Oral Glutamine Supplementation Reduces Obesity, Pro-Inflammatory Markers, and Improves Insulin Sensitivity in DIO Wistar Rats and Reduces Waist Circumference in Overweight and Obese Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Study
2.2. Animal Study
Determination of the Nuclear Factor Kappa B (NF-κB) Activation
2.3. Statistical Analysis
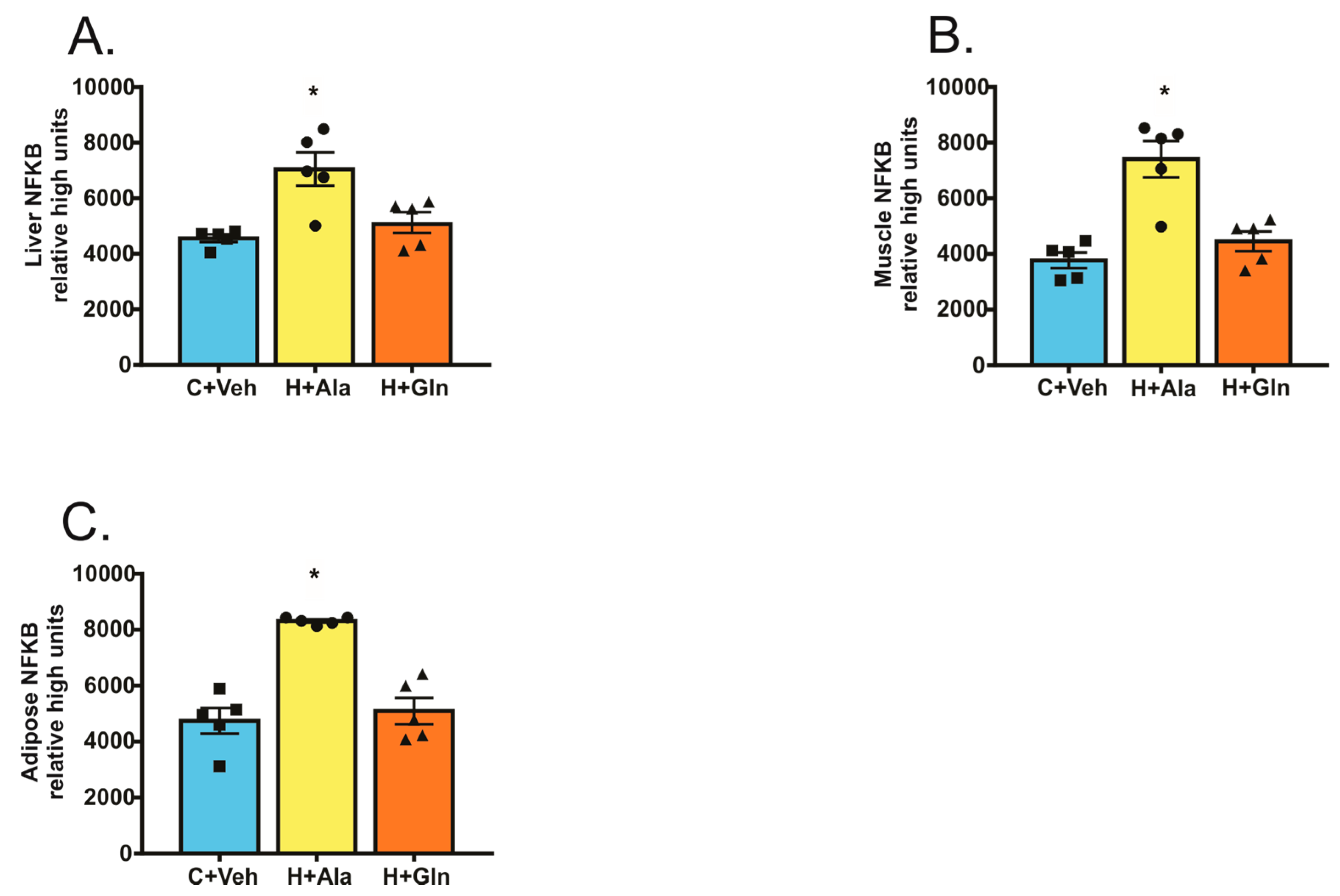
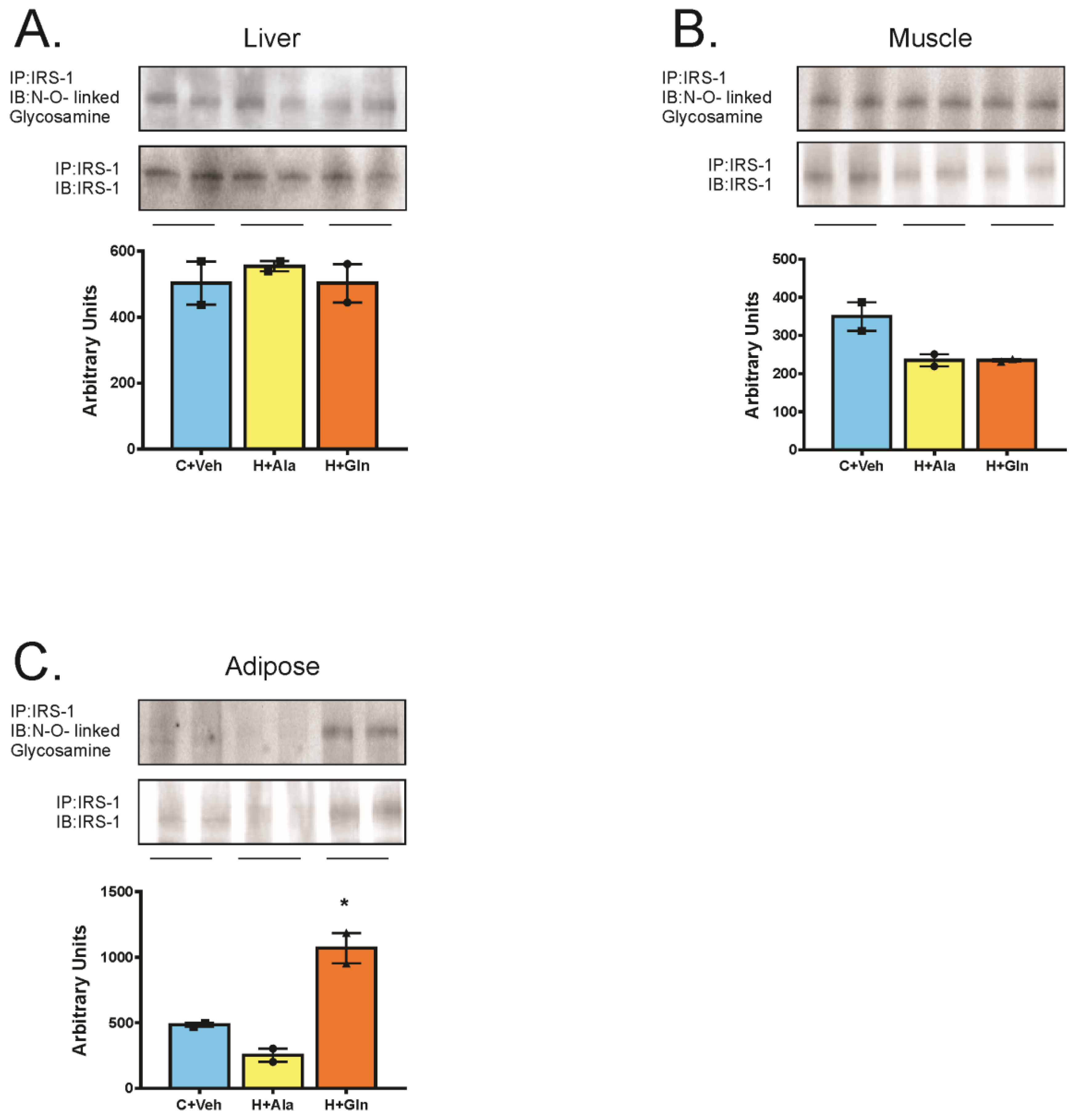
3. Results
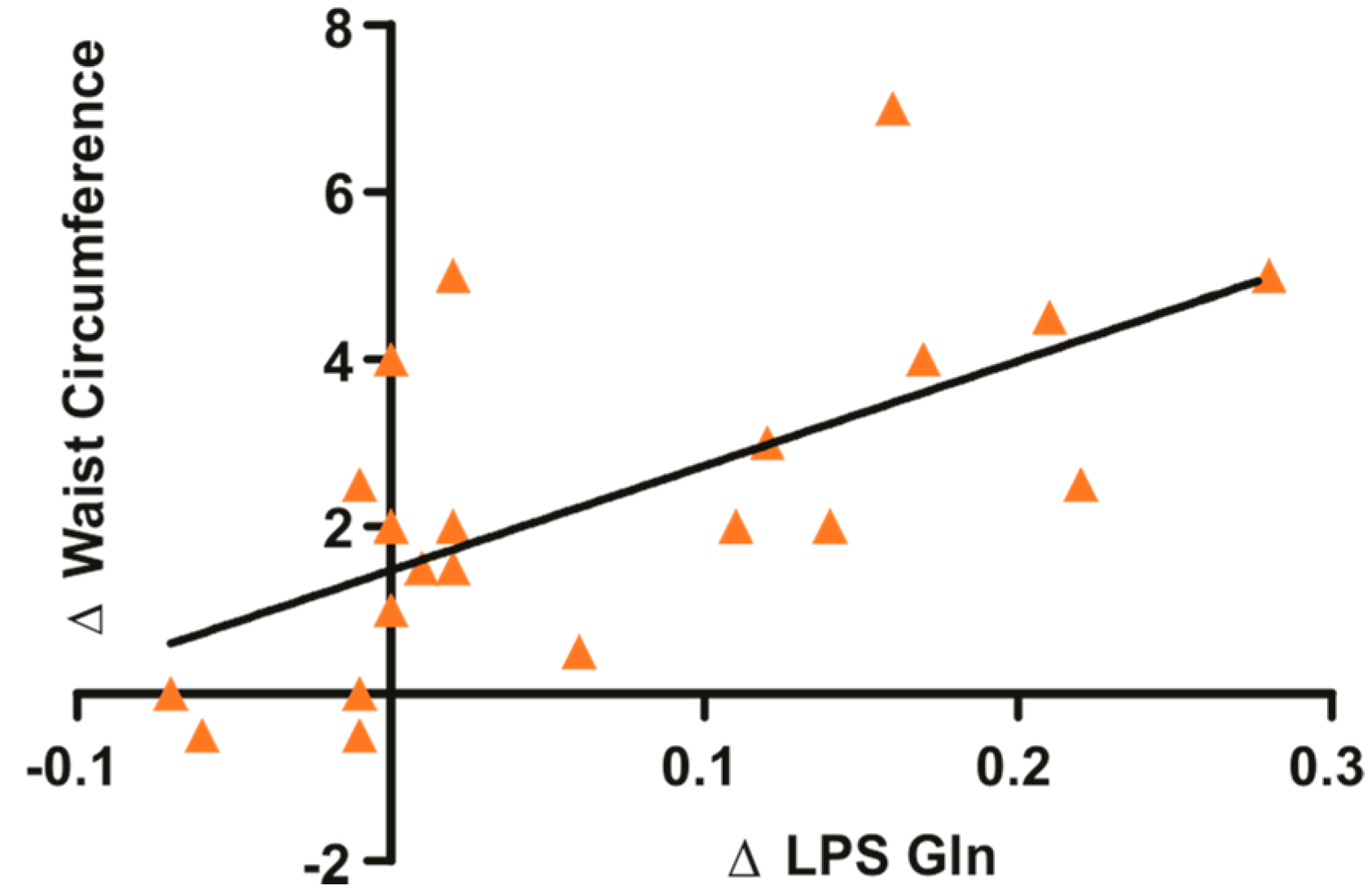
3.1. Glutamine Supplementation in Overweight and Obese Subjects Reduce Waist Circumference and Circulating LPS
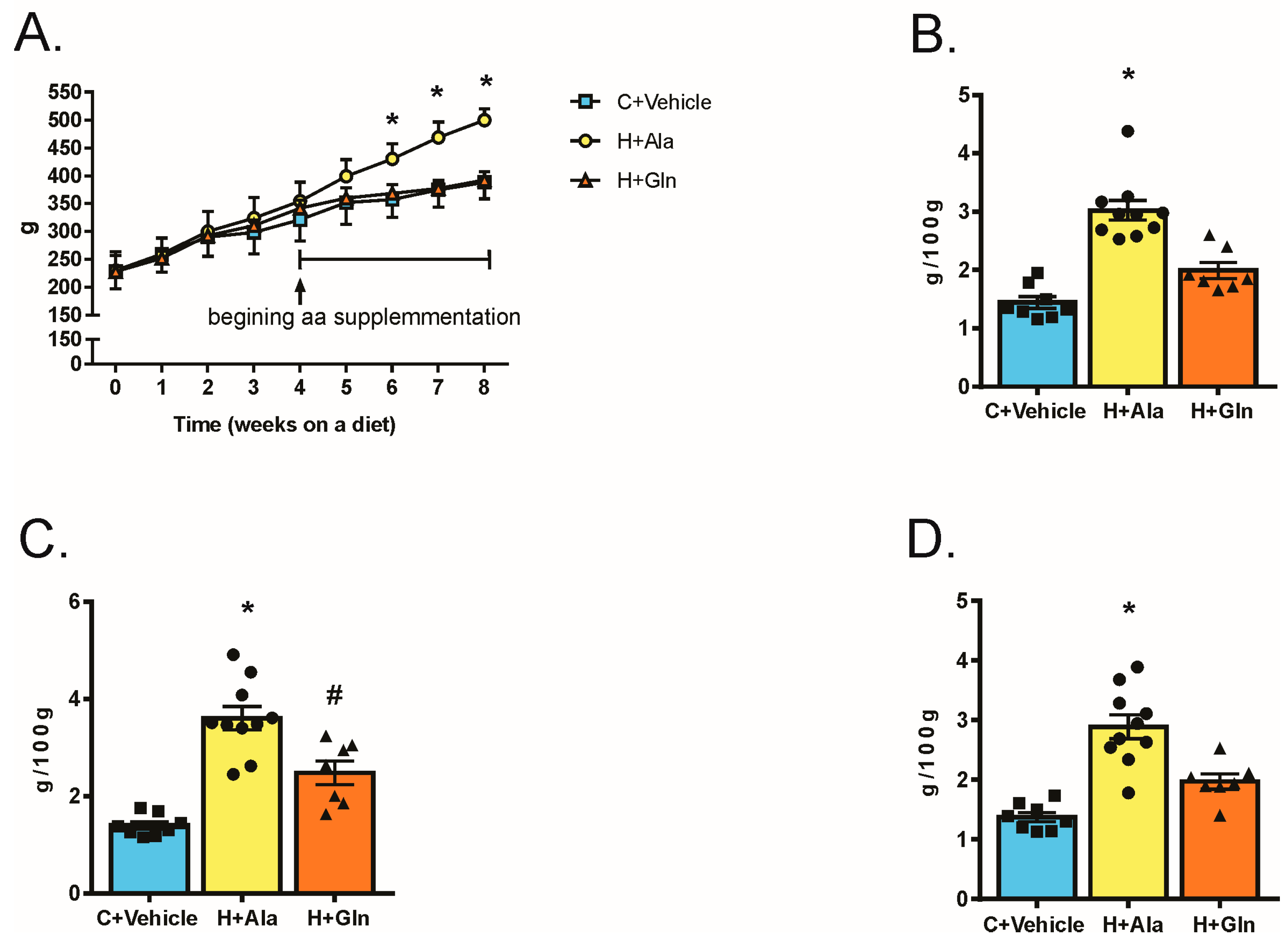
3.2. Effects of Glutamine Supplementation on Animal Characteristics
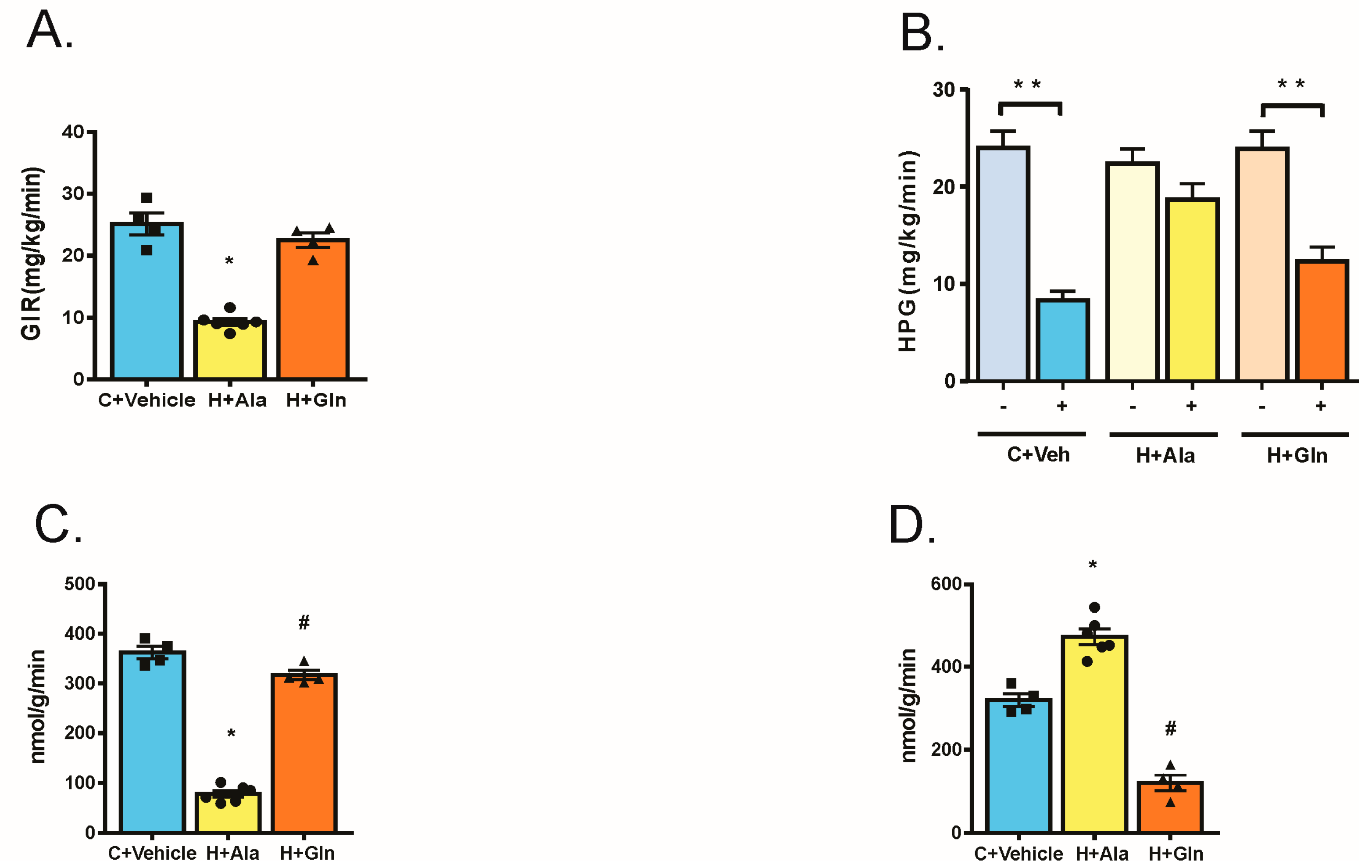
3.3. Effect of Glutamine Supplementation on Insulin-Induced Glucose Uptake in Muscle and Adipose Tissue and on the Suppression of Hepatic Glucose Output
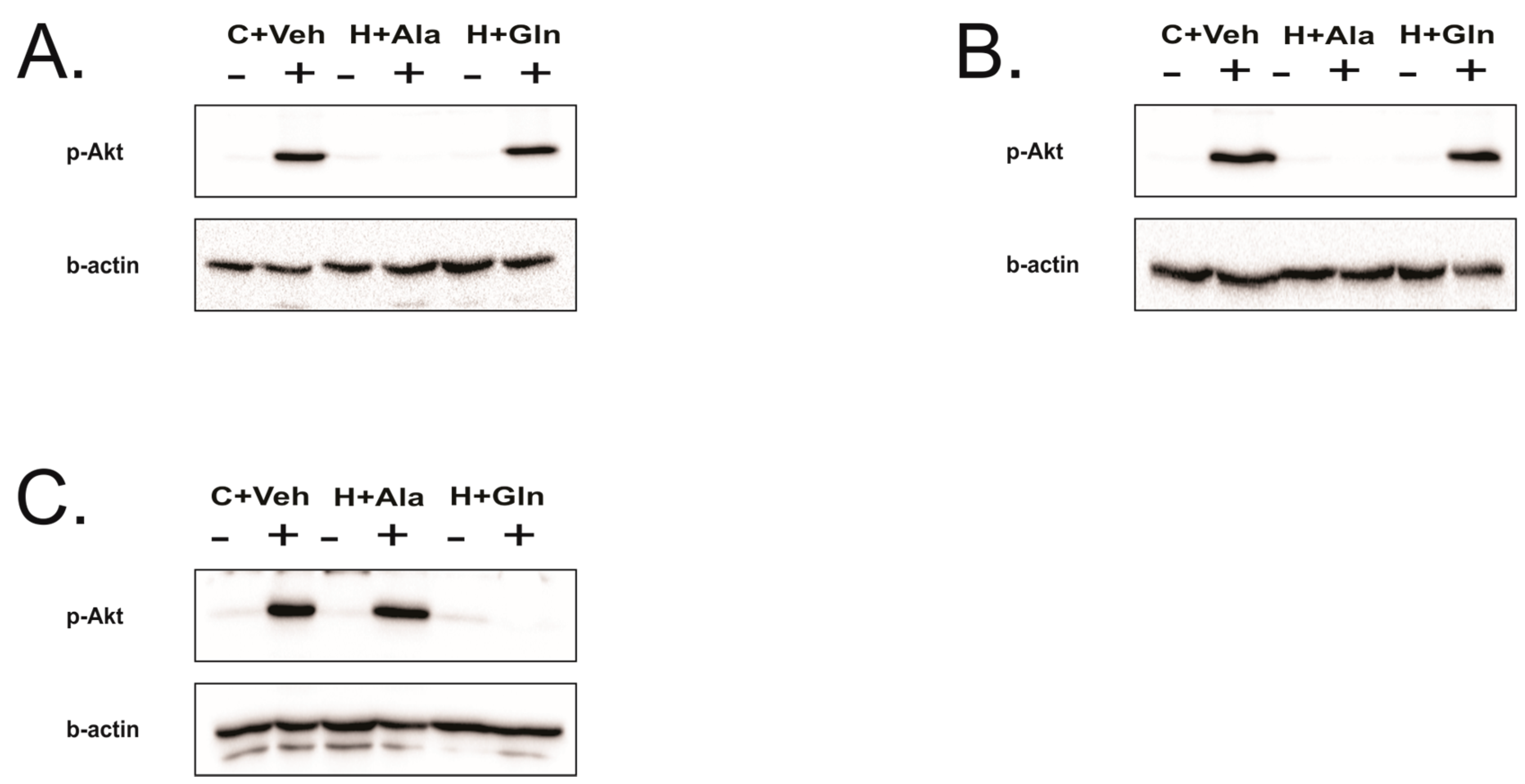
3.4. Insulin-Induced Insulin Signaling in the Tissues of Rats on HFD Supplemented with Glutamine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Collaboration, N.C. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Sellayah, D.; Cagampang, F.R.; Cox, R.D. On the evolutionary origins of obesity: A new hypothesis. Endocrinology 2014, 155, 1573–1588. [Google Scholar] [CrossRef] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.O.; Saad, M.J. Tyrosine kinase inhibitors as novel drugs for the treatment of diabetes. Expert Opin. Investig. Drugs 2013, 22, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Macrophages dispose of catecholamines in adipose tissue. Nat. Med. 2017, 23, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Saad, M.J. