Baicalein Suppresses Stem Cell-Like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through Up-Regulation of IFIT2
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Generation of Resistant Cells
2.3. Cell Viability
2.4. Clonogenic Assay
2.5. Mammosphere Assay
2.6. Wound Healing Assay
2.7. Invasion Assay
2.8. Flow Cytometry
2.9. Western Blot Assay
2.10. Transcriptomic Analysis
2.11. Pathway Analysis
2.12. Gene Expression Analysis
2.13. Statistical Analysis
3. Results
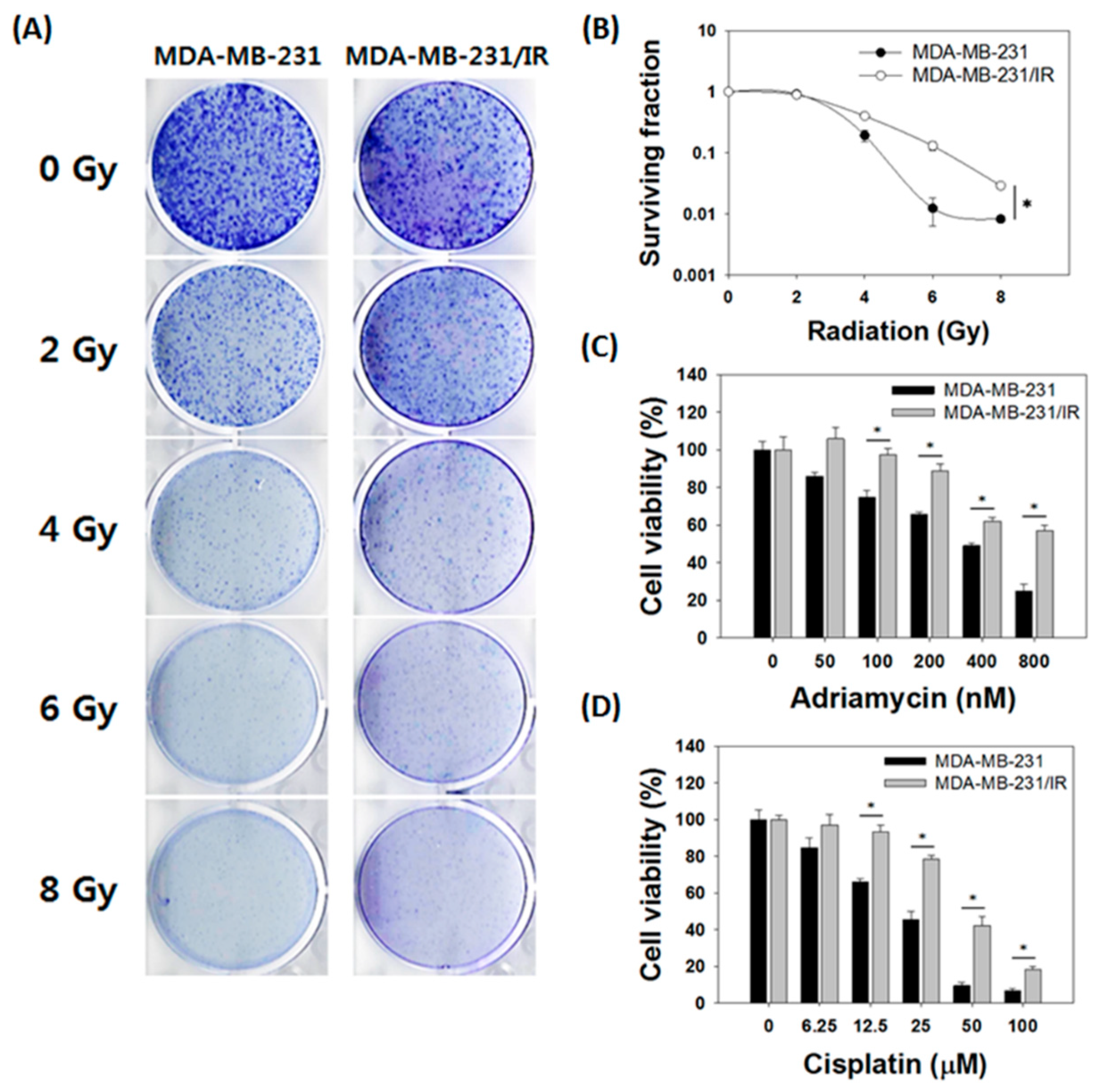
3.1. MDA-MB-231/IR Cells Exhibited Increased Radio- and Chemoresistance Compared to Parental Cells
3.2. Stem Cell Characteristics Were More Prominent in MDA-MB-231/IR Cells than Parent Cells
3.3. Transcriptomic Analysis of MDA-MB-231/IR Cells
3.4. Baicalein Treatment Reversed the Level of IFIT2 Expression in MDA-MB-231/IR Cells
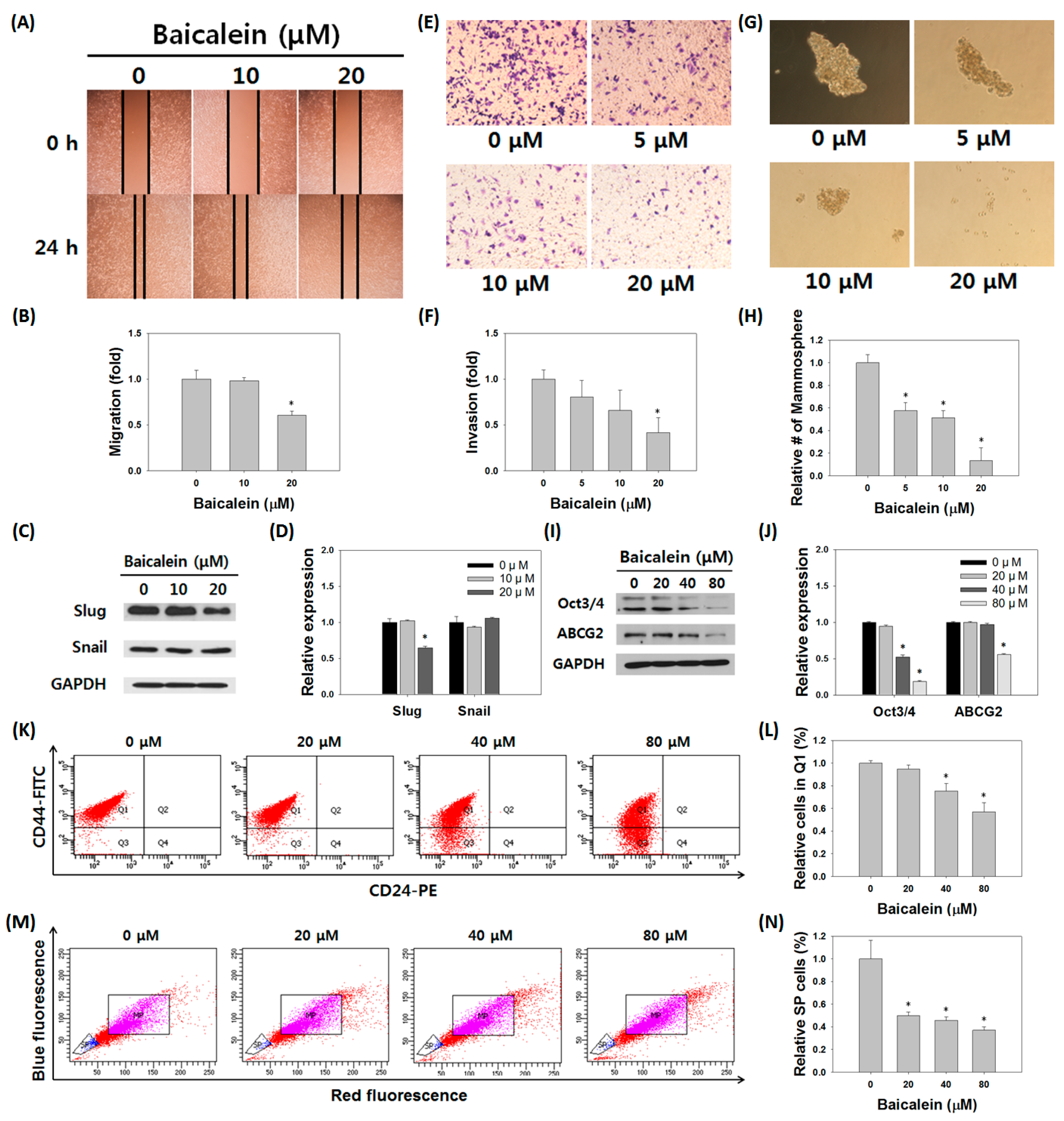
3.5. Baicalein Suppressed the Stem Cell-Like Characteristics of MDA-MB-231/IR Cells
3.6. Baicalein Induced Apoptosis and Reversed Radio- and Chemoresistance in MDA-MB-231/IR Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Adenosine triphosphate-binding cassette |
| ABCG | 2ATP-binding cassette super-family G member 2 |
| AKR1C1 | Aldo-keto reductase 1C1 |
| AKR1C2 | Aldo-keto reductase 1C2 |
| AKR1C3 | Aldo-keto reductase 1C3 |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| BSA | Bovine serum albumin |
| CCDC69 | Coiled-coil domain-containing 69 |
| CHOP | CCAAT/enhancer-binding protein homologous protein |
| CI | Combination index |
| CSCs | Cancer stem cells |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| DEGs | Differentially expressed genes |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| DR5 | Death receptor 5 |
| eIF3 | Eukaryotic initiation factor 3 |
| EMT | Epithelial-mesenchymal transition |
| ER | Estrogen receptor |
| FACS | Fluorescence-activated cell sorting |
| FBS | Fetal bovine serum |
| FPKM | Fragments per kilobase of transcript per million mapped reads |
| FTL | Ferritin light chain |
| GFPT2 | Glutamine-fructose-6-phosphate transaminase 2 |
| GO | Gene ontology |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hazard ratio |
| IC50 | Inhibitory concentration of 50 |
| IFIT2 | Interferon-induced protein with tetratricopeptide repeats 2 |
| IL-1 | Interleukin-1 |
| IR | Irradiation |
| ISG54 | Interferon-stimulated gene 54 |
| KAAS | Automatic Annotation Server |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LG3BP | Galectin-3-binding protein |
