Efficacy of Polydextrose Supplementation on Colonic Transit Time, Bowel Movements, and Gastrointestinal Symptoms in Adults: A Double-Blind, Randomized, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
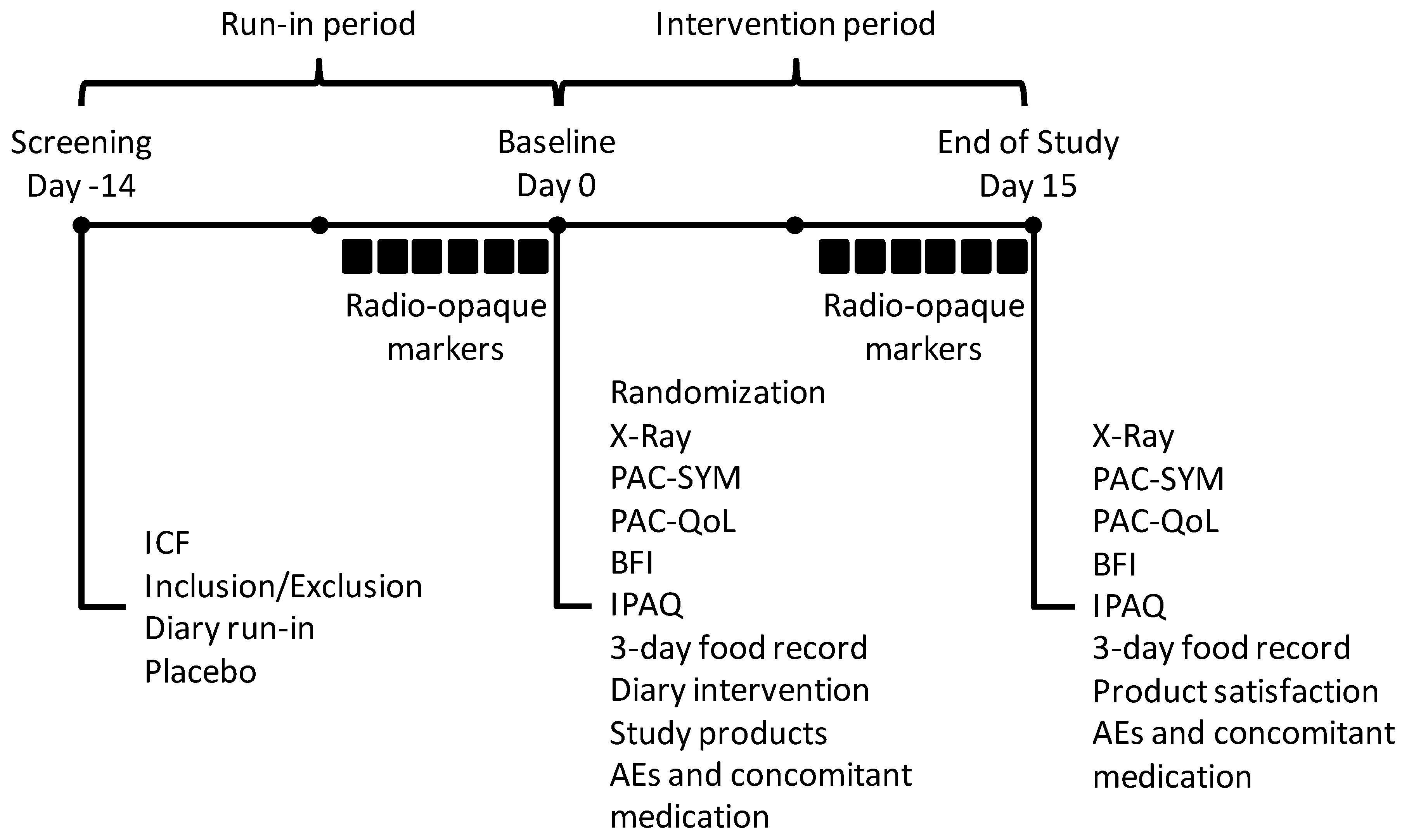
2.1. Study Design
2.2. Study Subjects
2.3. Study Products
2.4. Study Outcomes
2.5. Statistical Analyses
3. Results
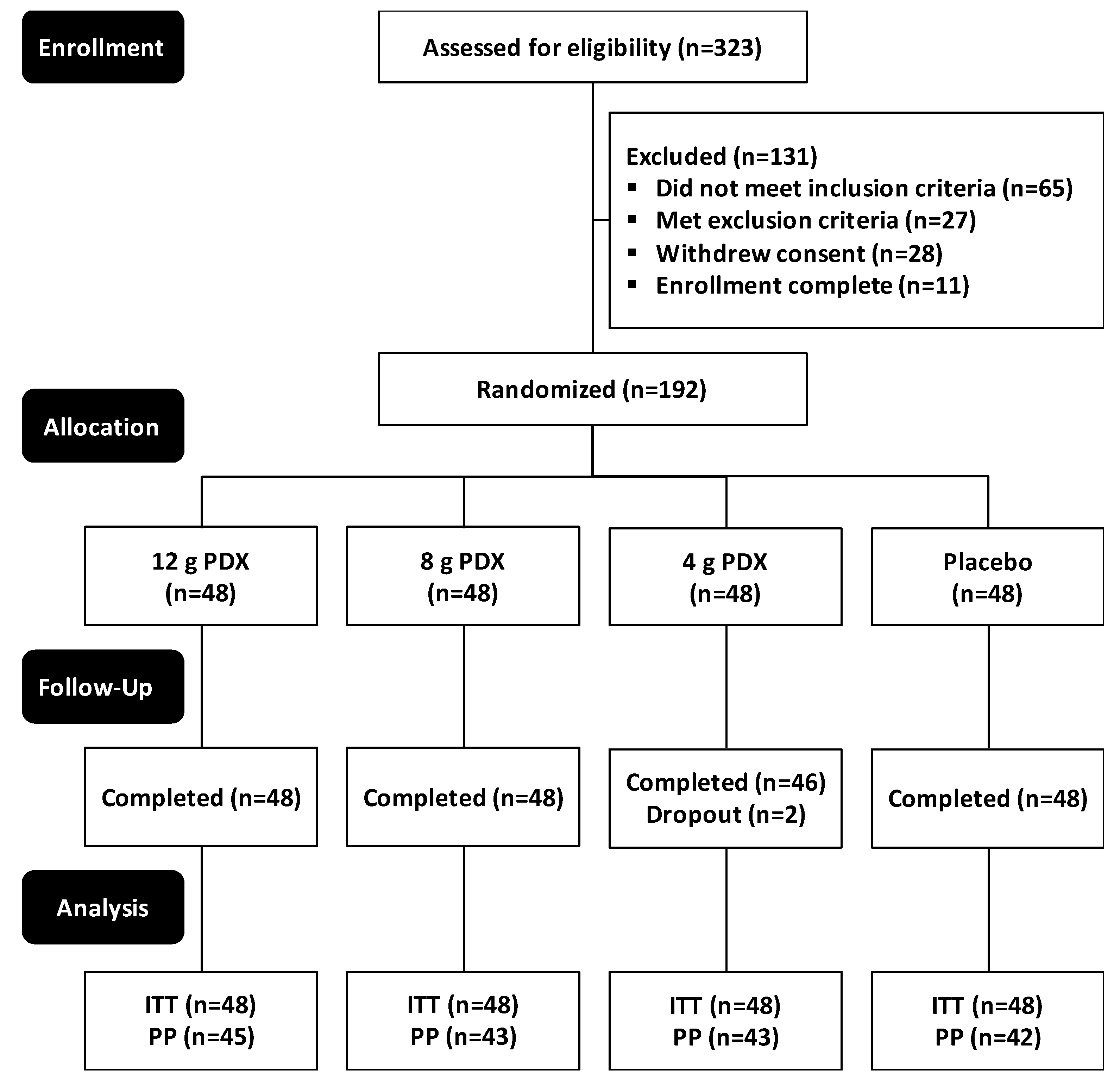
3.1. Participant Characteristics and Compliance
3.2. Colonic Transit Time (CTT)
3.3. Patient Assessment of Constipation Symptoms (PAC-SYM)
3.4. Patient Assessment of Constipation Quality of Life (PAC-QoL)
3.5. Bowel Function Index (BFI)
3.6. Constipation Relief Questionnaire
3.7. Bowel Habits Diary
3.8. The Severity of Abdominal Discomfort and Bloating
3.9. Product Satisfaction
3.10. Safety Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| BFI | Bowel Function Index |
| BMF | bowel movement frequency |
| BSS | Bristol stool scale |
| CSBM | Complete spontaneous bowel movements |
| CTT | colonic transit time |
| EMA | European Medicines Agency |
| IPAQ | International Physical Activity Questionnaire |
| PAC-QoL | Patient Assessment of Constipation Quality of Life |
| PAC-SYM | Patient Assessment of Constipation Symptoms |
| PDX | polydextrose |
References
- Locke, G.R., III; Pemberton, J.H.; Phillips, S.F. Aga technical review on constipation. Gastroenterology 2000, 119, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.D.; Johanson, J.F. Epidemiology of constipation in north america: A systematic review. Am. J. Gastroenterol. 2004, 99, 750. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.A. Prevalence and ramifications of chronic constipation. Manag. Care Interface 2005, 18, 23–30. [Google Scholar] [PubMed]
- Belsey, J.; Greenfield, S.; Candy, D.; Geraint, M. Systematic review: Impact of constipation on quality of life in adults and children. Aliment. Pharmacol. Ther. 2010, 31, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S. Constipation: Evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol. Clin. North Am. 2007, 36, 687–711. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.; Prasad, M.; Lloyd, A.; Bhattacharyya, S.K.; Dhawan, R.; Coyne, K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005, 23, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Orozco, J.F.; Foxx-Orenstein, A.E.; Sterler, S.M.; Stoa, J.M. Chronic constipation in the elderly. Am. J. Gastroenterol. 2012, 107, 18. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D. Symposium report: An evidence-based approach to ibs and cic: Applying new advances to daily practice: A review of an adjunct clinical symposium of the american college of gastroenterology meeting october 16, 2016. Las vegas, nevada. Gastroenterol. Hepatol. 2017, 13, 1. [Google Scholar]
- Flood, M.; Auerbach, M.; Craig, S. A review of the clinical toleration studies of polydextrose in food. Food Chem. Toxicol. 2004, 42, 1531–1542. [Google Scholar] [CrossRef]
- Murphy, O. Non-polyol low-digestible carbohydrates: Food applications and functional benefits. Br. J. Nutr. 2001, 85, S47–S53. [Google Scholar] [CrossRef]