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm. 2013, 2013, 986734. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.K.; Wallace, J.M.; Livingstone, M.B. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008, 21, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Obesity in the new millennium. Nature 2000, 404, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Pedersen-Skovsgaard, T.; Mortensen, O.H.; van Hall, G.; Moseley, P.L.; Pedersen, B.K. The effect of glutamine infusion on the inflammatory response and HSP70 during human experimental endotoxaemia. Crit. Care 2009, 13, R7. [Google Scholar] [CrossRef] [PubMed]
- Beutheu, S.; Ouelaa, W.; Guérin, C.; Belmonte, L.; Aziz, M.; Tennoune, N.; Bôle-Feysot, C.; Galas, L.; Déchelotte, P.; Coëffier, M. Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin. Nutr. 2014, 33, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cai, F.; Lin, N.; Ye, J.; Zheng, Q.; Ding, G. Effects of glutamine on oxidative stress and nuclear factor-kappaB expression in the livers of rats with nonalcoholic fatty liver disease. Exp. Ther. Med. 2014, 7, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Shanware, N.P.; Mullen, A.R.; De Berardinis, R.J.; Abraham, R.T. Glutamine: Pleiotropic roles in tumor growth and stress resistance. J. Mol. Med. 2011, 89, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, X.; Yin, Y.; Zhang, C.; He, L. Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids 2012, 43, 813–821. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.Z.; Zambom, A.Z.; Abboud, K.Y.; Reis, S.K.; Tannihao, F.; Guadagnini, D.; Saad, M.J.; Prada, P.O. Oral supplementation with L-glutamine alters gut microbiota of obese and overweight adults: A pilot study. Nutrition 2015, 31, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Molfino, A.; Lacaria, M.T.; Canelli, A.; De Leo, S.; Preziosa, I.; Rossi Fanelli, F. Glutamine supplementation favors weight loss in nondieting obese female patients. A pilot study. Eur. J. Clin. Nutr. 2014, 68, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Qorbani, M.; Heshmat, R.; Larijani, B.; Hosseini, S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015, 31, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Michael, M.D.; Peroni, O.D.; Ueki, K.; Carter, N.; Kahn, B.B.; Kahn, C.R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 2002, 3, 25–38. [Google Scholar] [CrossRef]
- Prada, P.O.; Zecchin, H.G.; Gasparetti, A.L.; Torsoni, M.A.; Ueno, M.; Hirata, A.E.; Corezola do Amaral, M.E.; Hoer, N.F.; Boschero, A.C.; Saad, M.J. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology 2005, 146, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Thirone, A.C.; Carvalheira, J.B.; Hirata, A.E.; Velloso, L.A.; Saad, M.J. Regulation of Cbl-associated protein/Cbl pathway in muscle and adipose tissues of two animal models of insulin resistance. Endocrinology 2004, 145, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Garvey, W.T.; Traxinger, R.R. New insights into the metabolic regulation of insulin action and insulin resistance: Role of glucose and amino acids. FASEB J. 1991, 5, 3031–3036. [Google Scholar] [CrossRef] [PubMed]
- Traxinger, R.R.; Marshall, S. Role of amino acids in modulating glucose-induced desensitization of the glucose transport system. J. Biol. Chem. 1989, 264, 20910–20916. [Google Scholar] [PubMed]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [PubMed]