| Mcl-1 | Myeloid leukemia 1 |
| MDR1 | Multidrug-resistance protein 1 |
| MK | Midkine |
| MP | Main population |
| MRP1 | Multidrug-resistance-associated protein 1 |
| mTOR | Mammalian target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| PBS | Phosphate buffered saline |
| PI | Propidium iodide |
| PI3K | Phosphatidylinositol 3-kinase |
| PKP3 | Plakophilin 3 |
| PLZF | Promyelocytic leukemia zinc-finger protein |
| PR | Progesterone receptor |
| RFS | Relapse-free survival |
| RIG-1 | Retinoic acid-inducible gene-1 |
| RNA-seq | RNA-sequencing |
| ROS | Reactive oxygen species |
| RSEM | RNA sequencing by Expectation Maximization |
| SATB1 | Special AT-rich sequence binding protein 1 |
| SP | Side population |
| STAR | Spliced Transcripts Alignment to a Reference |
| TCGA | The Cancer Genome Atlas |
| TGF | Transforming growth factor |
| TGFBI | Transforming growth factor beta induced |
| TICs | Tumor initiating cells |
| TLR | Toll-like receptor |
| TNBCs | Triple-negative breast cancers |
| TNF | Tumor necrosis factor |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TWF1 | Twinfilin actin binding protein 1 |
| ULK | Unc-51-like kinase 1 |
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Langlands, F.E.; Horgan, K.; Dodwell, D.D.; Smith, L. Breast cancer subtypes: Response to radiotherapy and potential radiosensitisation. Br. J. Radiol. 2013, 86, 1023. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Gao, Y.; Zhang, X.; Wang, J.; Ding, D.; Zhang, Y.; Zhang, J.; Chen, H. Niclosamide sensitizes triple-negative breast cancer cells to ionizing radiation in association with the inhibition of Wnt/β-catenin signaling. Oncotarget 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Speers, C.; Zhao, S.G.; Chandler, B.; Liu, M.; Wilder-Romans, K.; Olsen, E.; Nyati, S.; Ritter, C.; Alluri, P.G.; Kothari, V.; et al. Androgen receptor as a mediator and biomarker of radioresistance in triple-negative breast cancer. NPJ Breast Cancer 2017, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Roudkenar, M.H.; Urushihara, Y.; Saito, Y.; Tomita, K.; Roushandeh, A.M.; Sato, T.; Kurimasa, A.; Fukumoto, M. Clinically relevant radioresistant cell line: A simple model to understand cancer radioresistance. Med. Mol. Morphol. 2017, 50, 194–204. [Google Scholar] [CrossRef]
- Martin, H.L.; Smith, L.; Tomlinson, D.C. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer 2014, 10, 1–13. [Google Scholar]
