Growth Patterns of Neonates Treated with Thermal Control in Neutral Environment and Nutrition Regulation to Meet Basal Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Settings
2.2. Thermal Control and Nutrition Regulation to Meet Basal Metabolism
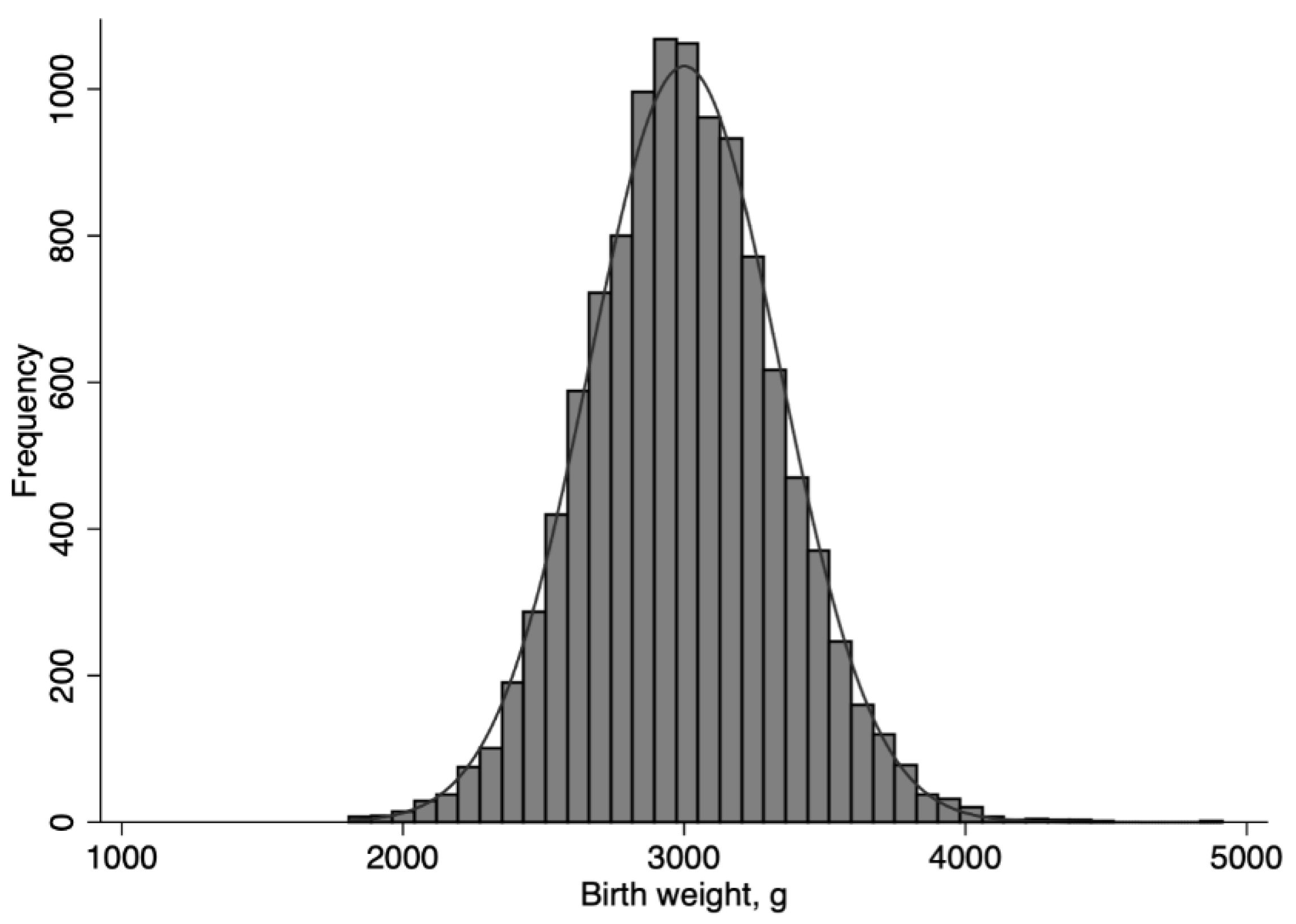
2.3. Birth Weight Categories
2.4. Assessment of Clinical Outcomes and Changes in Body Weight
2.5. Statistical Analysis
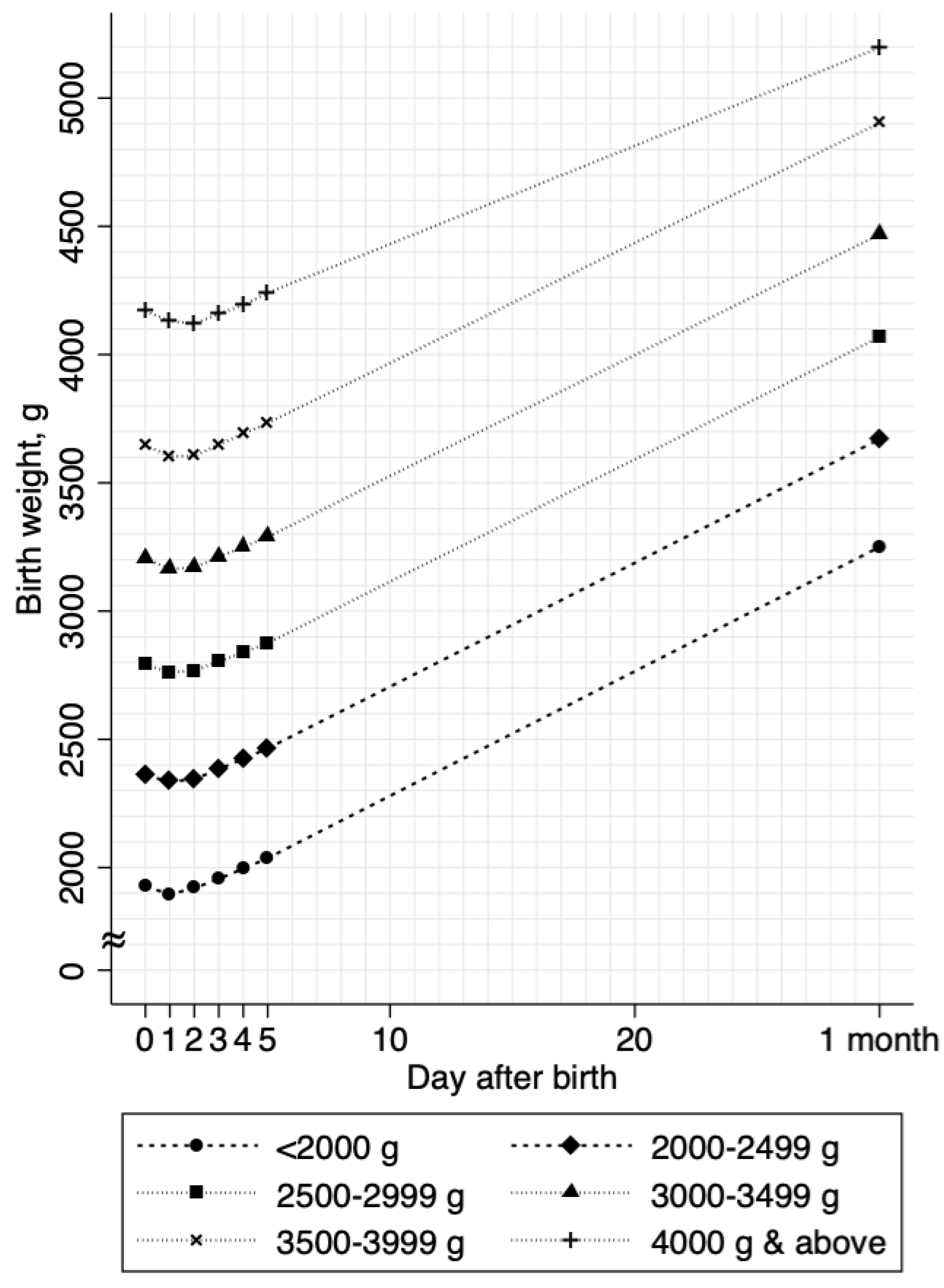
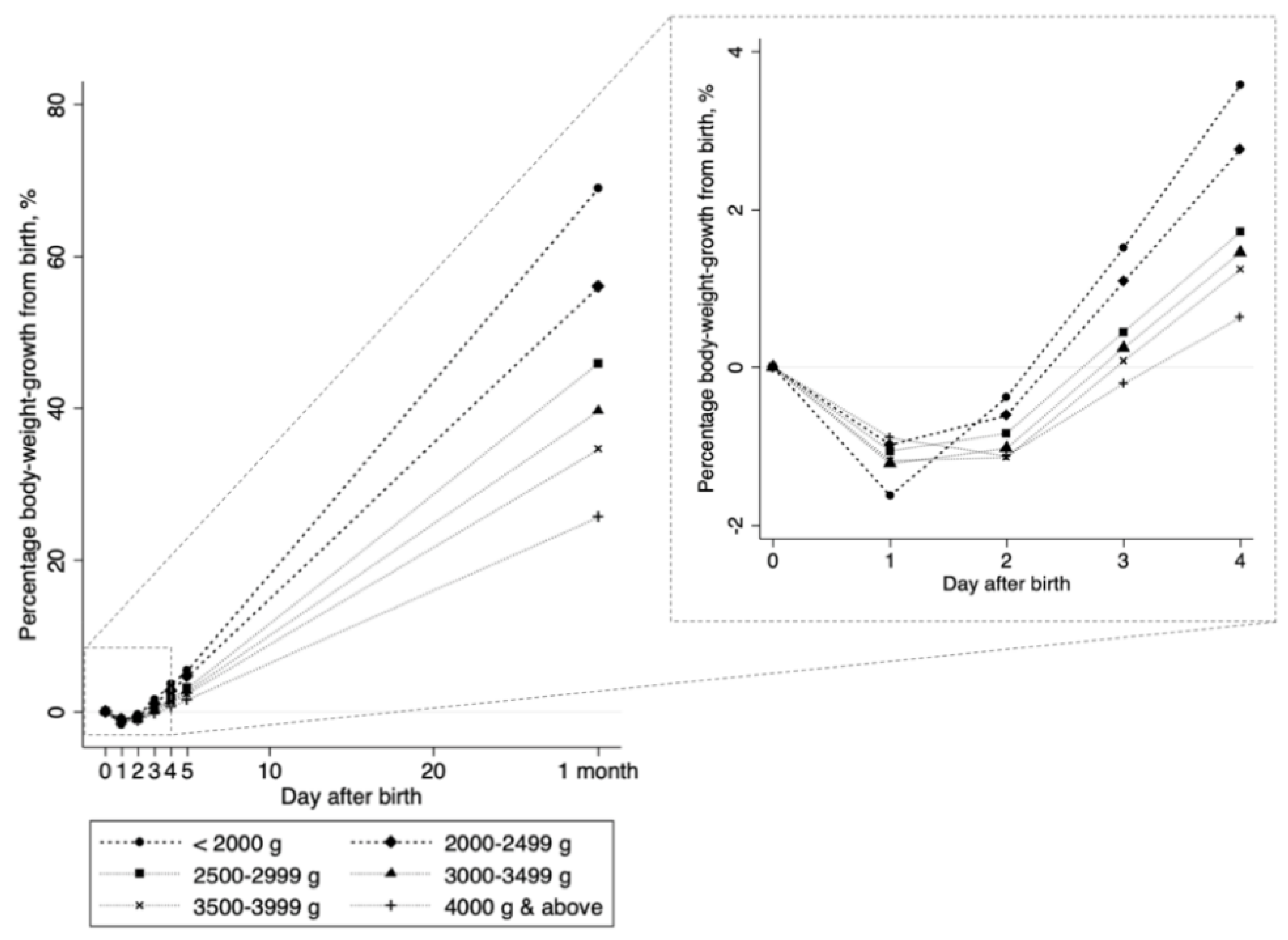
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maisels, M.J.; McDonagh, A.F. Phototherapy for neonatal jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. American Academy of Pediatrics Section on Breastfeeding. Pediatrics 2005, 115, 496–506. [Google Scholar] [PubMed]
- Sato, H.; Uchida, T.; Toyota, K.; Kanno, M.; Hashimoto, T.; Watanabe, M.; Nakamura, T.; Tamiya, G.; Aoki, K.; Hayasaka, K. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G>A (G71R) mutation becomes a risk factor under inadequate feeding. J. Hum. Genet. 2013, 58, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, W.C.; Chen, C.M. Risk factors for hyperbilirubinemia in breastfed term neonates. Eur. J. Pediatr. 2012, 171, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Hsu, M.C.; Shen, C.H.; Wang, C.L.; Chang, S.C.; Wu, K.G.; Wu, S.C.; Chen, S.J. Influence of breast-feeding on weight loss, jaundice, and waste elimination in neonates. Pediatr. Neonatol. 2011, 52, 85–92. [Google Scholar] [CrossRef]
- Seske, L.M.; Merhar, S.L.; Haberman, B.E. Late-onset hypoglycemia in term newborns with poor breastfeeding. Hosp. Pediatr. 2015, 5, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, M.; Yoshihara, T.; Nakai, H.; Kubota, S. Optimal thermal control with sufficient nutrition may reduce the incidence of neonatal jaundice by preventing body-weight loss among NLBW infants not admitted to neonatal intensive care