Dianthus superbus Improves Glomerular Fibrosis and Renal Dysfunction in Diabetic Nephropathy Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Dianthus Superbus
2.2. Extraction and Isolation
2.3. High Performance Liquid Chromatography (HPLC) Analysis
2.3.1. Sample Preparation
2.3.2. Chromatography Condition
2.4. Experimental Animals
2.5. Monitoring of Renal Function
2.6. Plasma Biochemical Analysis
2.7. Measurement of Blood Glucose and Insulin Resistance
2.8. Quantitative Histopathology
2.9. Immunohistochemical Staining
2.10. Protein preparation and Western Blot Analysis
2.11. Cell Cultures
2.12. Measurement of Cell Proliferation
2.13. RNA Isolation and Real-Time PCR
2.14. Reactive Oxygen Species (ROS) Measurement
2.15. Statistical Analysis
3. Results
3.1. Calibration Curve and Quantitative Analysis of Standards
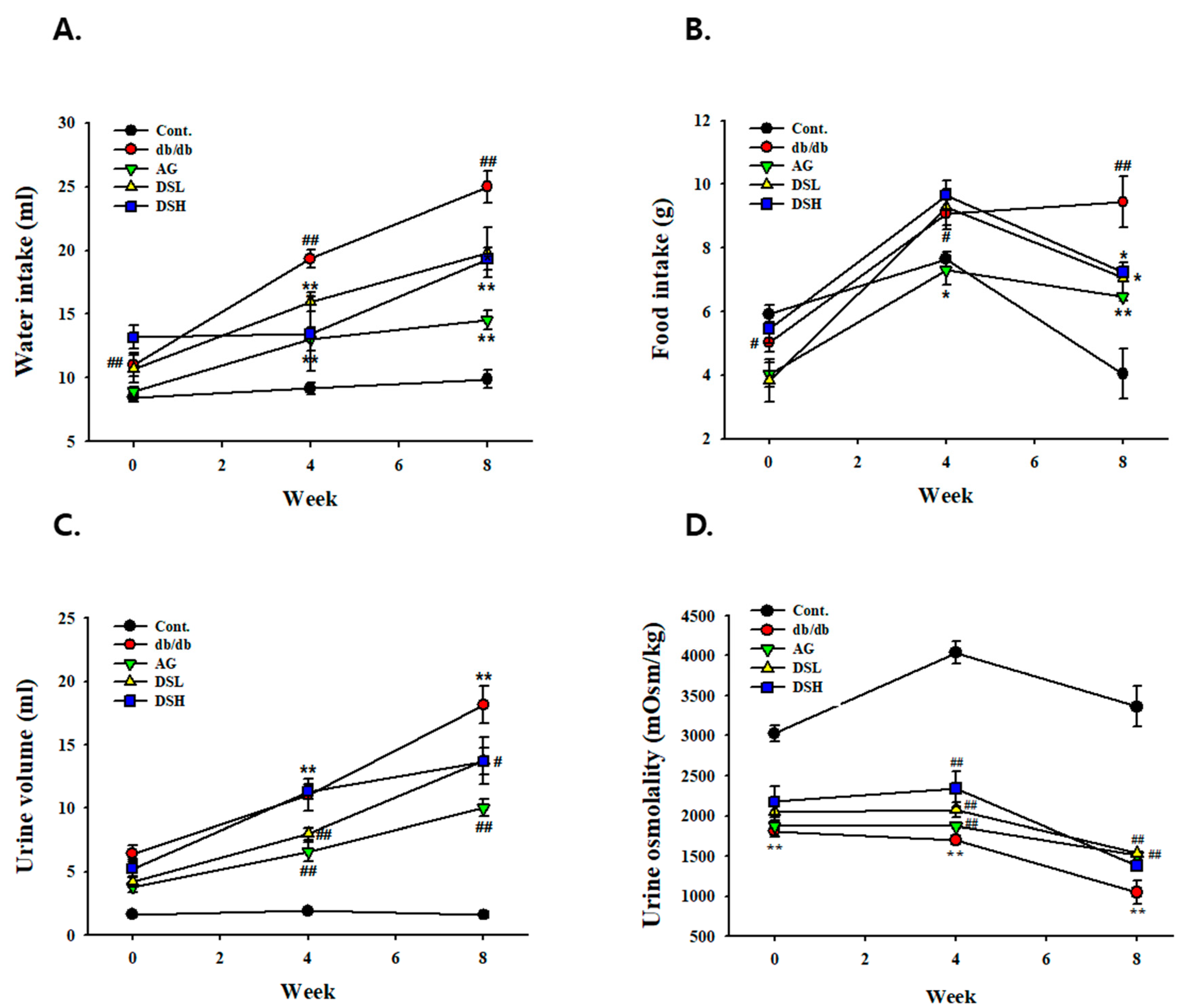
3.2. Effect of DS-EA on Fluid Metabolism in db/db Mice
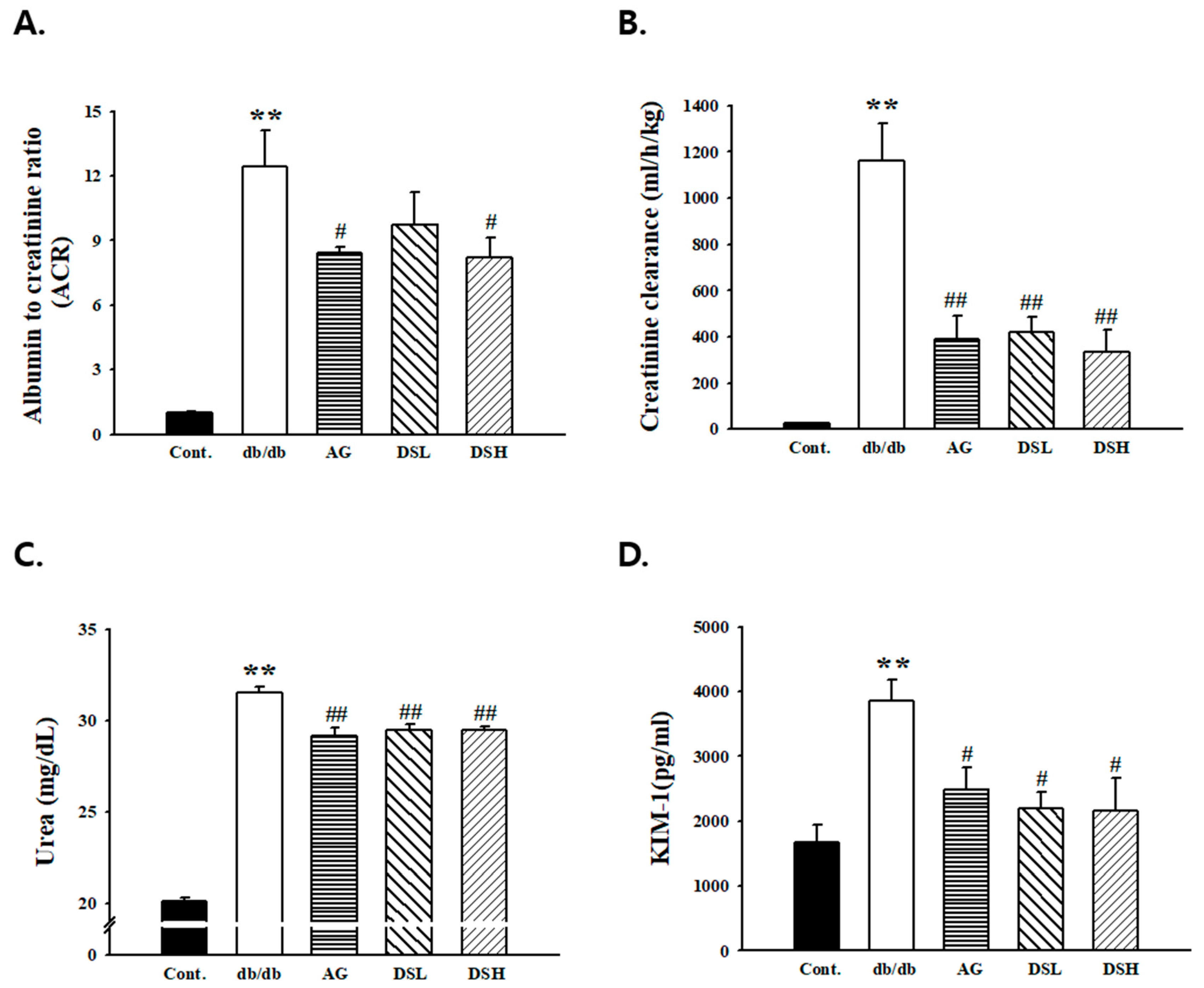
3.3. Effects of DS-EA on Renal Function and Glomerular Morphology
3.4. DS-EA Ameliorated Glucose Tolerance and Insulin Resistance in db/db Mice
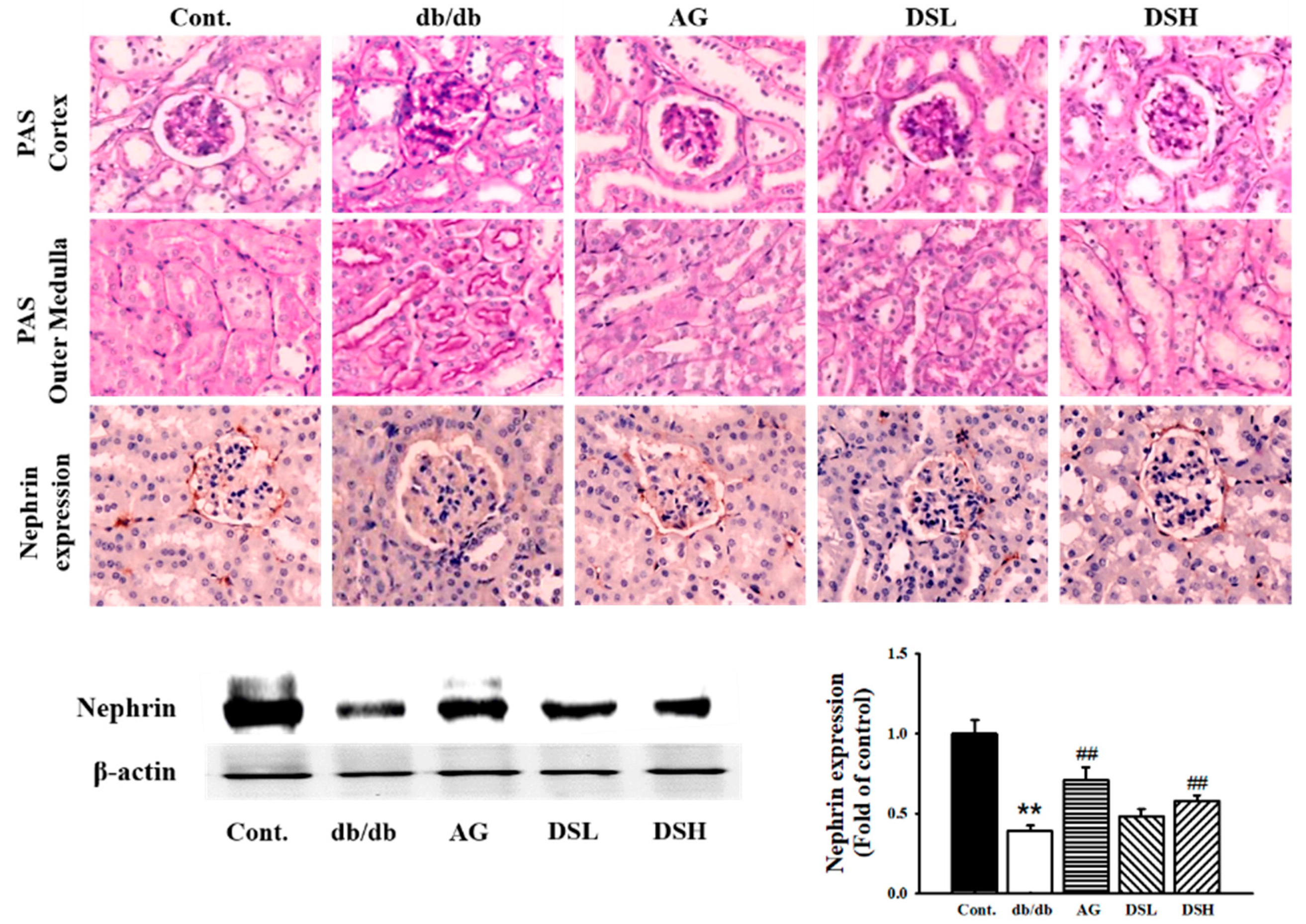
3.5. Effect of DS-EA on Renal Injury and Glomerular Morphological Changes
3.6. Effect of DS-EA on Renal Fibrosis and Inflammation in db/db Mice
3.7. Effect of DS-EA on Human Renal Mesangial Cell Proliferation
3.8. Effect of DS-EA on Renal Mesangial Cell Fibrosis