- Liu, J.; Chen, X.; Ward, T.; Pegram, M.; Shen, K. Combined niclosamide with cisplatin inhibits epithelial-mesenchymal transition and tumor growth in cisplatin-resistant triple-negative breast cancer. Tumour Biol. 2016, 37, 9825–9835. [Google Scholar] [CrossRef]
- Kang, Y.; Park, M.A.; Heo, S.W.; Park, S.Y.; Kang, K.W.; Park, P.H.; Kim, J.A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochem. Biophys. Acta 2013, 1830, 2638–2648. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirad, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into scid mice. Nature 1994, 367, 645. [Google Scholar] [CrossRef]
- Liu, T.J.; Sun, B.C.; Zhao, X.L.; Zhao, X.M.; Sun, T.; Gu, Q.; Yao, Z.; Dong, X.Y.; Zhao, N.; Liu, N. Cd133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 2013, 32, 544. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.; Zhang, H.; Damelin, M.; Geles, K.G.; Grindley, J.C.; Dirks, P.B. Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 2009, 8, 806–823. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Schatton, T.; Frank, N.Y.; Frank, M.H. Identification and targeting of cancer stem cells. Bioessays 2009, 31, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, C.W.; Harkey, M.A.; Torok-Storb, B. The abcg2 transporter is an efficient hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 2002, 99, 507–512. [Google Scholar] [CrossRef]
- Kim, B.W.; Lee, E.R.; Min, H.M.; Jeong, H.S.; Ahn, J.Y.; Kim, J.H.; Choi, H.Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained erk activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-d culture condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Li, J.; Guo, Y.; Jiang, W.; Huang, C.; Yang, J.; Li, Z. Baicalein, a natural anti-cancer compound, alters microrna expression profiles in bel-7402 human hepatocel-lular carcinoma cells. Cell. Physiol. Biochem. 2017, 41, 1519–1531. [Google Scholar] [CrossRef]
- Wang, L.; Ling, Y.; Chen, Y.; Li, C.L.; Feng, F.; You, Q.D.; Lu, N.; Guo, Q.L. Flavonoid baicalein suppresses adhesion, migration and invasion of mda-mb-231 human breast cancer cells. Cancer Lett. 2010, 297, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, J.; Huang, D.; Wang, W.; Chen, Y.; Liao, Y.; Tang, X.; Xie, H.; Tang, F. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through ezrin in a431 cells. BMC Cancer 2011, 11, 527. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, C.; Schmidt, B.; Park, D.J.; Zhang, A.Y.; Erkizan, H.V.; Toretsky, J.A.; Kirsch, D.G.; Yoon, S.S. Combining parp-1 inhibition and radiation in ewing sarcoma results in lethal dna damage. Mol. Cancer Ther. 2013, 12, 2591–2600. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Dai, Z.; Gao, X.; Ma, Y.; Xu, Q.; Jiang, J.; Zhang, S. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des. Dev. Ther. 2016, 10, 1419–1441. [Google Scholar] [CrossRef]
- Taniguchi, H.; Yoshida, T.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Konishi, M.; Wakada, M.; Kataoka, K.; Yoshikawa, T.; Sakai, T. Baicalein overcomes tumor necrosis factor–related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008, 68, 8918–8927. [Google Scholar] [CrossRef]
- Mai, T.T.; Moon, J.Y.; Song, Y.W.; Viet, P.Q.; Phuc, P.V.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2008, 321, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Hung, L.V.M.; Unno, T.; Cho, S.K. Nobiletin Enhances Chemosensitivity to Adriamycin through Modulation of the Akt/GSK3β/β–Catenin/MYCN/MRP1 Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. Nutrients 2018, 10, 1829. [Google Scholar] [CrossRef]
- Trinotate: Transcriptome Functional Annotation and Analysis. Available online: https://trinotate.github.io/ (accessed on 13 March 2019).
- DAVID Bioinformatics Resources 6.8. Available online: https://david.ncifcrf.gov/ (accessed on 13 March 2019).
- Xena Browser. Available online: https://xenabrowser.net (accessed on 13 March 2019).
- Kaplan-Meier Plotter. Available online: http://kmplot.com/analysis/ (accessed on 13 March 2019).
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cells 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, J.H.; Park, M.; Won, H.Y.; Joo, H.; Lee, C.H.; Lee, J.Y.; Kong, G. UTX inhibits EMT-induced breast CSC properties by epigenetic repression of EMT genes in cooperation with LSD1 and HDAC1. EMBO Rep. 2015, 16, 1288–1298. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Pouget, C.; Lauthier, F.; Simon, A.; Fagnere, C.; Basly, J.P.; Delage, C.; Chulia, A.J. Flavonoids: Structural Requirements for Antiproliferative Activity on Breast Cancer Cells. Bioorg. Med. Chem. Lett. 2001, 11, 3095–3097. [Google Scholar] [CrossRef]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef]