- Costabile, A.; Fava, F.; Röytiö, H.; Forssten, S.D.; Olli, K.; Klievink, J.; Rowland, I.R.; Ouwehand, A.C.; Rastall, R.A.; Gibson, G.R. Impact of polydextrose on the faecal microbiota: A double-blind, crossover, placebo-controlled feeding study in healthy human subjects. Br. J. Nutr. 2012, 108, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Achour, L.; Flourie, B.; Briet, F.; Pellier, P.; Marteau, P.; Rambaud, J. Gastrointestinal effects and energy value of polydextrose in healthy nonobese men. Am. J. Clin. Nutr. 1994, 59, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Hengst, C.; Ptok, S.; Roessler, A.; Fechner, A.; Jahreis, G. Effects of polydextrose supplementation on different faecal parameters in healthy volunteers. Int. J. Food Sci. Nutr. 2009, 60, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Bang-Yao, L.; Ming-Jie, X.; Hai-Wei, L.; Zu-Kang, Z.; Ting-Song, W.; Craig, S.A. Studies on the effects of polydextrose intake on physiologic functions in chinese people. Am. J. Clin. Nutr. 2000, 72, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Matsuike, T.; Suzuki, Y.; Nozawa, H.; Nishino, H.; Matsushima, M.; Mori, Y. Effects of polydextrose on habitual constipation with melanosis coli. J. Jpn. Assoc. Diet. Fiber Res. 2002, 6, 55–60. [Google Scholar]
- Matsuike, T.; Suzuki, Y.; Nozawa, H.; Nishino, H.; Mori, Y. Clinical research of polydextrose on habitual constipation. J. Jpn. Assoc. Diet. Fiber Res. 2002, 6, 17–20. [Google Scholar]
- Nakagawa, Y.; Okamatsu, H.; Fujii, Y. Effects of polydextrose feeding on the frequency and feeling of defecation in healthy female volunteers. J. Jpn. Soc. Nutr. Food Sci. 1990, 43, 95–101. [Google Scholar] [CrossRef]
- Timm, D.A.; Thomas, W.; Boileau, T.W.; Williamson-Hughes, P.S.; Slavin, J.L. Polydextrose and soluble corn fiber increase five-day fecal wet weight in healthy men and women. J. Nutr. 2013, 143, 473–478. [Google Scholar] [CrossRef]
- Tomlin, J.; Read, N. A comparative study of the effects on colon function caused by feeding ispaghula husk and polydextrose. Aliment. Pharmacol. Ther. 1988, 2, 513–519. [Google Scholar] [CrossRef]
- Shimada, M.; Nagano, N.; Goto, S.; Ito, K.; Tsutsui, T.; Ando, T.; Kamioka, H.; Ogawa, T. Effect of polydextrose intake on constipation in japanese dialysis patients: A triple-blind, randomized, controlled trial. J. Nutr. Sci. Vitaminol. 2015, 61, 345–353. [Google Scholar] [CrossRef]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73, 415s–420s. [Google Scholar] [CrossRef]
- Tramonte, S.M.; Brand, M.B.; Mulrow, C.D.; Amato, M.G.; O’Keefe, M.E.; Ramirez, G. The treatment of chronic constipation in adults. J. Gen. Intern. Med. 1997, 12, 15–24. [Google Scholar] [CrossRef]
- Drossman, D.A.; Dumitrascu, D.L. Rome iii: New standard for functional gastrointestinal disorders. J. Gastrointestin. Liver Dis. 2006, 15, 237. [Google Scholar] [PubMed]
