Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Magnetic Resonance Imaging (MRI)
2.1.2. Hepatic Indices
2.1.3. Dietary Information
2.1.4. Other Variables
2.1.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Pardee, P.E.; Lavine, J.E.; Blumkin, A.K.; Cook, S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008, 118, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Diagnosis of non-alcoholic fatty liver disease (NAFLD). Diabetologia 2016, 59, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Afendy, M.; Fang, Y.; Younossi, Y.; Mir, H.; Srishord, M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011, 9, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 2013, 162, 496–500.e1. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Velazquez, J.A.; Silva-Vidal, K.V.; Ponciano-Rodriguez, G.; Chavez-Tapia, N.C.; Arrese, M.; Uribe, M.; Mendez-Sanchez, N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann. Hepatol. 2014, 13, 166–178. [Google Scholar] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.T.; Ahmed, A.Y.; Rezk, D.G.; Hamed, A.I. Dietary fructose intake in obese children and adolescents: Relation to procollagen type III N-terminal peptide (P3NP) and non-alcoholic fatty liver disease. J. Pediatr. Endocrinol. Metab. 2016, 29, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Kantartzis, K.; Häring, H.U. Causes and metabolic consequences of Fatty liver. Endocr. Rev. 2008, 29, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.M.; Fryhofer, S.A.; Osbahr, A.J., III; Robinowitz, C.B.; The Council on Science and Public Health, American Medical Association. The effects of high fructose syrup. J. Am. Coll. Nutr. 2009, 28, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Nasser, G.; Kamayse, I.; Nseir, W.; Beniashvili, Z.; Djibre, A.; Grosovski, M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can. J. Gastroenterol. 2008, 22, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Barquera, S.; Hernandez-Barrera, L.; Tolentino, M.L.; Espinosa, J.; Ng, S.W.; Rivera, J.A.; Popkin, B.M. Energy intake from beverages is increasing among Mexican adolescents and adults. J. Nutr. 2008, 138, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Aburto, T.C.; Pedraza, L.S.; Sanchez-Pimienta, T.G.; Batis, C.; Rivera, J.A. Discretionary Foods Have a High Contribution and Fruit, Vegetables, and Legumes Have a Low Contribution to the Total Energy Intake of the Mexican Population. J. Nutr. 2016, 146, 1881S–1887S. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pimienta, T.G.; Batis, C.; Lutter, C.K.; Rivera, J.A. Sugar-Sweetened Beverages Are the Main Sources of Added Sugar Intake in the Mexican Population. J. Nutr. 2016, 146, 1888S–1896S. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cossio, T.; Peterson, K.E.; Sanin, L.H.; Fishbein, E.; Palazuelos, E.; Aro, A.; Hernandez-Avila, M.; Hu, H. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics 1997, 100, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Roldan-Valadez, E.; Favila, R.; Martinez-Lopez, M.; Uribe, M.; Mendez-Sanchez, N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann. Hepatol. 2008, 7, 212–220. [Google Scholar] [PubMed]
- Nasr, P.; Forsgren, M.F.; Ignatova, S.; Dahlstrom, N.; Cedersund, G.; Leinhard, O.D.; Noren, B.; Ekstedt, M.; Lundberg, P.; Kechagias, S. Using a 3% Proton Density Fat Fraction as a Cut-Off Value Increases Sensitivity of Detection of Hepatic Steatosis, Based on Results from Histopathology Analysis. Gastroenterology 2017, 153, 53–55.e7. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Shiehmorteza, M.; Hamilton, G.; Wolfson, T.; Schroeder, M.E.; Middleton, M.S.; Bydder, M.; Gamst, A.C.; Kono, Y.; Kuo, A.; et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 2011, 258, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Consultation, W.I. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia; World Health Organization: Geneva, Switzerland, 2006; pp. 17–20. [Google Scholar]
- Denova-Gutierrez, E.; Ramirez-Silva, I.; Rodriguez-Ramirez, S.; Jimenez-Aguilar, A.; Shamah-Levy, T.; Rivera-Dommarco, J.A. Validity of a food frequency questionnaire to assess food intake in Mexican adolescent and adult population. Salud Pública México 2016, 58, 617–628. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Public Health. The Compiled México-INSP Food Composition Data Bank; National Institute of Public Health: Saitama, Japan, 2002.
- Asociación Mexicana de Agencias de Investigación de Mercado (AMAI). Descarga la App Oficial de la revista AMAI. Available online: http://www.amai.org/ (accessed on 25 March 2018).
- IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short Form; IPAQ Research Committee: USA, 2004. [Google Scholar]
- Secretaría de Salud. National Addictions Survey, Alcohol Report; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2011. [Google Scholar]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Weickert, M.O.; Lythgoe, D.; Sprung, V.S.; Dobson, R.; Shoajee-Moradie, F.; Umpleby, M.; Pfeiffer, A.F.; Thomas, E.L.; Bell, J.D.; et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur. J. Endocrinol. 2014, 171, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Sviklane, L.; Olmane, E.; Dzerve, Z.; Kupcs, K.; Pirags, V.; Sokolovska, J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J. Gastroenterol. Hepatol. 2018, 33, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Briseño-Bass, P.; Chávez-Pérez, R.; López-Zendejas, M. Prevalence of liver steatosis and its relation to liver function tests and lipid profile in patients at medical check-up. Rev. Gastroenterol. Méx. 2018. [Google Scholar] [CrossRef]
- Aeberli, I.; Hochuli, M.; Gerber, P.A.; Sze, L.; Murer, S.B.; Tappy, L.; Spinas, G.A.; Berneis, K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: A randomized controlled trial. Diabetes Care 2013, 36, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.H.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Undereating and underrecording of habitual food intake in obese men: Selective underreporting of fat intake. Am. J. Clin. Nutr. 2000, 71, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Lee, J.S.; Kuller, L.H. Underreporting of dietary intake by body mass index in premenopausal women participating in the Healthy Women Study. Nutr. Res. Pract. 2007, 1, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.M.; Dong, H.J.; Li, Z.; Cai, W.; Altomonte, J.; Thung, S.N.; Zeng, F.; Fisher, E.A.; Vlassara, H. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002, 51, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; McKeown, N.M.; Rogers, G.; Meigs, J.B.; Saltzman, E.; D’Agostino, R.; Jacques, P.F. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J. Nutr. 2007, 137, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell. Metab. 2018, 27, 351–361.e3. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Nassar, F.; Assy, N. Soft drinks consumption and nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J. Dietary carbohydrate’s effects on lipogenesis and the relationship of lipogenesis to blood insulin and glucose concentrations. Br. J. Nutr. 2002, 87, S247–S253. [Google Scholar] [CrossRef] [PubMed]