unit. Neonatology 2018, 114, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Koyanagi, T.; Hori, E.; Hara, K.; Shimokawa, H.; Nakano, H. Homeothermal adjustment in the immediate post delivered infant monitored by continuous and simultaneous measurement of core and peripheral body temperatures. Biol. Neonate 1988, 54, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Tsukimori, K.; Wake, N.; Nakano, H. The influence of thermal environment on pulmonary hemodynamic acclimation to extrauterine life in normal full-term neonates. J. Perinat. Med. 2007, 35, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Hey, E.N.; Katz, G. The optimum thermal environment for naked babies. Arch. Dis. Child 1970, 45, 328–334. [Google Scholar] [CrossRef]
- Gross, S.J.; David, R.J.; Bauman, L.; Tomarelli, R.M. Nutritional composition of milk produced by mothers delivering preterm. J. Pediatr. 1980, 96, 641–644. [Google Scholar] [CrossRef]
- Aoshima, N.; Fujie, Y.; Itoh, T.; Tukey, R.H.; Fujiwara, R. Glucose induces intestinal human UDP-glucuronosyltransferase (UGT) 1A1 to prevent neonatal hyperbilirubinemia. Sci. Rep. 2014, 4, 6343. [Google Scholar] [CrossRef] [PubMed]
- Dancis, J.; O’Connell, J.R.; Holt, L.E., Jr. A grid for recording the weight of premature infants. J. Pediatr. 1948, 33, 570–572. [Google Scholar] [CrossRef]
- Brosius, K.K.; Ritter, D.A.; Kenny, J.D. Postnatal growth curve of the infant with extremely LBW who was fed enterally. Pediatrics 1984, 74, 778–782. [Google Scholar] [PubMed]
- Itabashi, K.; Takeuchi, T.; Okuyama, K.; Kuriya, N.; Ohtani, Y. Postnatal growth curves of very LBW Japanese infants. Acta Paediatr. Jpn. 1992, 34, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, K.; Takeuchi, T.; Hayashi, T.; Okuyama, K.; Kuriya, N.; Otani, Y. Postnatal reference growth curves for very LBW infants. Early Hum. Dev. 1994, 37, 151–160. [Google Scholar] [CrossRef]