- Drossman, D.A. The functional gastrointestinal disorders and the rome iii process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Devroede, G.; Faye, A.; Le Toumelin, P.; Arhan, P.; Arsac, M. Colonic response to food in constipation. Int. J. Colorectal. Dis. 2006, 21, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Riegler, G.; Esposito, I. Bristol scale stool form. A still valid help in medical practice and clinical research. Tech. Coloproctol. 2001, 5, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Kleinman, L.; Farup, C.; Taylor, L.; Miner, P. Psychometric validation of a constipation symptom assessment questionnaire. Scand. J. Gastroenterol. 1999, 34, 870–877. [Google Scholar] [PubMed]
- Marquis, P.; De La Loge, C.; Dubois, D.; McDermott, A.; Chassany, O. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand. J. Gastroenterol. 2005, 40, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Rentz, A.; Van Hanswijck De Jonge, P.; Leyendecker, P.; Hopp, M. Observational, nonintervention, multicenter study for validation of the bowel function index for constipation in european countries. Curr. Med. Res. Opin. 2011, 27, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191. [Google Scholar]
- ICH Expert Working Group. I.C.H. Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6 (R1); International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, 1996. [Google Scholar]
- Ibarra, A.; Olli, K.; Ouwehand, A.C. Correcting for non-compliance when determining colonic transit time with radio-opaque markers. World J. Gastroenterol. 2017, 23, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, M.; Oja, P.; Sjöström, M. The international physical activity questionnaire (ipaq): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.A.; Gopal, P.K.; Leyer, G.J.; Ouwehand, A.C.; Reifer, C.; Stewart, M.E.; Miller, L.E. Dose-response effect of bifidobacterium lactis hn019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 2011, 46, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Johanson, J.F.; Kralstein, J. Chronic constipation: A survey of the patient perspective. Aliment. Pharmacol. Ther. 2007, 25, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.; Latreille-Barbier, M.; Donazzolo, Y.; Pelletier, X.; Ouwehand, A.C. Effects of 28-day bifidobacterium animalis subsp. Lactis hn019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Ibarra, A.; Ouwehand, A.C. Normative values for colonic transit time and patient assessment of constipation in adults with functional constipation: Systematic review with meta-analysis. Clin. Med. Insights Gastroenterol. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Ibarra, A.; Ouwehand, A.C.; Zimmermann, A.K. Normative values for stool frequency and form using rome iii diagnostic criteria for functional constipation in adults: Systematic review with meta-analysis. Ann. Gastroenterol. Q. Publ. Hellen. Soc. Gastroenterol. 2017, 30, 161. [Google Scholar] [CrossRef]
- Duncan, P.; Enters-Weijnen, C.; Emami, N.; McLean, P.; Nunes, T.; Beaumont, M.; Crabbe, R.; Whelan, K.; Scott, S.; deWit, N. Short-term daily intake of polydextrose fiber does not shorten intestinal transit time in constipated adults: A randomized controlled trial. Nutrients 2018, 10, 920. [Google Scholar] [CrossRef]
- FDA, U.S. Guidance for Industry: Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2012.