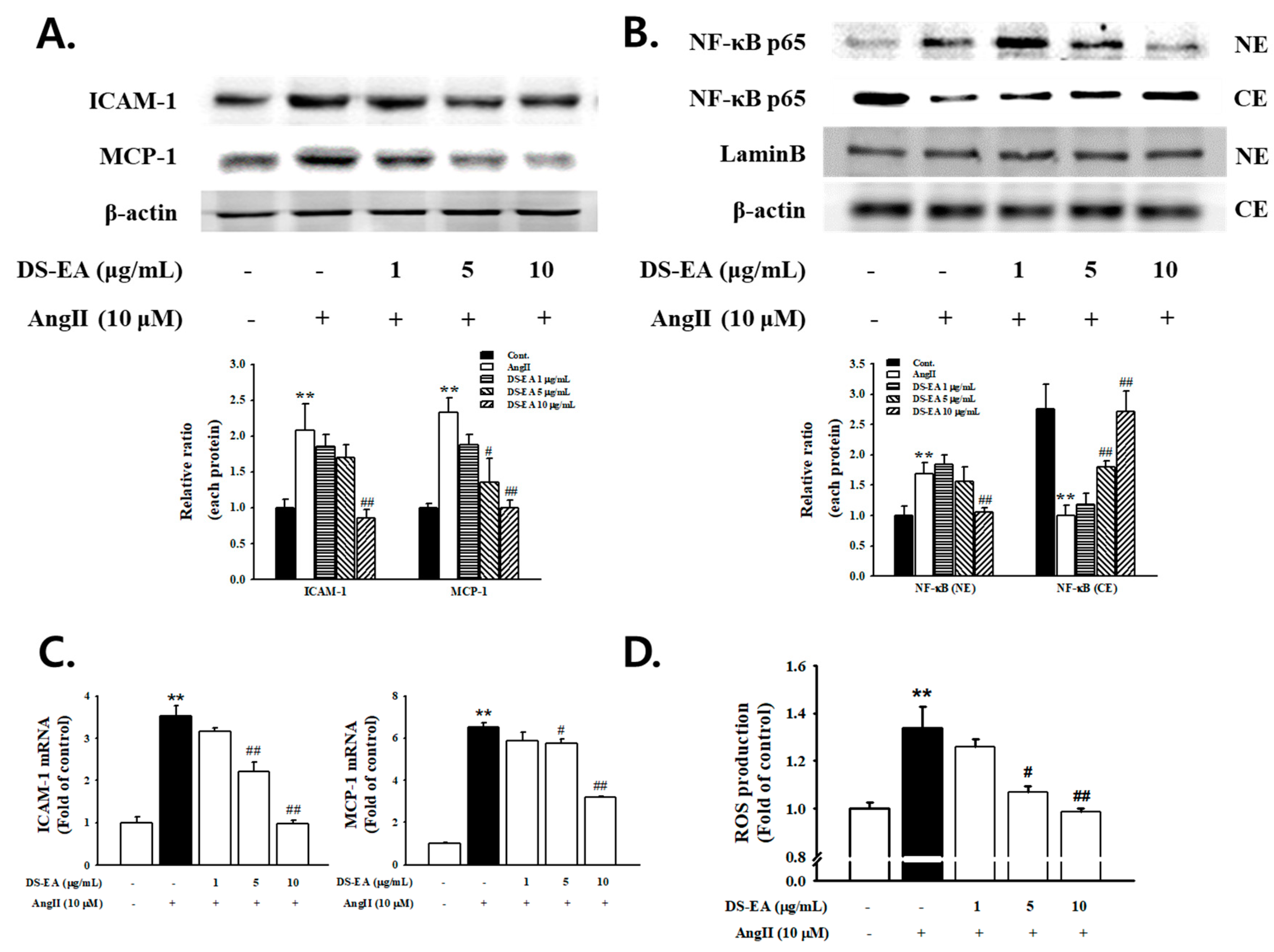
3.9. Effect of DS-EA on Renal Mesangial Cell Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Deepa, B.; Venkatraman Anuradha, C. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.E.; Sharma, K. New pharmacological treatments for improving renal outcomes in diabetes. Nat. Rev. Nephrol. 2010, 6, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Arai, H.; Yanagita, M.; Matsubara, T.; Kanamori, H.; Nakano, T.; Iehara, N.; Fukatsu, A.; Kita, T.; Doi, T. Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J. Biol. Chem. 2003, 278, 18229–18234. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Wang, J.Y.; Huang, Y.T.; Wang, S.Y.; Wang, F.S. Cannabinoid receptor 1 disturbance of PPAR2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J. Mol. Med. 2014, 92, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, W. Protective effect of berberine on renal fibrosis caused by diabetic nephropathy. Mol. Med. Rep. 2017, 16, 1055–1062. [Google Scholar]
- Kolset, S.O.; Reinholt, F.P.; Jenssen, T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in regulation of the tumor microenvironment and drug delivery. Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.; Doody, J.; Timokhina, I.; Massagué, J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev. 1999, 13, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Ziyadeh, F.N.; Wang, A.; Pyagay, P.E.; Kanwar, Y.S.; Chen, S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J. Am. Soc. Nephrol. 