- Garcia, R.F.; Gazola, V.A.; Barrena, H.C.; Hartmann, E.M.; Berti, J.; Toyama, M.H.; Boschero, A.C.; Carneiro, E.M.; Manso, F.C.; Bazotte, R.B. Blood amino acids concentration during insulin induced hypoglycemia in rats: The role of alanine and glutamine in glucose recovery. Amino Acids 2007, 33, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Silveira, L.R.; Alberici, L.C.; Leandro, C.V.; Lambertucci, R.H.; Polimeno, G.C.; Cury Boaventura, M.F.; Procopio, J.; Vercesi, A.E.; Curi, R. Acute effect of fatty acids on metabolism and mitochondrial coupling in skeletal muscle. Biochim. Biophys. Acta 2006, 1757, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.; Folli, F.; Araki, E.; Hashimoto, N.; Csermely, P.; Kahn, C.R. Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol. Endocrinol. 1994, 8, 545–557. [Google Scholar] [PubMed]
- Thirone, A.C.; Carvalho, C.R.; Saad, M.J. Growth hormone stimulates the tyrosine kinase activity of JAK2 and induces tyrosine phosphorylation of insulin receptor substrates and Shc in rat tissues. Endocrinology 1999, 140, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Franchi, M.; de Oliveira, L.F.; da Eira Silva, V.; da Silva, R.P.; de Salles Painelli, V.; Costa, L.A.R.; Sale, C.; Harris, R.C.; Roschel, H.; et al. 24-Week beta-alanine ingestion does not affect muscle taurine or clinical blood parameters in healthy males. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E. Relationship between regional fat distribution and insulin resistance. Int. J. Obes. Relat. Metab. Disord. 2000, 24 (Suppl. 2), S32–S35. [Google Scholar] [CrossRef] [PubMed]