- EMA, E.M.A. Guideline on the Evaluation of Medicinal Products for the Treatment of Chronic Constipation (Including Opioid Induced Constipation) and for Bowel Cleansing; European Medicines Agency: London, UK, 2015. [Google Scholar]
- EFSA Journal. Scientific Opinion on the Substantiation of a Health Claim Related to “Native Chicory Inulin” and Maintenance of Normal Defecation by Increasing Stool Frequency Pursuant to Article 13.5 of Regulation (EC) No 1924/2006; European Food Safety Authority: Parma, Italy, 2015. [Google Scholar]
- de Vries, J.; Le Bourgot, C.; Calame, W.; Respondek, F. Effects of β-fructans fiber on bowel function: A systematic review and meta-analysis. Nutrients 2019, 11, 91. [Google Scholar] [CrossRef]
- Bosaeus, I. Fibre effects on intestinal functions (diarrhoea, constipation and irritable bowel syndrome). Clin. Nutr. Suppl. 2004, 1, 33–38. [Google Scholar] [CrossRef]

| All (n = 192) | 12 g PDX (n = 48) | 8 g PDX (n = 48) | 4 g PDX (n = 48) | Placebo (n = 48) | p-Value σ | |
|---|---|---|---|---|---|---|
| Age Mean ± SD | 42.7 ± 18.8 | 42.9 ± 16.3 | 4297 ± 12.5 | 41.5 ± 17.1 | 43.6 ± 13.4 | 0.912 § |
| BMI (kg/m2) Mean ± SD | 25.28 ± 2.85 | 25.27 ± 3.06 | 25.31 ± 3.09 | 25.32 ± 2.82 | 25.23 ± 2.48 | 0.999 § |
| Gender (n (%)) | 0.985 | |||||
| Female | 133 (69%) | 34 (71%) | 34 (71%) | 32 (67%) | 33 (69%) | |
| Male | 59 (31%) | 14 (29%) | 14 (29%) | 16 (33%) | 15 (31%) | |
| Alcohol Use (n (%)) | 0.706 | |||||
| Daily | 5 (3%) | 0 (0%) | 2 (4%) | 2 (4%) | 1 (2%) | |
| None | 32 (17%) | 9 (19%) | 5 (10%) | 9 (19%) | 9 (19%) | |
| Occasionally | 100 (52%) | 27 (56%) | 25 (52%) | 21 (44%) | 27 (56%) | |
| Weekly | 55 (29%) | 12 (25%) | 16 (33%) | 16 (33%) | 11 (23%) | |
| Smoking Status (n (%)) | 0.673 | |||||
| Ex-Smoker | 25 (13%) | 7 (15%) | 8 (17%) | 3 (6%) | 7 (15%) | |
| Non-Smoker | 145 (76%) | 34 (71%) | 36 (75%) | 40 (83%) | 35 (73%) | |
| Current Smoker | 22 (11%) | 7 (15%) | 4 (8%) | 5 (10%) | 6 (12%) | |
| Race (n (%)) | 0.835 | |||||
| Black or African-American | 2 (1%) | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Central American | 4 (2%) | 2 (4%) | 1 (2%) | 0 (0%) | 1 (2%) | |
| East Asian | 4 (2%) | 1 (2%) | 0 (0%) | 1 (2%) | 2 (4%) | |
| Eastern European White | 8 (4%) | 0 (0%) | 3 (6%) | 0 (0%) | 5 (10%) | |
| Middle Eastern | 6 (3%) | 2 (4%) | 1 (2%) | 2 (4%) | 1 (2%) | |
| North American Indian | 2 (1%) | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | |
| South American | 12 (6%) | 0 (0%) | 6 (12%) | 3 (6%) | 3 (6%) | |
| South Asian | 5 (3%) | 3 (6%) | 1 (2%) | 0 (0%) | 1 (2%) | |
| South East Asian | 2 (1%) | 2 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Western European White | 147 (77%) | 36 (75%) | 36 (75%) | 40 (83%) | 35 (73%) | |
| Ethnicity (n (%)) | 0.841 | |||||
| Hispanic or Latino | 18 (9%) | 3 (6%) | 6 (12%) | 4 (8%) | 5 (10%) | |
| Not Hispanic or Latino | 174 (91%) | 45 (94%) | 42 (88%) | 44 (92%) | 43 (90%) |
| 12 g PDX | 8 g PDX | 4 g PDX | Placebo | p-Value Δ | |
|---|---|---|---|---|---|
| Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | ||
| Total Energy (kcal) | |||||
| Baseline | 1707 ± 494 (48) | 1712 ± 486 (48) | 1822 ± 531 (48) | 1766 ± 560 (48) | - |
| End of Study | 1695 ± 520 (48) | 1698 ± 529 (48) | 1723 ± 448 (46) | 1649 ± 500 (48) | 0.709 * |