2006, 17, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.Q.; Li, H.; Cai, W.M.; Tao, J.; Li, Q.; Ruan, Y.; Zheng, F.P.; Zhang, Z. Effects of pioglitazone on expressions of matrix metalloproteinases 2 and 9 in kidneys of diabetic rats. Chin. Med. J. 2004, 117, 1040–1044. [Google Scholar] [PubMed]
- Youhua, L. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar]
- Twigg, S.M.; Cooper, M.E. 2004. The time has come to target connective tissue growth factor in diabetic complications. Diabetologia 2004, 47, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Twigg, S.M.; Joly, A.H.; Chen, M.M.; Tsubaki, J.; Kim, H.S.; Hwa, V.; Oh, Y.; Rosenfeld, R.G. Connective tissue growth factor/IGF-binding protein-related protein-2 is a mediator in the induction of fibronectin by advanced glycosylation end-products in human dermal fibroblasts. Endocrinology 2002, 143, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bruno, G.; Grimaldi, S.; Gruden, G. Inflammation in diabetic nephropathy: Moving toward clinical biomarkers and targets for treatment. Endocrine 2015, 48, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. (Lond) 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.S.; Kim, H.J.; Ahn, C.W.; Park, C.W.; Cha, B.S.; Lim, S.K.; Kim, K.R.; Lee, H.C. Relationship of serum high sensitivity C-reactive protein to metabolic syndrome and microvascular complications in type 2 diabetes. Diabetes Res. Clin. Pract. 