- Gluz, O.; Liedtke, C.; Gottschalk, N.; Pusztai, L.; Nitz, U.; Harbeck, N. Triple-negative breast cancer—current status and future directions. Ann. Oncol. 2009, 20, 1913–1927. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Criscitiello, C.; Azim, H.A., Jr.; Schouten, P.C.; Linn, S.C.; Sotiriou, C. Understanding the biology of triple-negative breast cancer. Ann. Oncol. 2012, 23 (Suppl. 6), vi13–vi18. [Google Scholar] [CrossRef]
- Chao, J.I.; Su, W.C.; Liu, H.F. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol. Cancer Ther. 2007, 6, 3039–3048. [Google Scholar] [CrossRef]
- Aryal, P.; Kim, K.; Park, P.H.; Ham, S.; Cho, J.; Song, K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef]
- Takahashi, H.; Chen, M.C.; Pham, H.; Angst, E.; King, J.C.; Park, J.; Brovman, E.Y.; Ishiguro, H.; Harris, D.M.; Reber, H.A.; et al. Baicalein, a component of scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim. Biophys. Acta 2011, 1813, 1465–1474. [Google Scholar] [CrossRef]
- Velculescu, V.E.; Zhang, L.; Zhou, W.; Vogelstein, J.; Basrai, M.A.; Bassett, D.E., Jr.; Hieter, P.; Vogelstein, B.; Kinzler, K.W. Characterization of the yeast transcriptome. Cell 1997, 88, 243–251. [Google Scholar] [CrossRef]
- Carninci, P.; Kasukawa, T.; Katayama, S.; Gough, J.; Frith, M.C.; Maeda, N.; Oyama, R.; Ravasi, T.; Lenhard, B.; Wells, C.; et al. The transcriptional landscape of the mammalian genome. Science 2005, 309, 1559–1563. [Google Scholar] [PubMed]
- Cie’slik, M.; Chinnaiyan, A.M. Cancer transcriptome profiling at the juncture of clinical translation. Nat. Rev. Genet. 2018, 19, 93–109. [Google Scholar] [CrossRef]
- Hughes, T.R.; Marton, M.J.; Jones, A.R.; Roberts, C.J.; Stoughton, R.; Armour, C.D.; Bennett, H.A.; Coffey, E.; Dai, H.; He, Y.D.; et al. Functional discovery via a compendium of expression profiles. Cell 2000, 102, 109–126. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Cordes, N. Focal adhesion signaling and therapy resistance in cancer. Semin. Cancer Biol. 2015, 31, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.M.; Varghese, S.; Xu, H.; Alexander, H.R. Interleukin-1 and cancer progression: The emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J. Transl. Med. 2006, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Blatch, G.L.; Lässle, M. The tetratricopeptide repeat: A structural motif mediating protein-protein interactions. Bioessays 1999, 21, 932–939. [Google Scholar] [CrossRef]
- Pichlmair, A.; Lassnig, C.; Eberle, C.A.; Górna, M.W.; Baumann, C.L.; Burkard, T.R.; Bürckstümmer, T.; Stefanovic, A.; Krieger, S.; Bennett, K.L.; et al. Ifit1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nat. Immunol. 2011, 12, 624–630. [Google Scholar] [CrossRef]