| Change from Day 0 to Day 15 | −12 ± 435 (48) p = 0.686 * | −15 ± 375 (48) p = 0.657 * | −99 ± 400 (46) p = 0.132 * | −117 ± 404 (48) p = 0.065 * | - |
| Protein Mass (g) | |||||
| Baseline | 75.1 ± 34.3 (48) | 76.0 ± 27.8 (48) | 75.5 ± 27.6 (48) | 76.7 ± 23.2 (48) | - |
| End of Study | 75.2 ± 28.9 (48) | 75.0 ± 27.6 (48) | 73.5 ± 27.3 (46) | 70.6 ± 27.3 (48) | 0.205 * |
| Change from Day 0 to Day 15 | 0.1 ± 23.3 (48) p = 0.585 * | −1.0 ± 18.9 (48) p = 0.810 * | −1.8 ± 21.8 (46) p = 0.580 * | −6.1 ± 24.0 (48) p = 0.027 * | - |
| Carbohydrate Mass (g) | |||||
| Baseline | 207 ± 72 (48) | 202 ± 69 (48) | 219 ± 77 (48) | 209 ± 76 (48) | - |
| End of Study | 199 ± 80 (48) | 207 ± 82 (48) | 209 ± 75 (46) | 204 ± 78 (48) | 0.764 * |
| Change from Day 0 to Day 15 | −8 ± 68 (48) p = 0.182 * | 5 ± 56 (48) p = 0.887 * | −10 ± 48 (46) p = 0.177 * | −5 ± 63 (48) p = 0.503 * | - |
| Fibre Mass (g) | |||||
| Baseline | 17.7 ± 9.6 (48) | 18.0 ± 8.4 (48) | 18.0 ± 9.1 (48) | 16.8 ± 7.4 (48) | - |
| End of Study | 16.5 ± 7.8 (48) | 16.8 ± 7.4 (48) | 16.4 ± 7.4 (46) | 16.5 ± 7.3 (48) | 0.962 * |
| Change from Day 0 to Day 15 | −1.3 ± 7.7 (48) p = 0.357 * | −1.2 ± 6.6 (48) p = 0.198 * | −1.4 ± 6.0 (46) p = 0.266 * | −0.3 ± 7.1 (48) p = 0.779 * | - |
| Lipid Mass (g) | |||||
| Baseline | 64.9 ± 23.1 (48) | 66.2 ± 27.5 (48) | 71.7 ± 29.2 (48) | 67.2 ± 29.5 (48) | - |
| End of Study | 65.2 ± 23.2 (48) | 61.6 ± 22.2 (48) | 65.8 ± 25.0 (46) | 60.1 ± 23.5 (48) | 0.567 * |
| Change from Day 0 to Day 15 | 0.3 ± 26.1 (48) p = 0.916 * | −4.7 ± 23.5 (48) p = 0.237 * | −6.2 ± 26.8 (46) p = 0.187 * | −7.1 ± 24.3 (48) p = 0.106 * | - |
| Water Mass (g) | |||||
| Baseline | 1389 ± 800 (48) | 1356 ± 760 (48) | 1563 ± 846 (48) | 1645 ± 809 (48) | - |
| End of Study | 1344 ± 728 (48) | 1290 ± 901 (48) | 1375 ± 746 (46) | 1528 ± 888 (48) | 0.752* |
| Change from Day 0 to Day 15 | −46 ± 709 (48) p = 0.927 * | −66 ± 516 (48) p = 0.067 * | −157 ± 798 (46) p = 0.217 * | −117 ± 848 (48) p = 0.110 * | - |
| All | 12 g PDX | 8 g PDX | 4 g PDX | Placebo | p-Value Δ | |
|---|---|---|---|---|---|---|
| Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | ||
| Baseline | 42 ± 32 (192) | 39 ± 26 (48) | 40 ± 31 (48) | 46 ± 31 (48) | 43 ± 39 (48) | 0.779 λ § |
| End of Study | 44 ± 35 (192) | 38 ± 28 (48) | 44 ± 35 (48) | 50 ± 43 (48) | 45 ± 31 (48) | 0.328 λ |
| Change from Day 0 to Day 15 | 2.1 ± 24.8 (192) p = 0.569 λ | −1.8 ± 23.9 (48) p = 0.268 λ | 4.2 ± 23.6 (48) p = 0.409 λ | 4.2 ± 23.9 (48) p = 0.698 λ | 1.7 ± 27.9 (48) p = 0.298 λ | 0.328 λ |
| All | 12 g PDX | 8 g PDX | 4 g PDX | Placebo | p-Value Δ | |
|---|---|---|---|---|---|---|
| Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | ||
| PAC-SYM Total Score | ||||||
| Baseline | 1.07 ± 0.56 (192) | 1.11 ± 0.71 (48) | 1.01 ± 0.47 (48) | 1.08 ± 0.54 (48) | 1.08 ± 0.51 (48) | 0.928 λ § |
| End of Study | 0.90 ± 0.54 (192) | 0.89 ± 0.60 (48) | 0.83 ± 0.48 (48) | 1.03 ± 0.57 (48) | 0.86 ± 0.51 (48) | 0.202 λ |
| Change from Day 0 to Day 15 | −0.17 ± 0.50 (192) p < 0.001 | −0.23 ± 0.58 (48) p = 0.004 λ | −0.18 ± 0.42 (48) p = 0.003 λ | −0.05 ± 0.42 (48) p = 0.315 λ | −0.22 ± 0.54 (48) p = 0.004 λ | 0.202 λ |
| PAC-SYM Abdominal Symptoms Score | ||||||
