolysaccharide-binding protein attenuates the development of diet-induced non-alcoholic fatty liver disease in mice. J. Gastroenterol. Hepatol. 2017, 32, 708–715. [Google Scholar] [CrossRef]
- Hill, R.J.; Davies, P.S.W. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br. J. Nutr. 2007, 85, 415. [Google Scholar] [CrossRef]
- Kohl, H.W.; Fulton, J.E.; Caspersen, C.J. Assessment of physical activity among children and adolescents: A review and synthesis. Prev. Med. 2000, 31, S54–S76. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Jarrett, K.V.; Crawley, H.F. The development of waist circumference percentiles in british children aged 5.0–16.9 y. Eur. J. Clin. Nutr. 2001, 55, 902–907. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Saggiani, F.; Targher, G.; Zenere, M.B.; Alberiche, M.; Monauni, T.; Bonadonna, R.C.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity—Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Gishti, O.; Gaillard, R.; Durmus, B.; Abrahamse, M.; van der Beek, E.M.; Hofman, A.; Franco, O.H.; de Jonge, L.L.; Jaddoe, V.W. BMI, total and abdominal fat distribution, and cardiovascular risk factors in school-age children. Pediatr. Res. 2015, 77, 710–718. [Google Scholar] [CrossRef] [PubMed]
| MH | MUH | |
|---|---|---|
| n | 36 | 9 |
| Sex (male/female) | 20/16 | 4/5 |
| Ethnicity (Caucasian/Asian) | 27/9 | 6/3 |
| Age (years) | 7.3 ± 0.2 | 8.0 ± 0.4 |
| Weight (kg) | 27 ± 1 | 29 ± 5 |
| Height (m) | 1.26 ± 0.01 | 1.30 ± 0.02 |
| BMI (kg/m2) | 16.7 ± 0.2 | 17.1 ± 0.5 |
| BMI-SD score | 0.4 ± 0.1 | 0.4 ± 0.3 |
| Waist circumference (cm) | 59 ± 1 | 64 ± 2 * |
| Waist to Height Ratio | 0.48 ± 0.01 | 0.48 ± 0.01 |
| Systolic blood pressure (mmHg) | 103 ± 1 | 109 ± 3 * |
| Diastolic blood pressure (mmHg) | 62 ± 1 | 70 ± 3 * |
| ALT (U/L) | 19 ± 1 | 20 ± 3 |
| AST (U/L) | 33 ± 1 | 33 ± 2 |
| Triglycerides (mg/dL) | 57 ± 3 | 77 ± 13 * |
| HDL cholesterol (mg/dL) | 57 ± 1 | 56 ± 3 |
| LDL cholesterol (mg/dL) | 100 ± 3 | 113 ± 4 |
| Total cholesterol (mg/dL) | 170 ± 4 | 187 ± 3 |
| Insulin (µIU/mL) | 9 ± 0.4 | 12 ± 1 * |
| Fasting Glucose | 85 ± 0.9 | 87 ± 2.3 |
| HOMA-IR | 1.9 ± 0.1 | 2.6 ± 1.8 * |
| SIBO (with/without) # | 2/32 | 0/7 |
| MH | MUH | |
|---|---|---|
| TNF α (pg/mL) | 0.12 ± 0.004 | 0.12 ± 0.007 |
| IL 6 (pg/mL) | 0.68 ± 0.12 | 1.12 ± 0.47 |
| PAI-1 (U/L) | 6.8 ± 0.8 | 8.7 ± 1.0 |
| CRP (mg/L) | 0.36 ± 0.12 | 0.39 ± 0.17 |
| MH | MUH | |
|---|---|---|
| Total Energy Intake (kcal/day) | 1900 ± 70 | 2043 ± 168 |
| Total Fat Intake (g/day) | 78 ± 4 | 72 ± 8 |
| PUFA (g/day) | 10 ± 1 | 8 ± 1 |
| MUFA (g/day) | 21 ± 1 | 18 ± 3 |
| SFA (g/day) | 29 ± 2 | 22 ± 3 |
| Total Protein Intake (g/day) | 59 ± 3 | 60 ± 6 |
| Animal Protein (g/day) | 27 ± 2 | 30 ± 4 |
| Plant Protein (g/day) | 16 ± 1 | 20 ± 2 |
| Total Carbohydrate Intake (g/day) | 242 ± 10 | 280 ± 30 |
| Starch (g/day) | 99 ± 6 | 96 ± 11 |
| Fructose (g/day) § | 55 ± 3 | 76 ± 10 * |
| Glucose (g/day) § | 49 ± 2 | 67 ± 9 * |
| Fiber Intake (g/day) | 15 ± 1 | 17 ± 2 |
| MH | MUH | |
|---|---|---|
| Beverages (kcal/day) | 161 ± 18 | 265 ± 47 * |
| Fruits/Dried Fruits (kcal/day) | 86 ± 12 | 148 ± 26 * |
| Vegetables/Legumes (kcal/day) | 27 ± 5 | 22 ± 4 |
| Potatoes/Pasta/Rice (kcal/day) | 210 ± 33 | 194 ± 46 |
| Bread (kcal/day) | 259 ± 22 | 239 ± 50 |
| Spreads (kcal/day) | 141 ± 23 | 79 ± 27 |
| Bakery Goods (kcal/day) | 206 ± 28 | 210 ± 54 |
| Meat (kcal/day) | 216 ± 24 | 156 ± 19 |
| Milk and Dairy (kcal/day) | 161 ± 16 | 204 ± 54 |
| Cheese and Quark (kcal/day) | 92 ± 15 | 39 ± 17 |
| Oils, Margarines and Butter (kcal/day) | 94 ± 12 | 65 ±12 |
| Sweets and Sugar (kcal/day) | 154 ± 26 | 239 ± 38 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nier, A.; Brandt, A.; Baumann, A.; Conzelmann, I.B.; Özel, Y.; Bergheim, I. Metabolic Abnormalities in Normal Weight Children Are Associated with Increased Visceral Fat Accumulation, Elevated Plasma Endotoxin Levels and a Higher Monosaccharide Intake. Nutrients 2019, 11, 652. https://doi.org/10.3390/nu11030652
Nier A, Brandt A, Baumann A, Conzelmann IB, Özel Y, Bergheim I. Metabolic Abnormalities in Normal Weight Children Are Associated with Increased Visceral Fat Accumulation, Elevated Plasma Endotoxin Levels and a Higher Monosaccharide Intake. Nutrients. 2019; 11(3):652. https://doi.org/10.3390/nu11030652
Chicago/Turabian StyleNier, Anika, Annette Brandt, Anja Baumann, Ina Barbara Conzelmann, Yelda Özel, and Ina Bergheim. 2019. "Metabolic Abnormalities in Normal Weight Children Are Associated with Increased Visceral Fat Accumulation, Elevated Plasma Endotoxin Levels and a Higher Monosaccharide Intake" Nutrients 11, no. 3: 652. https://doi.org/10.3390/nu11030652
APA StyleNier, A., Brandt, A., Baumann, A., Conzelmann, I. B., Özel, Y., & Bergheim, I. (2019). Metabolic Abnormalities in Normal Weight Children Are Associated with Increased Visceral Fat Accumulation, Elevated Plasma Endotoxin Levels and a Higher Monosaccharide Intake. Nutrients, 11(3), 652. https://doi.org/10.3390/nu11030652