- Diekmann, M.; Genzel-Boroviczeny, O.; Zoppelli, L.; von Poblotzki, M. Postnatal growth curves for extremely LBW infants with early enteral nutrition. Eur. J. Pediatr. 2005, 164, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Kuboi, T.; Kusaka, T.; Kawada, K.; Koyano, K.; Nakamura, S.; Okubo, K.; Yasuda, S.; Isobe, K.; Itoh, S. Hour-specific nomogram for transcutaneous bilirubin in Japanese neonates. Pediatr. Int. 2013, 55, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Kondo, M.; Kusaka, T.; Isobe, K.; Onishi, S. Differences in transcutaneous bilirubin readings in Japanese term infants according to feeding method. Pediatr. Int. 2001, 43, 12–15. [Google Scholar] [CrossRef]
- Hammarlund, K.; Sedin, G.; Stromberg, B. Transepidermal water loss in newborn infants. VIII. Relation to gestational age and post-natal age in appropriate and small for gestational age infants. Acta Paediatr. Scand. 1983, 72, 721–728. [Google Scholar] [CrossRef]
- Paul, I.M.; Schaefer, E.W.; Miller, J.R.; Kuzniewicz, M.W.; Li, S.X.; Walsh, E.M.; Flaherman, V.J. Weight Change Nomograms for the First Month After Birth. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- Laing, I.A.; Wong, C.M. Hypernatraemia in the first few days: Is the incidence rising? Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F158–F162. [Google Scholar] [CrossRef]
- Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #3: Hospital guidelines for the use of supplementary feedings in the healthy term breastfed neonate, revised 2009. Breastfeed Med. 2009, 4, 175–182. [Google Scholar]
- Miller, J.R.; Flaherman, V.J.; Schaefer, E.W.; Kuzniewicz, M.W.; Li, S.X.; Walsh, E.M.; Paul, I.M. Early weight loss nomograms for formula fed newborns. Hosp. Pediatr. 2015, 5, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.J.; Romphf, L. Factors associated with newborn in-hospital weight loss: Comparisons by feeding method, demographics, and birthing procedures. J. Hum. Lact. 2007, 23, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; Kring, E. Length of Stay, Jaundice, and Hospital Readmission. Pediatrics 1998, 101, 995–998. [Google Scholar] [CrossRef]