| Baseline | 1.15 ± 0.69 (192) | 1.18 ± 0.82 (48) | 1.07 ± 0.61 (48) | 1.19 ± 0.65 (48) | 1.16 ± 0.69 (48) | 0.926 λ § |
| End of Study | 1.04 ± 0.71 (192) | 1.05 ± 0.77 (48) | 0.97 ± 0.72 (48) | 1.16 ± 0.66 (48) | 0.97 ± 0.68 (48) | 0.406 λ |
| Change from Day 0 to Day 15 | −0.11 ± 0.58 (192) p = 0.008 | −0.14 ± 0.67 (48) p = 0.055 λ | −0.09 ± 0.51 (48) p = 0.014 λ | −0.03 ± 0.54 (48) p = 0.531 λ | −0.19 ± 0.59 (48) p = 0.016 λ | 0.406 λ |
| PAC-SYM Rectal Symptoms Score | ||||||
| Baseline | 0.58 ± 0.65 (192) | 0.69 ± 0.82 (48) | 0.55 ± 0.59 (48) | 0.54 ± 0.62 (48) | 0.56 ± 0.53 (48) | 0.866 λ § |
| End of Study | 0.44 ± 0.54 (192) | 0.43 ± 0.46 (48) a,b | 0.33 ± 0.42 (48) a,b | 0.61 ± 0.71 (48) a | 0.37 ± 0.50 (48) b | 0.034 λ |
| Change from Day 0 to Day 15 | −0.15 ± 0.60 (192) p = 0.001 | −0.26 ± 0.69 (48) a,b p = 0.035 λ | −0.22 ± 0.52 (48) a,b p = 0.011 λ | 0.07 ± 0.57 (48) a p = 0.488 λ | −0.19 ± 0.58 (48) b p = 0.017 λ | 0.034 λ |
| PAC-SYM Stool Symptoms Score | ||||||
| Baseline | 1.48 ± 0.73 (192) | 1.48 ± 0.88 (48) | 1.41 ± 0.59 (48) | 1.52 ± 0.73 (48) | 1.52 ± 0.70 (48) | 0.787 λ § |
| End of Study | 1.24 ± 0.77 (192) | 1.19 ± 0.84 (48) | 1.20 ± 0.76 (48) | 1.34 ± 0.69 (48) | 1.23 ± 0.78 (48) | 0.706 λ |
| Change from Day 0 to Day 15 | −0.24 ± 0.72 (192) p < 0.001 | −0.28 ± 0.82 (48) p = 0.0012 λ | −0.22 ± 0.68 (48) p = 0.008 λ | −0.19 ± 0.59 (48) p = 0.029 λ | −0.29 ± 0.80 (48) p = 0.007 λ | 0.706 λ |
| PAC-QoL Overall Score | ||||||
| Baseline | 1.10 ± 0.43 (192) | 1.16 ± 0.48 (48) | 1.00 ± 0.44 (48) | 1.14 ± 0.41 (48) | 1.10 ± 0.38 (48) | 0.273 λ § |
| End of Study | 1.03 ± 0.40 (192) | 1.12 ± 0.46 (48) | 0.95 ± 0.36 (48) | 1.06 ± 0.40 (48) | 1.00 ± 0.38 (48) | 0.470 λ |
| Change from Day 0 to Day 15 | −0.07 ± 0.37 (192) p = 0.008 λ | −0.04 ± 0.35 (48) p = 0.642 λ | −0.05 ± 0.44 (48) p = 0.546 λ | −0.08 ± 0.31 (48) p = 0.078 λ | −0.11 ± 0.37 (48) p = 0.039 λ | 0.470 λ |
| PAC-QoL Worries and Concerns Score | ||||||
| Baseline | 1.06 ± 0.68 (192) | 1.16 ± 0.77 (48) | 0.89 ± 0.61 (48) | 1.17 ± 0.74 (48) | 1.01 ± 0.58 (48) | 0.187 λ § |
| End of Study | 0.88 ± 0.63 (192) | 0.90 ± 0.65 (48) | 0.70 ± 0.56 (48) | 1.02 ± 0.68 (48) | 0.88 ± 0.62 (48) | 0.312 λ |
| Change from Day 0 to Day 15 | −0.18 ± 0.56 (192) p < 0.001 λ | −0.26 ± 0.62 (48) p = 0.005 λ | −0.19 ± 0.52 (48) p = 0.004 λ | −0.14 ± 0.45 (48) p = 0.046 λ | −0.13 ± 0.64 (48) p = 0.087 λ | 0.312λ |
| PAC-QoL Physical Discomfort Score | ||||||
| Baseline | 1.29 ± 0.73 (192) | 1.41 ± 0.80 (48) | 1.14 ± 0.71 (48) | 1.34 ± 0.66 (48) | 1.28 ± 0.73 (48) | 0.358 λ § |
| End of Study | 1.09 ± 0.79 (192) | 1.18 ± 0.87 (48) | 0.92 ± 0.76 (48) | 1.20 ± 0.76 (48) | 1.05 ± 0.73 (48) | 0.372 λ |
| Change from Day 0 to Day 15 | −0.20 ± 0.69 (192) p < 0.001 λ | −0.23 ± 0.73 (48) p = 0.008 λ | −0.22 ± 0.72 (48) p = 0.019 λ | −0.14 ± 0.56 (48) p = 0.056 λ | −0.23 ± 0.75 (48) p = 0.047 λ | 0.372 λ |
| PAC-QoL Psychosocial Discomfort Score | ||||||
| Baseline | 0.58 ± 0.59 (192) | 0.61 ± 0.58 (48) | 0.47 ± 0.53 (48) | 0.66 ± 0.66 (48) | 0.59 ± 0.58 (48) | 0.411 λ § |
| End of Study | 0.46 ± 0.57 (192) | 0.52 ± 0.67 (48) | 0.32 ± 0.42 (48) | 0.51 ± 0.53 (48) | 0.50 ± 0.60 (48) | 0.502 λ |