- Ness-Abramof, R.; Apovian, C.M. Waist circumference measurement in clinical practice. Nutr. Clin. Pract. 2008, 23, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Hedberg, P.; Jonason, T.; Lonnberg, I.; Tenerz, A.; Forberg, R.; Ohrvik, J. Waist circumference alone predicts insulin resistance as good as the metabolic syndrome in elderly women. Eur. J. Intern. Med. 2008, 19, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Staiano, A.E.; Reeder, B.A.; Elliott, S.; Joffres, M.R.; Pahwa, P.; Kirkland, S.A.; Paradis, G.; Katzmarzyk, P.T. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int. J. Obes. 2012, 36, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Vogeser, M.; Konig, D.; Frey, I.; Predel, H.G.; Parhofer, K.G.; Berg, A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clin. Biochem. 2007, 40, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Colgan, S.P.; Frank, D.N. Of microbes and meals: The health consequences of dietary endotoxemia. Nutr. Clin. Pract. 2012, 27, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Bukh, A.R.; Melchjorsen, J.; Offersen, R.; Jensen, J.M.; Toft, L.; Stovring, H.; Ostergaard, L.; Tolstrup, M.; Sogaard, O.S. Endotoxemia is associated with altered innate and adaptive immune responses in untreated HIV-1 infected individuals. PLoS ONE 2011, 6, e21275. [Google Scholar] [CrossRef] [PubMed]
- Schaller, S.J.; Anstey, M.; Blobner, M.; Edrich, T.; Grabitz, S.D.; Gradwohl-Matis, I.; Heim, M.; Houle, T.; Kurth, T.; Latronico, N.; et al. Early, goal-directed mobilisation in the surgical intensive care unit: A randomised controlled trial. Lancet 2016, 388, 1377–1388. [Google Scholar] [CrossRef]