- Inoue, S.; Naruse, H.; Yorifuji, T.; Kato, T.; Murakoshi, T.; Doi, H.; Subramanian, S.V. Impact of Maternal and Paternal Smoking on Birth Outcomes. J. Public Health (Oxf.) 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Hung, T.H.; Hsieh, T.T.; Chen, S.F. Risk of Abnormal Fetal Growth in Women with Early- and Late-Onset Preeclampsia. Pregnancy Hypertens. 2018, 12, 201–206. [Google Scholar] [CrossRef]
- Koivunen, S.; Torkki, A.; Bloigu, A.; Gissler, M.; Pouta, A.; Kajantie, E.; Vaarasmaki, M. Towards National Comprehensive Gestational Diabetes Screening—Consequences for Neonatal Outcome and Care. Acta Obstet. Gynecol. Scand. 2017, 96, 106–113. [Google Scholar] [CrossRef]
- Fonseca, M.J.; Severo, M.; Santos, A.C. A new approach to estimating weight change and its reference intervals during the first 96 hours of life. Acta Paediatr. 2015, 104, 1028–1034. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Bhutta, Z.A.; Bertino, E.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Altman, D.G.; Victora, C.; Noble, J.A.; et al. Postnatal growth standards for preterm infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21(st) Project. Lancet Glob. Health 2015, 3, e681–e691. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Lancet Breastfeeding Series Group: Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Moschonis, G.; de Lauzon-Guillain, B.; Jones, L.; Oliveira, A.; Lambrinou, C.P.; Damianidi, L.; Lioret, S.; Moreira, P.; Lopes, C.; Emmett, P.; et al. The effect of early feeding practices on growth indices and obesity at preschool children from four European countries and UK schoolchildren and adolescents. Eur. J. Pediatr. 2017, 176, 1181–1192. [Google Scholar] [CrossRef]