| Change from Day 0 to Day 15 | −0.12 ± 0.56 (192) p = 0.003λ | −0.08 ± 0.57 (48) p = 0.060λ | −0.15 ± 0.60 (48) p = 0.062λ | −0.15 ± 0.49 (48) p = 0.115λ | −0.09 ± 0.56 (48) p = 0.106 λ | 0.502 λ |
| PAC-QoL Satisfaction Score | ||||||
| Baseline | 1.48 ± 0.69 (192) | 1.48 ± 0.74 (48) | 1.50 ± 0.77 (48) | 1.40 ± 0.63 (48) | 1.53 ± 0.61 (48) | 0.805 λ § |
| End of Study | 1.70 ± 0.71 (192) | 1.88 ± 0.75 (48) a | 1.85 ± 0.73 (48) a | 1.49 ± 0.72 (48) a | 1.56 ± 0.58 (48) a | 0.006 λ |
| Change from Day 0 to Day 15 | 0.22 ± 0.80 (192) p = 0.001λ | 0.40 ± 1.00 (48) a p = 0.010λ | 0.35 ± 0.78 (48) a p = 0.004λ | 0.10 ± 0.66 (48) a p = 0.555λ | −0.03 ± 0.66 (48) a p = 0.697 λ | 0.006 λ |
| All | 12 g PDX | 8 g PDX | 4 g PDX | Placebo | p-Value Δ | |
|---|---|---|---|---|---|---|
| Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | Mean ± SD (n) within Group p-Value δ | ||
| Baseline | 44.9 ± 25.6 (192) | 44.0 ± 28.1 (48) | 40.0 ± 25.3 (48) | 48.1 ± 26.6 (48) | 47.6 ± 21.8 (48) | 0.384 λ § |
| End of Study | 36.7 ± 25.0 (192) | 36.2 ± 26.0 (48) | 34.2 ± 27.2 (48) | 39.9 ± 24.0 (48) | 36.4 ± 23.3 (48) | 0.867 λ |
| Change from Day 0 to Day 15 | −8.6 ± 21.7 (192) p < 0.001 λ | −7.9 ± 24.0 (48) p = 0.028 λ | −7.2 ± 21.1 (48) p = 0.024 λ | −8.2 ± 19.3 (48) p = 0.005 λ | −11.2 ± 22.6 (48) p = 0.001 λ | 0.867 λ |
| All | 12 g PDX | 8 g PDX | 4 g PDX | Placebo | p-Value Δ | ||
|---|---|---|---|---|---|---|---|
| n (%) within Group p-Value σ | n (%) within Group p-Value σ | n (%) within Group p-Value σ | n (%) within Group p-Value σ | n (%) within Group p-Value σ | |||
| Baseline | No Yes | 95 (50%) 96 (50%) | 22 (46%) 26 (54%) | 20 (42%) 28 (58%) | 25 (52%) 23 (48%) | 28 (60%) 19 (40%) | 0.328 |
| End of Study | No Yes | 66 (34%) 126 (66%) p = 0.002 | 10 (21%) a 38 (79%) p = 0.009 | 14 (29%) a 34 (71%) p = 0.200 | 24 (50%) a 24 (50%) p = 0.838 | 18 (38%) a 30 (62%) p = 0.031 | 0.020 |
| All | 12 g PDX | 8 g PDX | 4 g PDX | Placebo | p-Value Δ | |
|---|---|---|---|---|---|---|
| Mean ± SD (n) Within Group p-Value δ | Mean ± SD (n) Within Group p-Value δ | Mean ± SD (n) Within Group p-Value δ | Mean ± SD (n) Within Group p-Value δ | Mean ± SD (n) Within Group p-Value δ | ||
| Average Weekly Number of Bowel Movements | ||||||
| Run-in | 8.7 ± 4.6 (192) | 8.5 ± 4.1 (48) | 8.8 ± 4.6 (48) | 7.8 ± 4.3 (48) | 9.7 ± 5.3 (48) | 0.196 * § |
| Week 1 | 8.7 ± 4.7 (192) | 8.8 ± 4.6 (48) | 8.8 ± 4.5 (48) | 7.9 ± 4.1 (48) | 9.4 ± 5.4 (48) | 0.918 * |
| Week 2 | 8.6 ± 4.5 (192) | 9.6 ± 5.2 (48) a | 8.6 ± 4.0 (48) a,b | 7.4 ± 3.7 (48) a,b | 8.8 ± 5.0 (48) b | 0.017 * |
| Change from Run-in to Week 1 | 0.00 ± 2.87 (192) p = 0.971 * | 0.25 ± 2.70 (48) p = 0.659 * | 0.02 ± 2.87 (48) p = 0.955 * | 0.08 ± 2.47 (48) p = 0.671 * | −0.31 ± 3.41 (48) p = 0.407 * | 0.918 * |
| Change from Run-in to Week 2 | −0.1 ± 3.2 (192) p = 0.550 * | 1.1 ± 3.3 (48) a p = 0.059 * | −0.1 ± 3.3 (48) a,b p = 0.987 * | −0.4 ± 2.1 (48) a,b p = 0.514 * | −1.0 ± 3.7 (48) b p = 0.031 * | 0.017 * |
| Average Stool Consistency | ||||||
| Run-in | 3.29 ± 1.05 (192) | 3.42 ± 1.23 (48) | 3.36 ± 1.00 (48) | 3.06 ± 0.92 (48) | 3.31 ± 1.03 (48) | 0.359 § |
| Week 1 | 3.36 ± 1.05 (192) | 3.47 ± 1.11 (48) | 3.26 ± 1.07 (48) | 3.40 ± 1.01 (48) | 3.30 ± 1.04 (48) | 0.338 |