2005, 69, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.K.; Forsblom, C.; Saraheimo, M.; Thorn, L.; Wadén, J.; Høyem, P.; Østergaard, J.; Flyvbjerg, A.; Groop, P.H.; FinnDiane Study Group. Association between mannose-binding lectin, high-sensitivity Creactive protein and the progression of diabetic nephropathy in type 1 diabetes. Diabetologia 2010, 53, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Shyu, K.G.; Wang, B.W.; Lai, L.P. C-reactive protein activates the nuclear factor-κB pathway and induces vascular cell adhesion molecule-1 expression through CD32 in human umbilical vein endothelial cells and aortic endothelial cells. J. Mol. Cell. Cardiol. 2006, 40, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Jin, Y.; Guo, J.; Ziyadeh, F.N. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996, 45, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Munehiro, K.; Yoshio, O.; Daisuke, K. Rodent models of diabetic nephropathy: their utility and limitations. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 279–290. [Google Scholar]
- Cove-Smith, A.; Hendry, B.M. The regulation of mesangial cell proliferation. Nephron Exp. Nephrol. 2008, 108, e74–e79. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R. Synthesis and biological activity of a cyclic hexapeptide from Dianthus superbus. Chem. Pap. 2008, 62, 527–535. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.G.; Kong, L.Y. Two new triterpenoid saponins from Dianthus superbus L. J. Asian Nat. Prod. Res. 2010, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.G.; Chen, X.C.; Kong, L.Y. Three new triterpenoid saponins from Dianthus superbus. Chem. Pharm. Bull. 2011, 59, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.O.; Liao, Z.X.; Lei, J.C.; Hu, X.M. Antioxidant and cytotoxic activities of various fractions of ethanol extract of Dianthus superbus. Food Chem. 2007, 104, 1215–1219. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, W.; Li, J.; Lei, J.; Yu, J. Cytotoxic constituents of ethyl acetate fraction from Dianthus superbus. Nat. Prod. Res. 2013, 27, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Exposito, I.; Castillo, A.; Yang, N.; Liang, B.; Li, X.M. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chin. Med. 2011, 16, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Lee, M.Y.; Ha, H.; Jeon, W.Y.; Seo, C.S.; Shin, H.K. Dianthus superbus fructus suppresses airway inflammation by downregulating of inducible nitric oxide synthase in an ovalbumin-induced murine model of asthma. J. Inflamm. 