- Flaherman, V.J.; Narayan, N.R.; Hartigan-O’Connor, D.; Cabana, M.D.; McCulloch, C.E.; Paul, I.M. The Effect of Early Limited Formula on Breastfeeding, Readmission, and Intestinal Microbiota: A Randomized Clinical Trial. J. Pediatr. 2018, 196, 84–90. [Google Scholar] [CrossRef]
- Suresh, G.K.; Clark, R.E. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics 2004, 114, 917–924. [Google Scholar] [CrossRef]
- Eggert, L.D.; Wiedmeier, S.E.; Wilson, J.; Christensen, R.D. The Effect of Instituting a Prehospital-Discharge Newborn Bilirubin Screening Program in an 18-Hospital Health System. Pediatrics 2006, 117, e855–e862. [Google Scholar] [CrossRef]
- Flaherman, V.; Schaefer, E.W.; Kuzniewicz, M.W.; Li, S.X.; Walsh, E.M.; Paul, I.M. Health Care Utilization in the First Month After Birth and its Relationship to Newborn Weight Loss and Method of Feeding. Acad. Pediatr. 2018, 18, 677–684. [Google Scholar] [CrossRef]
- Bhutani, V.K.; Johnson, L.H.; Schwoebel, A.; Gennaro, S. A Systems Approach for Neonatal Hyperbilirubinemia in Term and Near-Term Newborns. J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 444–455. [Google Scholar] [CrossRef]
| Characteristics | Mean (SD) or number (%) | p | ||
|---|---|---|---|---|
| Total n = 11,224 | NLBW Neonates (≥2500 g) n = 10,544 | LBW Neonates (<2500 g) n = 680 | ||
| Background characteristics | ||||
| Female | 5468 (49%) | 5066 (48%) | 402 (59%) | <0.001 |
| Gestational age (weeks) | 39.0 (1.2) | 39.0 (1.1) | 37.9 (1.4) | <0.001 |
| Birth weight (g) | 3000 (337) | 3,043 (300) | 2346 (136) | <0.001 |
| Apgar score at 1 min | 9.6 (0.6) | 9.6 (0.6) | 9.5 (0.6) | <0.001 |
| Caesarean delivery | 899 (8.0%) | 810 (7.7%) | 89 (13%) | <0.001 |
| Maternal age (years) | 31 (4) | 31 (4) | 31 (4) | 0.58 |
| Maternal BMI (kg/m2) | 20.0 (2.0) | 20.1 (2.0) | 19.7 (2.0) | <0.001 |