| Week 2 | 3.41 ± 1.01 (192) | 3.69 ± 0.85 (48) | 3.32 ± 0.94 (48) | 3.30 ± 0.99 (48) | 3.33 ± 1.20 (48) | 0.199 |
| Change from Run-in to Week 1 | 0.07 ± 0.98 (192) p = 0.329 | 0.05 ± 0.98 (48) p = 0.720 | −0.10 ± 0.96 (48) p = 0.465 | 0.33 ± 0.96 (48) p = 0.019 | 0.01 ± 1.01 (48) p = 0.971 | 0.338 |
| Change from Run-in to Week 2 | 0.12 ± 1.00 (192) p = 0.087 | 0.27 ± 1.01 (48) p = 0.068 | −0.04 ± 0.99 (48) p = 0.780 | 0.24 ± 0.95 (48) p = 0.083 | 0.02 ± 1.04 (48) p = 0.889 | 0.199 |
| Average Degree of Straining | ||||||
| Run-in | 2.13 ± 0.73 (192) | 2.11 ± 0.76 (48) | 2.21 ± 0.82 (48) | 2.19 ± 0.68 (48) | 2.02 ± 0.66 (48) | 0.616 * § |
| Week 1 | 2.01 ± 0.74 (192) | 2.05 ± 0.86 (48) | 1.99 ± 0.71 (48) | 2.04 ± 0.77 (48) | 1.95 ± 0.64 (48) | 0.922 * |
| Week 2 | 1.88 ± 0.71 (192) | 1.83 ± 0.72 (48) | 1.78 ± 0.81 (48) | 2.01 ± 0.60 (48) | 1.92 ± 0.68 (48) | 0.065 * |
| Change from Run-in to Week 1 | −0.13 ± 0.74 (192) p = 0.010* | −0.06 ± 0.80 (48) p = 0.492* | −0.22 ± 0.89 (48) p = 0.080* | −0.15 ± 0.78 (48) p = 0.125* | −0.07 ± 0.44 (48) p = 0.254* | 0.922* |
| Change from Run-in to Week 2 | −0.25 ± 0.73 (192) p < 0.001 * | −0.29 ± 0.65 (48) p = 0.002 * | −0.44 ± 0.94 (48) p < 0.001 * | −0.18 ± 0.63 (48) p = 0.072 * | −0.10 ± 0.65 (48) p = 0.218 * | 0.065 * |
| Average Proportion of Complete Bowel Movements (%) | ||||||
| Run-in | 53 ± 33 (192) | 54 ± 35 (48) | 50 ± 34 (48) | 49 ± 32 (48) | 58 ± 31 (48) | 0.520 § |
| Week 1 | 57 ± 32 (192) | 61 ± 33 (48) | 56 ± 33 (48) | 53 ± 31 (48) | 60 ± 33 (48) | 0.809 |
| Week 2 | 58 ± 33 (192) | 64 ± 34 (48) | 60 ± 35 (48) | 56 ± 32 (48) | 54 ± 33 (48) | 0.084 |
| Change from Run-in to Week 1 | 4.5 ± 28.7 (192) p = 0.031 | 6.8 ± 31.1 (48) p = 0.138 | 5.6 ± 27.3 (48) p = 0.163 | 3.6 ± 29.1 (48) p = 0.392 | 2.0 ± 27.7 (48) p = 0.620 | 0.809 |
| Change from Run-in to Week 2 | 5.6 ± 29.4 (192) p = 0.009 | 9.4 ± 28.7 (48) p = 0.027 | 10.5 ± 25.8 (48) p = 0.007 | 6.8 ± 29.6 (48) p = 0.121 | −4.4 ± 31.7 (48) p = 0.337 | 0.084 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra, A.; Pelipyagina, T.; Rueffer, M.; Evans, M.; Ouwehand, A.C. Efficacy of Polydextrose Supplementation on Colonic Transit Time, Bowel Movements, and Gastrointestinal Symptoms in Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2019, 11, 439. https://doi.org/10.3390/nu11020439
Ibarra A, Pelipyagina T, Rueffer M, Evans M, Ouwehand AC. Efficacy of Polydextrose Supplementation on Colonic Transit Time, Bowel Movements, and Gastrointestinal Symptoms in Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients. 2019; 11(2):439. https://doi.org/10.3390/nu11020439
Chicago/Turabian StyleIbarra, Alvin, Tetyana Pelipyagina, Matthew Rueffer, Malkanthi Evans, and Arthur C. Ouwehand. 2019. "Efficacy of Polydextrose Supplementation on Colonic Transit Time, Bowel Movements, and Gastrointestinal Symptoms in Adults: A Double-Blind, Randomized, Placebo-Controlled Trial" Nutrients 11, no. 2: 439. https://doi.org/10.3390/nu11020439
APA StyleIbarra, A., Pelipyagina, T., Rueffer, M., Evans, M., & Ouwehand, A. C. (2019). Efficacy of Polydextrose Supplementation on Colonic Transit Time, Bowel Movements, and Gastrointestinal Symptoms in Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients, 11(2), 439. https://doi.org/10.3390/nu11020439