- Le, K.A.; Faeh, D.; Stettler, R.; Ith, M.; Kreis, R.; Vermathen, P.; Boesch, C.; Ravussin, E.; Tappy, L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006, 84, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Le, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Elliott, S.S.; Tschop, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’Alessio, D.; Havel, P.J. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Simon, M.C.; Strassburger, K.; Markgraf, D.F.; Buyken, A.E.; Szendroedi, J.; Mussig, K.; Roden, M.; Group, G.D.S. Habitual Fructose Intake Relates to Insulin Sensitivity and Fatty Liver Index in Recent-Onset Type 2 Diabetes Patients and Individuals without Diabetes. Nutrients 2018, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S. Sugar sweetened beverages and cardiometabolic health. Curr. Opin. Cardiol. 2017, 32, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Vetrani, C.; Lombardi, G.; Bozzetto, L.; Annuzzi, G.; Rivellese, A.A. Isocaloric Dietary Changes and Non-Alcoholic Fatty Liver Disease in High Cardiometabolic Risk Individuals. Nutrients 2017, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. Non-alcoholic fatty liver disease: A massive problem. Clin. Med. 2011, 11, 176–178. [Google Scholar] [CrossRef]
- Machado, M.; Marques-Vidal, P.; Cortez-Pinto, H. Hepatic histology in obese patients undergoing bariatric surgery. J. Hepatol. 2006, 45, 600–606. [Google Scholar] [CrossRef] [PubMed]
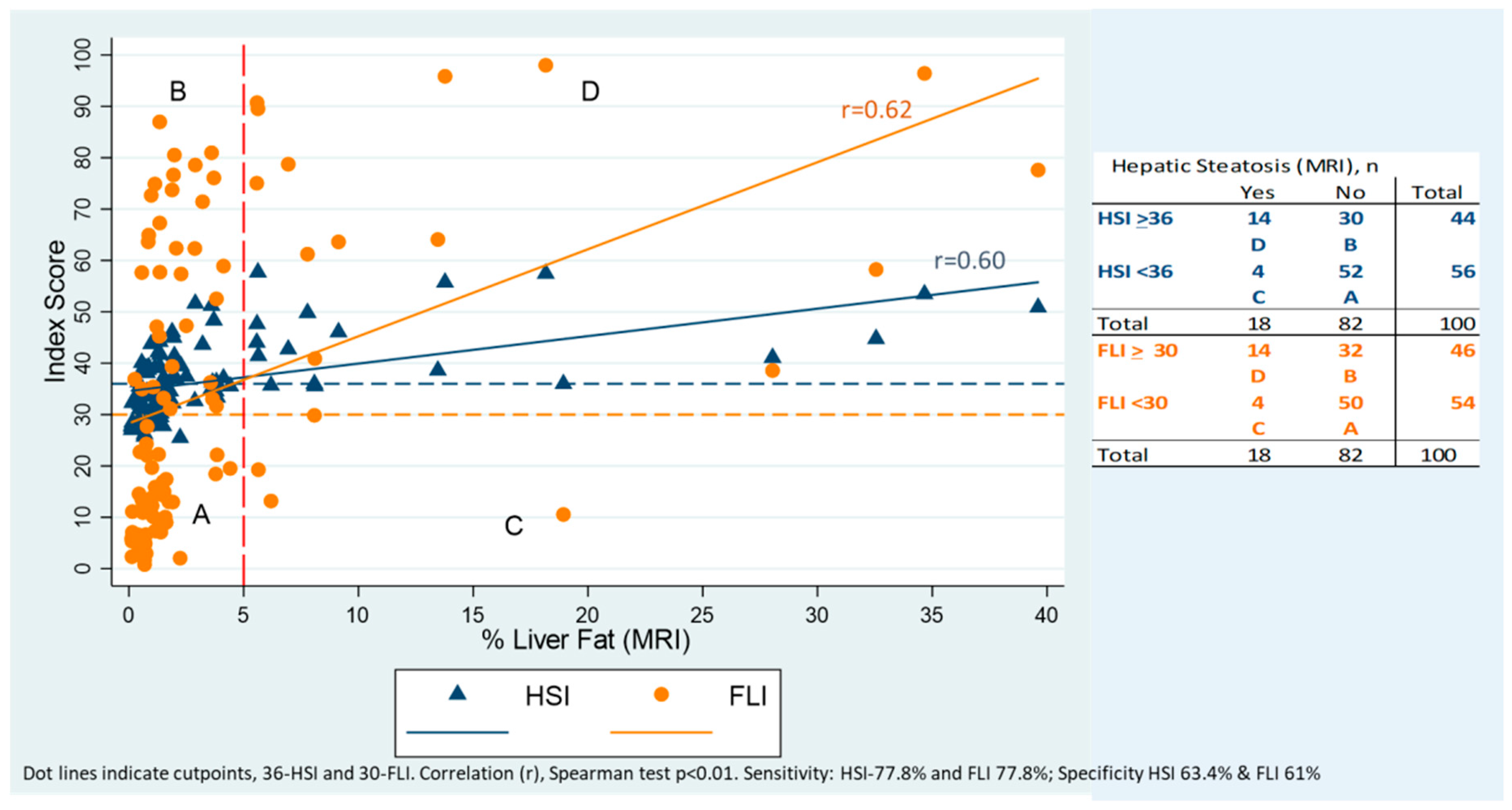
| HSI | FLI | NAFLD | |||||
|---|---|---|---|---|---|---|---|
| All | <36 points | >=36 points | <30 points | >=30 points | <5% of liver fat content | >=5% of liver fat content | |
| n = 100 | n = 56 | n = 44 | n = 54 | n = 46 | n = 82 | n = 18 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age, years | 21.4 (0.5) | 21.5 (0.5) | 21.4 (0.5) | 21.5 (0.5) | 21.4 (0.5) | 21.5 (0.5) | 21.4 (0.5) |
| Sex, male (%) | 54 | 29 (54) | 25 (46) | 25 (46) | 29 (54) | 25 (46) | 29 (54) |
| Marital status | |||||||
| Single | 80 | 47 (59) | 33 (41) | 44 (55) | 36 (45) | 44 (55) | 36 (45) |
| Married/cohabiting | 20 | 9 (45) | 11 (55) | 10 (50) | 10 (50) | 10 (50) | 10 (50) |
| Occupation | |||||||
| Student | 33 | 19 (58) | 14 (42) | 17 (51) | 16(49) | 17 (51) | 16(49) |
| Employee/home/Other | 67 | 37 (55) | 30 (45) | 37 (55) | 30 (45) | 37 (55) | 30 (45) |
| Socio economic status | |||||||
| Low | 52 | 22 (56) | 23 (44) | 28 (54) | 24 (46) | 28 (54) | 24 (46) |
| Meddium | 48 | 27 (56) | 21 (44) | 26 (54) | 22 (46) | 26 (54) | 22 (46) |
| Smoke cigarettes | |||||||
| Never | 14 | 8 (57) | 6 (43) | 9 (64) | 5 (36) | 14 (100) | 0 (0) |