| Multipara | 5,256 (47%) | 4,982 (47%) | 274 (40%) | <0.001 |
| Hypertensive disorders of pregnancy | 43 (0.4%) | 36 (0.3%) | 7 (1.0%) | 0.005 |
| Birth year | 2001 (8) | 2001 (8) | 2002 (8) | 0.001 |
| Clinical outcomes | ||||
| Maximum weight loss (%) | 1.9 (1.5) | 1.9 (1.5) | 1.8 (1.5) | 0.056 |
| Excess weight loss 7% and above | 44 (0.4%) | 39 (0.4%) | 5 (0.7%) | 0.14 |
| Day of peak weight loss | 1.4 (0.9) | 1.4 (0.9) | 1.3 (0.9) | 0.004 |
| Body-weight loss per day (g) | 40 (36) | 41 (36) | 31 (29) | <0.001 |
| Percentage body-weight loss per day (%) 1 | 1.3 (1.2) | 1.3 (1.2) | 1.3 (1.2) | 0.52 |
| Body-weight gain per day (g) | 41 (24) | 41 (25) | 39 (21) | 0.06 |
| Percentage body-weight gain per day (%) 2 | 1.4 (0.8) | 1.4 (0.8) | 1.7 (0.9) | <0.001 |
| Peak bilirubin level at day 4 (mg/dL) | 8.5 (2.7) | 8.5 (2.7) | 8.4 (2.7) | 0.42 |
| Phototherapy | 30 (0.3%) | 30 (0.3%) | 0 (0.0%) | 0.16 |
| Characteristics | Model 1 3 | Model 2 4 | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Percentage body weight loss per day1 | ||||
| LBW (<2500 g) | −0.03 (−0.12 to 0.06) | 0.52 | 0.03 (−0.06 to 0.13) | 0.48 |
| Female | 0.05 (0.004 to 0.09) | 0.03 | ||
| Gestational week | 0.03 (0.01 to 0.05) | 0.001 | ||
| Apgar score | 0.13 (0.09 to 0.17) | <0.001 | ||
| Caesarean delivery | 0.12 (0.03 to 0.20) | 0.005 | ||
| Maternal age | 0.003 (−0.003 to 0.01) | 0.34 | ||
| Maternal body mass index | −0.03 (−0.04 to −0.01) | <0.001 | ||
| Multipara | 0.21 (0.16 to 0.26) | <0.001 | ||
| Hypertensive disorders of pregnancy | 0.24 (−0.11 to 0.59) | 0.18 | ||
| Birth year | −0.02 (−0.02 to −0.02) | <0.001 | ||
| Percentage body weight gain per day2 | ||||
| LBW (<2500 g) | 0.33 (0.26 to 0.39) | <0.001 | 0.52 (0.46 to 0.58) | <0.001 |
| Female | −0.12 (−0.15 to −0.10) | <0.001 | ||
| Gestational week | 0.15 (0.14 to 0.16) | <0.001 | ||
| Apgar score | −0.02 (−0.04 to 0.01) | 0.17 | ||