2012, 9, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Soulis-Liparota, T.; Cooper, M.; Papazoglou, D.; Clarke, B.; Jerums, G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes 1991, 40, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Gilbert, R.E.; Cox, A.J.; Soulis, T.; Jerums, G.; Cooper, M.E. Aminoguanidine Ameliorates Overexpression of Prosclerotic Growth Factors and Collagen Deposition in Experimental Diabetic Nephropathy. J. Am. Soc. Nephrol. 2001, 12, 2098–2107. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Brand, K.; Edgington, T.S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J. Exp. Med. 1991, 174, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, F.; Xia, Z. Chemical constituents of Gynostemma pentaphyllum. Nat. Prod. Res. Dev. 2013, 8, 1067–1069. [Google Scholar]
- Shahbazian, H.; Rezaii, I. Diabetic kidney disease; review of the current knowledge. J. Renal Inj. Prev. 2013, 2, 73–80. [Google Scholar] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br. Med. J. 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Adler, S. Structure–function relationships associated with extracellular matrix alternationsin diabetic glomerulopathy. J. Am. Soc. Nephrol. 1994, 5, 1165–1172. [Google Scholar] [PubMed]
- Ibrahim, H.M.; Hostetter, T.H. Diabetic nephropathy. J. Am. Soc. Nephrol. 1997, 8, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Coward, R.J.; Welsh, G.I.; Koziell, A.; Hussain, S.; Lennon, R.; Ni, L.; Tavaré, J.M.; Mathieson, P.W.; Saleem, M.A. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 2007, 56, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Giunti, S.; Barit, D.; Cooper, M.E. Mechanisms of diabetic nephropathy: role of hypertension. Hypertension 2006, 48, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.P.; Lautenslager, G.T.; Shearman, C.W. Increased collagen IV excretion in diabetes. A marker of compromised filtration function. Diabetes Care 2001, 24, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Andy, K.L.H.; Gregory, H.T. Inflammation in Diabetic Nephropathy. Mediat. Inflamm. 2012, 2012, 12. [Google Scholar]
- Chow, F.Y.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol. Dial. Transplant. 2014, 19, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Deepak, Y.K.; Denis, X.; Alben, S.; Prem, P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: An Indian perspective. Indian J. Med. Res. 2015, 142, 261–268. [Google Scholar]
- Liu, F.; Chen, H.Y.; Huang, X.R.; Chung, A.C.K.; Zhou, L.; Fu, P.; Szalai, A.J.; Lan, H.Y. C-reactive protein promotes diabetic kidney disease in a mouse model of type 1 diabetes. Diabetologia 2011, 54, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
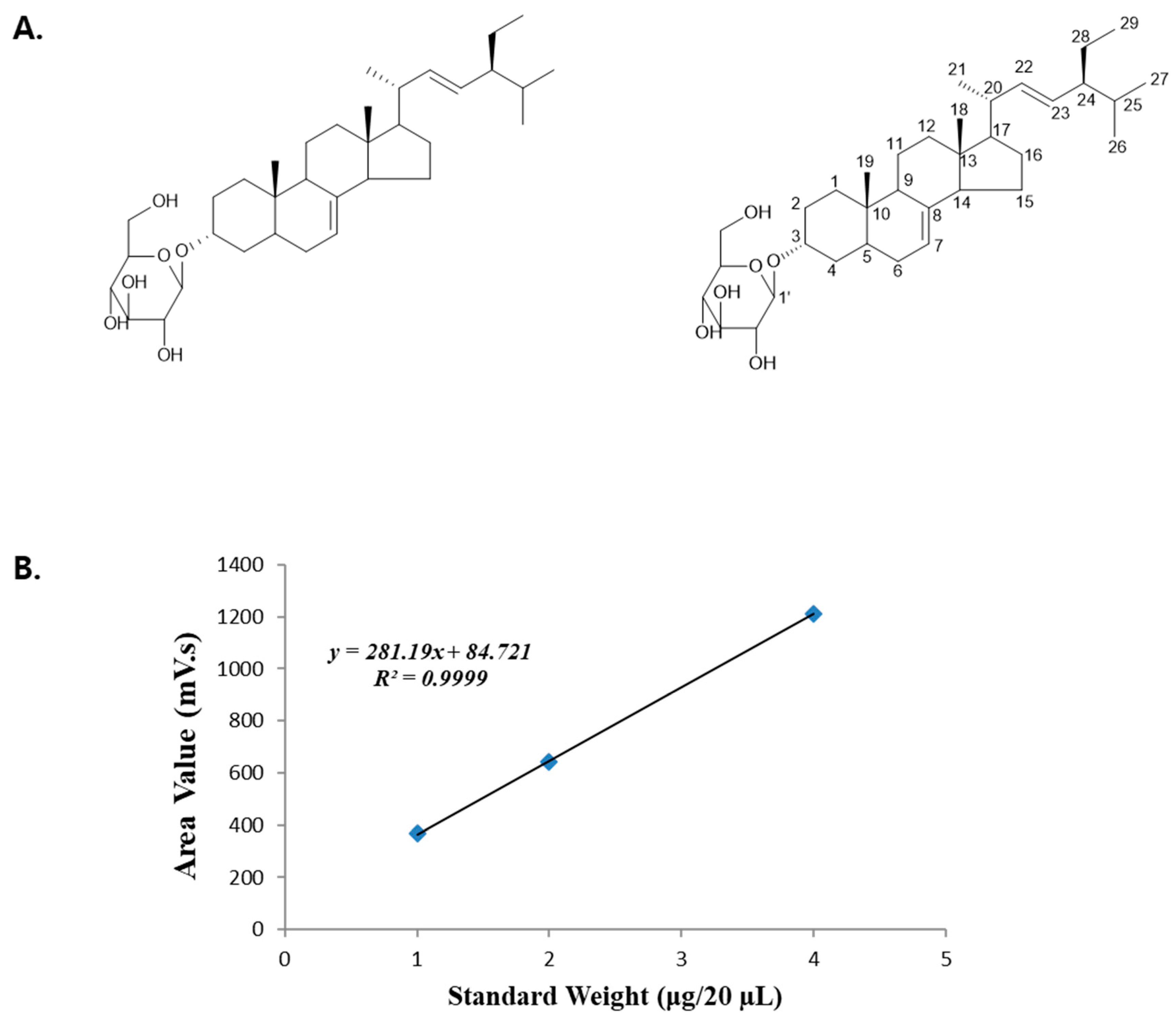




| Standard Weight (μg/20 µL) | Area Value (mV·s) |
|---|---|
| 4 | 1210.772 |
| 2 | 643.183 |
| 1 | 368.516 |