| Past smoker | 41 | 26 (63) | 15 (37) | 27 (66) | 14 (34) | 34 (82.9) | 7 (17.1) |
| Active smoker | 45 | 22 (49) | 23 (51) | 10 (40) | 27 (60) | 34 (75.6) | 11 (24.4) |
| Alcohol intake | |||||||
| Never/Annualy | 48 | 25 (52) | 23 (48) | 28 (58) | 20 (42) | 39 (81.2) | 9 (18.8) |
| Monthly | 32 | 20 (63) | 12 (37) | 17 (53) | 15 (47) | 24 (75) | 8 (25) |
| Weekly | 20 | 11 (55) | 9 (45) | 9 (45) | 11 (55) | 19 (95) | 1 (5) |
| Physical Activity | |||||||
| Inactive | 18 | 8 (44) | 10 (56) | 7 (39) | 11 (61) | 15 (83.3) | 3 (16.7) |
| Minimal active | 38 | 24 (63) | 14 (37) | 25 (66) | 13 (24) | 31 (81.6) | 7 (18.4) |
| Active | 44 | 22 (55) | 20 (45) | 22 (50) | 22 (50) | 36 (82) | 18 (18) |
| Risk Factors Considered in the Index | Subgroup of Study and Reference | False Positive HSI Or at Risk versus Healthy | True Positive HSI versus Healthy | True Positive HSI versus False Positive HSI | False Positive FLI Or at Risk versus Healthy | True Positive FLI versus Healthy | True Positive FLI versus False Positive FLI |
| Risk factor | RRR (CI 95%) | RRR (CI 95%) | RRR (CI 95%) | RRR (CI 95%) | RRR (CI 95%) | RRR (CI 95%) | |
| HSI and FLI | BMI | 3.01 (1.74, 5.21) £ | 3.64 (2.03, 6.54) £ | 1.21 (0.96, 1.52) | 2.35 (1.55, 3.54) £ | 2.80 (1.78, 4.39) £ | 1.19 (0.96, 1.47) |
| FLI | Waist Circumference (cm) | 1.41 (1.09, 1.83) £ | 1.34 (1.01, 1.79) £ | 0.95 (0.80, 1.13) | 1.27 (1.02, 1.59) £ | 1.12 (0.87, 1.45) | 0.88 (0.74, 1.06) |
| HSI | ALT (U/L) | 1.20 (1.06, 1.35) £ | 1.32 (1.15, 1.52) £ | 1.10 (1.02, 1.19) £ | 1.10 (1.02, 1.20) £ | 1.21 (1.09, 1.35) £ | 1.10 (1.03, 1.18) £ |
| HSI | AST (U/L) | 1.11 (0.98, 1.27) | 1.20 (1.05, 1.38) £ | 1.08 (1.01, 1.16) £ | 1.10 (0.97, 1.23) | 1.20 (1.06, 1.37) £ | 1.10 (1.02, 1.18) £ |
| FLI | GGT (U/L) | 1.16 (1.05, 1.29) £ | 1.16 (1.04, 1.29) £ | 0.98 (0.95, 1.04) | 1.22 (1.09, 1.37) £ | 1.22 (1.08, 1.38) £ | 0.99 (0.96, 1.04) |
| FLI | Triglicerydes (mg/dL) | 1.00 (0.99, 1.01) | 1.02 (1.00, 1.03) £ | 1.01 (1.00, 1.03) £ | 1.13 (1.02, 1.25) £ | 1.14 (1.04, 1.26) £ | 1.01 (0.99, 1.03) |
| HSI | Glucose (mg/dL) | 1.03 (0.96, 1.11) | 1.14 (1.01, 1.30) £ | 1.11 (1.00, 1.23) £ | 1.03 (0.95, 1.10) | 1.14 (1.01, 1.28) £ | 1.11 (1.00, 1.13) £ |
| Other Risk Factors for NAFLD | |||||||
| Subcutaneous fat (cm2) | 1.02 (1.00, 1.04) £ | 1.01 (0.99, 1.03) | 0.99 (0.98, 1.00) | 1.02 (0.99, 1.03) | 1.00 (0.98, 1.03) | 0.98 (0.98, 1.00) | |
| Visceral fat (cm2) | 1.00 (0.96, 1.04) | 1.03 (0.98, 1.08) | 1.03 (0.98, 1.07) | 0.99 (0.95, 1.03) | 1.01 (0.96, 1.06) | 1.02 (0.98, 1.05) | |