| Caesarean delivery | 0.001 (−0.05 to 0.05) | >0.99 | ||
| Maternal age | −0.005 (−0.005 to 0.004) | 0.82 | ||
| Maternal body mass index | 0.001 (−0.01 to 0.01) | 0.97 | ||
| Multipara | −0.08 (−0.11 to −0.05) | <0.001 | ||
| Hypertensive disorders of pregnancy | 0.05 (−0.18 to 0.29) | 0.66 | ||
| Birth year | −0.02 (−0.02 to −0.01) | <0.001 | ||
| Characteristics | Percentage Body-Weight-Loss per Day 1 | Percentage Body-Weight-Gain per Day 2 | ||
|---|---|---|---|---|
| β (95% CI) 3 | p | β (95% CI)3 | p | |
| Overall (n = 11,224) | ||||
| Birth weight (per 100 g) | 0.01 (−0.003 to 0.01) | 0.06 | −0.05 (−0.06 to −0.05) | <0.001 |
| Non-LBW (2500 g and above, n = 10,544) | ||||
| Birth weight (per 100 g) | 0.01 (0.001 to 0.02) | 0.02 | −0.04 (−0.05 to −0.03) | <0.001 |
| LBW (<2500 g, n = 680) | ||||
| Birth weight (per 100 g) | 0.003 (−0.07 to 0.08) | 0.93 | −0.16 (−0.21 to −0.11) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubota, S.; Zaitsu, M.; Yoshihara, T. Growth Patterns of Neonates Treated with Thermal Control in Neutral Environment and Nutrition Regulation to Meet Basal Metabolism. Nutrients 2019, 11, 592. https://doi.org/10.3390/nu11030592
Kubota S, Zaitsu M, Yoshihara T. Growth Patterns of Neonates Treated with Thermal Control in Neutral Environment and Nutrition Regulation to Meet Basal Metabolism. Nutrients. 2019; 11(3):592. https://doi.org/10.3390/nu11030592
Chicago/Turabian StyleKubota, Shiro, Masayoshi Zaitsu, and Tatsuya Yoshihara. 2019. "Growth Patterns of Neonates Treated with Thermal Control in Neutral Environment and Nutrition Regulation to Meet Basal Metabolism" Nutrients 11, no. 3: 592. https://doi.org/10.3390/nu11030592
APA StyleKubota, S., Zaitsu, M., & Yoshihara, T. (2019). Growth Patterns of Neonates Treated with Thermal Control in Neutral Environment and Nutrition Regulation to Meet Basal Metabolism. Nutrients, 11(3), 592. https://doi.org/10.3390/nu11030592