| DS-EA (μg/20 µL) | Area Value (mV·s) | Contents of Standard (μg) | Retention Time (min) |
|---|---|---|---|
| 100 | 607.863 | 1.86 | 25.2 |
| Retention Time (min) | Contents in DS-EA (w/w) |
|---|---|
| 25.2 | 1.86% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.J.; Park, J.H.; Kim, H.J.; Jin, H.-G.; Kim, H.Y.; Ahn, Y.M.; Kim, Y.C.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Dianthus superbus Improves Glomerular Fibrosis and Renal Dysfunction in Diabetic Nephropathy Model. Nutrients 2019, 11, 553. https://doi.org/10.3390/nu11030553
Yoon JJ, Park JH, Kim HJ, Jin H-G, Kim HY, Ahn YM, Kim YC, Lee HS, Lee YJ, Kang DG. Dianthus superbus Improves Glomerular Fibrosis and Renal Dysfunction in Diabetic Nephropathy Model. Nutrients. 2019; 11(3):553. https://doi.org/10.3390/nu11030553
Chicago/Turabian StyleYoon, Jung Joo, Ji Hun Park, Hye Jin Kim, Hong-Guang Jin, Hye Yoom Kim, You Mee Ahn, Youn Chul Kim, Ho Sub Lee, Yun Jung Lee, and Dae Gill Kang. 2019. "Dianthus superbus Improves Glomerular Fibrosis and Renal Dysfunction in Diabetic Nephropathy Model" Nutrients 11, no. 3: 553. https://doi.org/10.3390/nu11030553
APA StyleYoon, J. J., Park, J. H., Kim, H. J., Jin, H.-G., Kim, H. Y., Ahn, Y. M., Kim, Y. C., Lee, H. S., Lee, Y. J., & Kang, D. G. (2019). Dianthus superbus Improves Glomerular Fibrosis and Renal Dysfunction in Diabetic Nephropathy Model. Nutrients, 11(3), 553. https://doi.org/10.3390/nu11030553