- Sun, L.; Yu, Z.; Ye, X.; Zou, S.; Li, H.; Yu, D.; Wu, H.; Chen, Y.; Dore, J.; Clement, K.; et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 2010, 33, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. 2003, 27 (Suppl. 3), S53–S55. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003, 112, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Virkamaki, A.; Landaker, E.J.; Kahn, C.R.; Yki-Jarvinen, H. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 1999, 48, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Scherer, P.E. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002, 13, 84–89. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Yamauchi, T.; Kubota, T.; Moroi, M.; Matsui, J.; Eto, K.; Yamashita, T.; Kamon, J.; Satoh, H.; et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002, 277, 25863–25866. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Yatagai, T.; Nagasaka, S.; Taniguchi, A.; Fukushima, M.; Nakamura, T.; Kuroe, A.; Nakai, Y.; Ishibashi, S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism 2003, 52, 1274–1278. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.W.; Baird, F.E.; Christie, G.R.; Stewart, A.; Low, S.Y.; Hundal, H.S.; Taylor, P.M. Mechanisms of glutamine transport in rat adipocytes and acute regulation by cell swelling. Cell. Physiol. Biochem. 2001, 11, 259–270. [Google Scholar] [CrossRef] [PubMed]
| Overweight Volunteers | ||||
|---|---|---|---|---|
| Ala Suppl Difference between After and Before (n = 8) | p-Value | Gln Supplemental Difference between After and Before (n = 11) | p-Value | |
| Height (m) | ||||
| Weight (kg) | 0.4 ± 0.74 | 0.17 | −0.44 ± 0.89 | 0.13 |
| BMI (kg/m2) | 0.16 ± 0.29 | 0.16 | −0.17 ± 0.32 | 0.10 |
| Serum amino acid levels (µmol/L) | 106.36 ± 12.55 | 0.0001 | 111.20 ± 13.83 | 0.0001 |
| WC (cm) | −0.19 ± 0.8 | 0.52 | −1.82 ± 1.4 | 0.001 * |
| Glucose (mmol/L) | −0.08 ± 0.21 | 0.34 | 0.06 ±0.6 | 0.83 |