- Zhou, X.; Michal, J.J.; Zhang, L.; Ding, B.; Lunney, J.K.; Liu, B.; Jiang, Z. Interferon induced IFIT family genes in host antiviral defense. Int. J. Biol. Sci. 2013, 9, 200–208. [Google Scholar] [CrossRef]
- Lai, K.C.; Chang, K.W.; Liu, C.J.; Kao, S.Y.; Lee, T.C. Ifn-induced protein with tetratricopeptide repeats 2 inhibits migration activity and increases survival of oral squamous cell carcinoma. Mol. Cancer Res. 2008, 6, 1431–1439. [Google Scholar] [CrossRef]
- Saha, S.; Sugumar, P.; Bhandari, P.; Rangarajan, P.N. Identification of Japanese encephalitis virus-inducible genes in mouse brain and characterization of garg39/ifit2 as a microtubule-associated protein. J. Gen. Virol. 2006, 87, 3285–3289. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Liu, C.J.; Lin, T.J.; Mar, A.C.; Wang, H.H.; Chen, C.W.; Hong, Z.X.; Lee, T.C. Blocking TNF-α inhibits angiogenesis and growth of IFIT2-depleted metastatic oral squamous cell carcinoma cells. Cancer Lett. 2016, 370, 207–215. [Google Scholar] [CrossRef]
- Shen, H.; Zhan, M.; Zhang, Y.; Huang, S.; Xu, S.; Huang, X.; He, M.; Yao, Y.; Man, M.; Wang, J. PLZF inhibits proliferation and metastasis of gallbladder cancer by regulating IFIT2. Cell Death Dis. 2018, 9, 71. [Google Scholar] [CrossRef]
- Jia, H.; Song, L.; Cong, Q.; Wang, J.; Xu, H.; Chu, Y.; Li, Q.; Zhang, Y.; Zou, X.; Zhang, C.; et al. The lim protein ajuba promotes colorectal cancer cell survival through suppression of JAK1/STAT1/IFIT2 network. Oncogene 2017, 36, 2655. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zheng, X.; Zhong, W.; Tian, X.; Yin, B.; Tian, K.; Zhang, W. Long non-coding RNA LINC00161 sensitises osteosarcoma cells to cisplatin-induced apoptosis by regulating the mir-645-IFIT2 axis. Cancer Lett. 2016, 382, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, Y.; Ma, Z.; Yang, R.; Liang, S.; Zhang, M.; Song, S.; Li, S.; Liu, G.; Fan, D.; et al. MicroRNA-645, up-regulated in human adencarcinoma of gastric esophageal junction, inhibits apoptosis by targeting tumor suppressor IFIT2. BMC Cancer 2014, 14, 633. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhai, W.; Zheng, X.; Xie, Q.; Zhou, Q.; Tao, M.; Zhu, Y.; Wu, C.; Jiang, J. Decreased ifit2 expression promotes gastric cancer progression and predicts poor prognosis of the patients. Cell. Physiol. Biochem. 2018, 45, 15–25. [Google Scholar] [CrossRef]