| Healthy | HSI | FLI | NAFLD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| False Positive | False Positive | |||||||||
| All | <36 points | >=36 points | >36 points + no-NAFDL | <30 points | >=30 points | >=30 points + no-NAFLD | <5% of liver fat | >=5% of liver fat | ||
| n = 100 | n = 46 | n = 56 | n = 44 | n = 30 | n = 54 | n = 46 | n = 32 | n = 82 | n = 18 | |
| Energy, Kcal/day | 2689 (1682) | 2430 (2083) | 2557 (1768) | 2801 (1482) | 2867 (1687) | 2380 (1885) | 2953 (1305) | 3020 (1518) | 2801 (1966) | 2363 (1471) |
| Proteins, g/day | 85.7 (54.5) | 84.9 (66.6) | 85.7 (49.6) | 86.2 (56.4) | 91.6 (55.4) | 82.4 (46.6) | 91.6 (57.5) | 93.4 (48.7) | 88.1 (57.5) | 81 (34.4) |
| Calories from proteins (%) | 13 (2.8) | 13.3 (3.6) | 13.3 (2.9) | 12.7 (3) | 12.5 (2.8) | 13.3 (3) | 12.6 (2.6) | 12.5 (2.7) | 13.1 (2.8) | 12.8 (2.5) |
| Carbohydrates (CHO), g/day | 375 (268.8) | 338.1 (294.8) | 347.9 (279.2) | 419 (237.8) | 435 (263.9) | 338.1 (298.8) | 442.9 (194.6) | 453.6 (198.5) | 375.7 (272.8) | 363.6 (238.1) |
| Calories from CHO (%) | 55.8 (12.4) | 53.8 (11.4) | 54.6 (12.9) | 58.5 (11.3) | 57.2 (11) | 54 (10.9) | 58.7 (13.8) | 58.7 (13.3) | 54.6 (13.3) | 58.9 (9.9) |
| Sugar (g/day) | 25.8 (31.9) | 24.1 (24.8) | 24.6 (28.8) | 28.7 (33.1) | 26.9 (33.3) | 23.6 (25.5) | 29.9 (29.8) | 28.3 (37.4) | 25 (32.7) | 31.9 (28.5) |
| Calories from sugar (%) | 3.9 (3.9) | 3.8 (3.4) | 3.8 (3.4) | 4.1 (4.2) | 4.1 (4.2) | 3.7 (3.4) | 4.2 (4.1) | 4.2 (4.1) | 3.9 (3.5) | 5 (4.9) |
| Total fiber, g/day | 26.1 (26.1) | 25.3 (29) | 25.3 (24.7) | 28 (26.2) | 25 (25.8) | 25.3 (29.5) | 27.1 (24.6) | 24.5 (21.5) | 24.8 (25.8) | 34.4 (25) |
| Lipids, g/day | 98.4 (66.4) | 98.6 (79.2) | 100.5 (66.9) | 94.2 (66.9) | 100.9 (66.7) | 90.9 (69) | 102.9 (64.3) | 108.6 (60.9) | 100.5 (70.3) | 80.1 (55.6) |
| Calories from lipids (%) | 33.1 (10.3) | 36.1 (9.8) | 34.5 (10) | 32.3 (10) | 32.4 (9.8) | 35.3 (9.3) | 30.4 (11.2) | 30.3 (10.4) | 34.2 (10.2) | 30.5 (7.5) |
| Fructose Food Groups | ||||||||||
| Sugar-sweetened beverages (SSB) (mL/day) | 720 (1037.1) | 518.6 (874.3) | 557.1 (921.4) | 895.7 (977.1) | 1045 (1440) | 535.7 (857.1) | 960 (959.9) | 1272.9 (1538.6) | 720 (1105.7) | 690.0 (565.7) |
| SSB with HFCS (mL/day) | 9 (107.2) | 9 (85.8) | 8.9 (94.3) | 16.9 (174.3) | 0 (107.2) | 0 (85.8) | 35 (177) | 52.2 (142.1) | 8.9 (107.1) | 17.1 (191.4) |
| Cereals, bars and bread (g/day) | 90.7 (88.9) | 105.9 (141.7) | 96.2 (128.9) | 97.0 (97.5) | 109.32 (85) | 81.4 (126.3) | 109.3 (84.3) | 111.5 (67) | 107.2 (112.5) | 76.2 (116.3) |
| Cereals, bars and bread with HFCS (g/day) | 10 (25.8) | 9.2 (32.1) | 12.2 (30.9) | 8.9 (24.9) | 9 (25.7) | 8 (25.7) | 12.5 (29.6) | 16 (28.3) | 11.3 (25.9) | 8.9 (18.2) |