| Insulin (μU/mL) | 0.16 ± 0.74 | 0.33 | −1.00 ± 2.89 | 0.41 |
| TNF-α (pg/mL) | 0.04 ± 0.96 | 0.91 | −2.88 ± 3.94 | 0.28 |
| IL-1β (pg/mL) | 0.01 ± 0.17 | 0.84 | −0.14 ± 0.33 | 0.31 |
| IL-6 (pg/mL) | −0.006 ± 0.38 | 0.96 | 0.271 ± 1.413 | 0.53 |
| LPS (EU/mL) | −0.001 ± 0.006 | >0.99 | −0.041 ± 0.059 | 0.04 * |
| Obese Volunteers | ||||
|---|---|---|---|---|
| Ala Suppl Difference between After and Before (n = 7) | p-Value | Gln Supplemented Difference between After and Before (n = 13) | p-Value | |
| Height (m) | ||||
| Weight (kg) | 0.3 ± 1.02 | 0.46 | −0.21 ± 0.76 | 0.34 |
| BMI (kg/m2) | 0.12 ± 0.39 | 0.44 | −0.08 ± 0.30 | 0.37 |
| Serum amino acid levels (µmol/L) | 110.97 ± 15.53 | 0.0001 | 105.99 ± 14.99 | 0.0001 |
| WC (cm) | 0.0 ± 1.19 | >0.99 | −2.61 ± 2.21 | 0.002 * |
| Glucose (mmol/L) | 0.25 ± 0.28 | 0.06 | 0.03 ± 0.41 | 0.84 |
| Insulin (μU/mL) | 0.34 ± 0.94 | 0.27 | −1.15 ± 1.7 | 0.037 * |
| TNF-α (pg/mL) | 0.11 ± 0.63 | 0.67 | −2.65 ± 4.18 | 0.09 |
| IL-1β (pg/mL) | 0.06 ± 0.45 | 0.74 | −0.24 ± 0.74 | 0.41 |
| IL-6 (pg/mL) | 0.07 ± 0.21 | 0.37 | −0.27 ± 0.74 | 0.22 |
| LPS (EU/mL) | −0.001 ± 0.01 | 0.78 | −0.08 ± 0.12 | 0.08 |
| C+vehicle | H+Ala | H+Gln | |
|---|---|---|---|
| Insulin (ng/mL) | 0.24 ± 0.01 (n = 5) | 1.14 ±0.21 * (n = 8) | 0.49 ±0.08 # (n = 6) |
| Adiponectin (μg/mL) | 8.16 ± 0.29 (n = 5) | 4.86 ± 0.22 * (n = 6) | 6.78 ± 0.4 # (n = 6) |
| TNF alpha (pg/mL) | 95.94 ± 7.06 (n = 4) | 499.60 ± 63.23 * (n = 5) | 250.00 ± 34.86 (n = 5) |
| IL6 (pg/mL) | 349.50 ± 5.80 (n = 4) | 609.50 ± 46.10 * (n = 5) | 347.50 ± 12.90 (n = 4) |