- Christian, S.L.; Zu, D.; Licursi, M.; Komatsu, Y.; Pongnopparat, T.; Codner, D.A.; Hirasawa, K. Suppression of IFN-induced transcription underlies IFN defects generated by activated Ras/MEK in human cancer cells. PLoS ONE 2012, 7, e44267. [Google Scholar] [CrossRef]
- Stawowczyk, M.; Van Scoy, S.; Kumar, K.P.; Reich, N.C. The interferon stimulated gene 54 promotes apoptosis. J. Biol. Chem. 2011, 286, 7257–7266. [Google Scholar] [CrossRef]




| No. | Difference | Fold | Gene | Full Name | Role in Cancer |
|---|---|---|---|---|---|
| 1 | 97.57 | 6.83 | AKR1C1 | Aldo-keto reductase 1C1 | Metastasis |
| 2 | 79.67 | 6.64 | FTL | Ferritin light chain | Drug resistance |
| 3 | 77.43 | 2.92 | MK | Midkine | Metastasis |
| 4 | 62.20 | 6.01 | LG3BP | Galectin-3-binding protein | Anti-differentiation |
| 5 | 46.92 | 3.04 | GFPT2 | Glutamine-fructose-6-phosphate Transaminase 2 | Metabolism |
| 6 | 42.64 | 3.34 | TGFBI | Transforming growth factor beta induced | Metastasis |
| 7 | 42.35 | 3.87 | AKR1C3 | Aldo-keto reductase 1C3 | Drug resistance |
| 8 | 37.59 | 2.96 | CCDC69 | Coiled-coil domain-containing 69 | Drug resistance |
| 9 | 14.51 | 4.13 | AKR1C2 | Aldo-keto reductase 1C2 | Metastasis |
| 10 | 12.55 | +Inf | TWF1 | Twinfilin actin binding protein 1 | Migration |
| 11 | −4.33 | −Inf | PKP3 | Plakophilin 3 | Invasion |
| 12 | −85.90 | −2.84 | IFIT2 | Interferon induced protein with tetratricopeptide repeats 2 | Apoptosis mediator |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, S.Y.; Moon, J.Y.; Unno, T.; Cho, S.K. Baicalein Suppresses Stem Cell-Like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through Up-Regulation of IFIT2. Nutrients 2019, 11, 624. https://doi.org/10.3390/nu11030624
Koh SY, Moon JY, Unno T, Cho SK. Baicalein Suppresses Stem Cell-Like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through Up-Regulation of IFIT2. Nutrients. 2019; 11(3):624. https://doi.org/10.3390/nu11030624
Chicago/Turabian StyleKoh, So Yae, Jeong Yong Moon, Tatsuya Unno, and Somi Kim Cho. 2019. "Baicalein Suppresses Stem Cell-Like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through Up-Regulation of IFIT2" Nutrients 11, no. 3: 624. https://doi.org/10.3390/nu11030624
APA StyleKoh, S. Y., Moon, J. Y., Unno, T., & Cho, S. K. (2019). Baicalein Suppresses Stem Cell-Like Characteristics in Radio- and Chemoresistant MDA-MB-231 Human Breast Cancer Cells through Up-Regulation of IFIT2. Nutrients, 11(3), 624. https://doi.org/10.3390/nu11030624