| Candies (g/day) | 6 (16.2) | 2.9 (14.5) | 6.8 (17.6) | 5.3 (14.7) | 5.3 (13.8) | 2.4 (14.5) | 7.8 (23.3) | 7.8 (18.2) | 5.3 (15.2) | 8.2 (23.6) |
| Candies with HFCS (g/day) | 8.6 (22.9) | 11.1 (25.8) | 11.1 (25.4) | 5.8 (19) | 4.7 (19.7) | 9.7 (22.2) | 8.6 (20) | 7.2 (20.2) | 8.6 (21.5) | 9.3 (28.6) |
| Natural fruit juices (mL/day) | 0 (102.9) | 0 (102.9) | 0 (102.9) | 0 (85.8) | 0 (0) | 0 (102.9) | 0 (102.9) | 0 (68.6) | 0 (68.6) | 145.8 (377.2) |
| Fruit (g/day) | 258 (335.4) | 223.6 (298.8) | 247.8 (297.3) | 415.8 (415.8) | 255.8 (349.1) | 230.9 (325.1) | 306.9 (390.6) | 281.3 (319.2) | 238.6 (318) | 383.2 (549.3) |
| SSB (ml) Detail | ||||||||||
| Coffe with sugar (mL/day) | 0 (102.8) | 0 (102.8) | 0 (102.8) | 0 (171.4) | 0 (240) | 0 (102.8) | 0 (235.7) | 0 (246.4) | 0 (102.8) | 25.7 (102.8) |
| Tea with sugar (mL/day) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Soda (mL/day) | 222.8 (445.7) | 154.2 (480) | 180 (471.4) | 257.1 (411.4) | 257.1 (360) | 154.2 (342.8) | 257.1 (385.7) | 281.8 (334.2) | 248.5 (445.7) | 205.7 (411.4) |
| Flavored water beverages and home-made fruit beverages (mL/day) | 0 (222.8) | 0 (137.1) | 0 (222.9) | 0 (338.5) | 0 (514.3) | 0 (137.1) | 68.6 (514.2) | 85.7 (582.8) | 0 (240) | 51.4 (205.7) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantoral, A.; Contreras-Manzano, A.; Luna-Villa, L.; Batis, C.; Roldán-Valadez, E.A.; Ettinger, A.S.; Mercado, A.; Peterson, K.E.; Téllez-Rojo, M.M.; Rivera, J.A. Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults. Nutrients 2019, 11, 522. https://doi.org/10.3390/nu11030522
Cantoral A, Contreras-Manzano A, Luna-Villa L, Batis C, Roldán-Valadez EA, Ettinger AS, Mercado A, Peterson KE, Téllez-Rojo MM, Rivera JA. Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults. Nutrients. 2019; 11(3):522. https://doi.org/10.3390/nu11030522
Chicago/Turabian StyleCantoral, Alejandra, Alejandra Contreras-Manzano, Lynda Luna-Villa, Carolina Batis, Ernesto A. Roldán-Valadez, Adrienne S. Ettinger, Adriana Mercado, Karen E. Peterson, Martha M Téllez-Rojo, and Juan A. Rivera. 2019. "Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults" Nutrients 11, no. 3: 522. https://doi.org/10.3390/nu11030522
APA StyleCantoral, A., Contreras-Manzano, A., Luna-Villa, L., Batis, C., Roldán-Valadez, E. A., Ettinger, A. S., Mercado, A., Peterson, K. E., Téllez-Rojo, M. M., & Rivera, J. A. (2019). Dietary Sources of Fructose and Its Association with Fatty Liver in Mexican Young Adults. Nutrients, 11(3), 522. https://doi.org/10.3390/nu11030522