| LPS (EU/mL) | 0.464 ± 0.05 (n = 5) | 1.15 ± 0.16 * (n = 6) | 0.74 ± 0.06 (n = 6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abboud, K.Y.; Reis, S.K.; Martelli, M.E.; Zordão, O.P.; Tannihão, F.; de Souza, A.Z.Z.; Assalin, H.B.; Guadagnini, D.; Rocha, G.Z.; Saad, M.J.A.; et al. Oral Glutamine Supplementation Reduces Obesity, Pro-Inflammatory Markers, and Improves Insulin Sensitivity in DIO Wistar Rats and Reduces Waist Circumference in Overweight and Obese Humans. Nutrients 2019, 11, 536. https://doi.org/10.3390/nu11030536
Abboud KY, Reis SK, Martelli ME, Zordão OP, Tannihão F, de Souza AZZ, Assalin HB, Guadagnini D, Rocha GZ, Saad MJA, et al. Oral Glutamine Supplementation Reduces Obesity, Pro-Inflammatory Markers, and Improves Insulin Sensitivity in DIO Wistar Rats and Reduces Waist Circumference in Overweight and Obese Humans. Nutrients. 2019; 11(3):536. https://doi.org/10.3390/nu11030536
Chicago/Turabian StyleAbboud, Kahlile Youssef, Sabrina Karen Reis, Maria Eduarda Martelli, Olivia Pizetta Zordão, Fabiana Tannihão, Alessandra Zanin Zambom de Souza, Heloisa Balan Assalin, Dioze Guadagnini, Guilherme Zweig Rocha, Mario Jose Abdalla Saad, and et al. 2019. "Oral Glutamine Supplementation Reduces Obesity, Pro-Inflammatory Markers, and Improves Insulin Sensitivity in DIO Wistar Rats and Reduces Waist Circumference in Overweight and Obese Humans" Nutrients 11, no. 3: 536. https://doi.org/10.3390/nu11030536
APA StyleAbboud, K. Y., Reis, S. K., Martelli, M. E., Zordão, O. P., Tannihão, F., de Souza, A. Z. Z., Assalin, H. B., Guadagnini, D., Rocha, G. Z., Saad, M. J. A., & Prada, P. O. (2019). Oral Glutamine Supplementation Reduces Obesity, Pro-Inflammatory Markers, and Improves Insulin Sensitivity in DIO Wistar Rats and Reduces Waist Circumference in Overweight and Obese Humans. Nutrients, 11(3), 536. https://doi.org/10.3390/nu11030536





