Requirements of an Application to Monitor Diet, Physical Activity and Glucose Values in Patients with Type 2 Diabetes: The Diameter
Abstract
1. Introduction
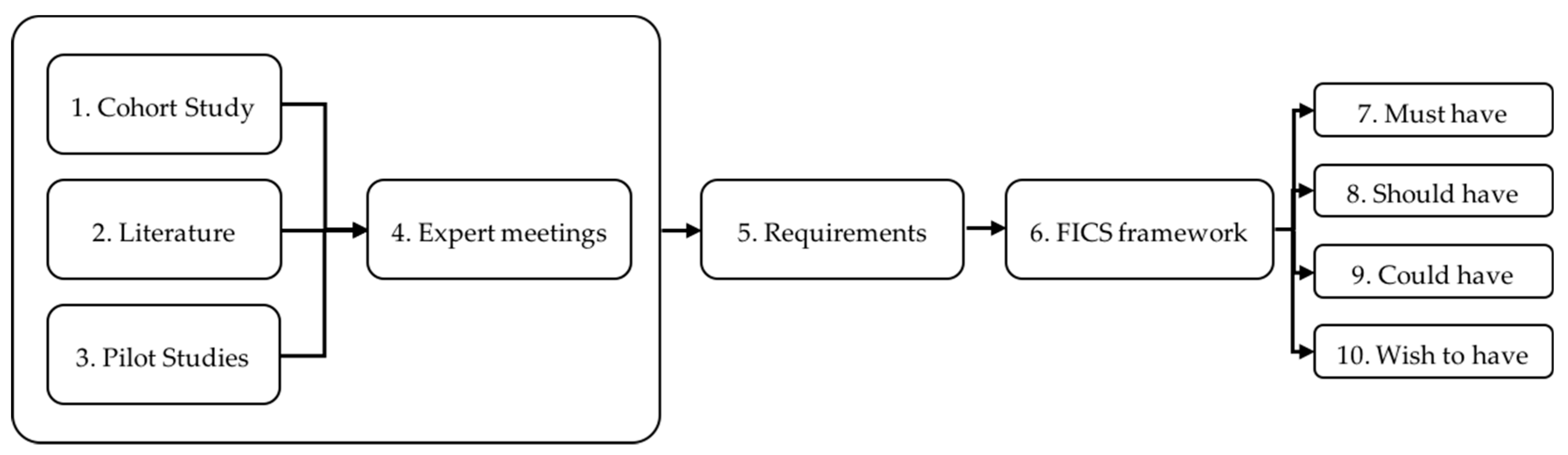
2. Materials and Methods
2.1. Cohort Study
2.2. Literature Search
2.3. Pilot Studies
2.4. Expert Meetings
3. Results
3.1. Requirement for Measuring Dietary Intake
3.1.1. Cohort Study
3.1.2. Literature Search
3.1.3. Pilot Studies
3.1.4. Expert Meetings
3.2. Requirements for Measuring Physical Activity and Sedentary Behaviour
3.2.1. Cohort Study
3.2.2. Literature Search
3.2.3. Expert Meetings
3.3. Requirements for Measuring Glucose Values
3.3.1. Cohort Study
3.3.2. Literature Search
3.3.3. Expert Meetings
3.4. Shared Requirements
3.4.1. Literature Search
3.4.2. Pilot Studies
3.4.3. Expert Meetings
3.5. Overview of the Requirements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th Edition 2017; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- World Health Organization. Global Report on Diabetes; WHO Press: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7. [Google Scholar]
- Marin-Penalver, J.J.; Martin-Timon, I.; Sevillano-Collantes, C.; Del Canizo-Gomez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Santulli, G. Integrating diet and inflammation to calculate cardiovascular risk. Atherosclerosis 2016, 253, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Ciccarelli, M.; Trimarco, B.; Iaccarino, G. Physical activity ameliorates cardiovascular health in elderly subjects: The functional role of the beta adrenergic system. Front. Physiol. 2013, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Rutten, G.E.H.M.; De Grauw, W.J.C.; Nijpels, G.; Houweling, S.T.; Van de Laar, F.A.; Bilo, H.J.; Holleman, F.; Burgers, J.S.; Wiersma, T.J.; Janssen, P.G.H. NHG-Standaard Diabetes mellitus type 2 (derde herziening). Huisarts Wet. 2013, 56, 512–525. [Google Scholar]
- Broom, D.; Whittaker, A. Controlling diabetes, controlling diabetics: Moral language in the management of diabetes type 2. Soc. Sci. Med. 2004, 58, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.M.; Chua, S.S.; Ng, C.J. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: A qualitative study. Patient Prefer. Adherence 2014, 8, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Fisher, L.; Schikman, C.H.; Hinnen, D.A.; Parkin, C.G.; Jelsovsky, Z.; Petersen, B.; Schweitzer, M.; Wagner, R.S. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: Results from the Structured Testing Program study. Diabetes Care 2011, 34, 262–267. [Google Scholar] [CrossRef]
- Wolpert, H.A.; Anderson, B.J. Management of diabetes: Are doctors framing the benefits from the wrong perspective? BMJ 2001, 323, 994–996. [Google Scholar] [CrossRef]
- Kadirvelu, A.; Sadasivan, S.; Ng, S.H. Social support in type II diabetes care: A case of too little, too late. Diabetes Metab. Syndr. Obes. 2012, 5, 407–417. [Google Scholar] [CrossRef]
- Gant, C.M.; Binnenmars, S.H.; Berg, E.V.D.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients 2017, 9, 709. [Google Scholar] [CrossRef]
- Gant, C.M.; Binnenmars, S.H.; Harmelink, M.; Soedamah-Muthu, S.S.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Real-life achievement of lipid-lowering treatment targets in the DIAbetes and LifEstyle Cohort Twente: Systemic assessment of pharmacological and nutritional factors. Nutr. Diabetes 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Oosterom, N.; Gant, C.M.; Ruiterkamp, N.; van Beijnum, B.F.; Hermens, H.; Bakker, S.J.L.; Navis, G.; Vollenbroek-Hutten, M.M.R.; Laverman, G.D. Physical Activity in Patients with Type 2 Diabetes: The Case for Objective Measurement in Routine Clinical Care. Diabetes Care 2018, 41, e50–e51. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, J.; Knittle, K.; Helf, C.; Zwickl, P.; Lusilla Palacios, P.; Castellano Tejedor, C.; Costa Requena, J.; Myllymäki, T.; Ravaja, N.; Haukkala, A. A Personalised, Sensor-Based Smart Phone Intervention for Physical Activity and Diet – PRECIOUS N-of-1 Trial. Front. Public Health 2016, 4. [Google Scholar] [CrossRef]
- Mezgec, S.; Korousic Seljak, B. NutriNet: A Deep Learning Food and Drink Image Recognition System for Dietary Assessment. Nutrients 2017, 9, 657. [Google Scholar] [CrossRef]
- Helbostad, J.L.; Vereijken, B.; Becker, C.; Todd, C.; Taraldsen, K.; Pijnappels, M.; Aminian, K.; Mellone, S. Mobile Health Applications to Promote Active and Healthy Ageing. Sensors 2017, 17, 622. [Google Scholar] [CrossRef] [PubMed]
- Holtz, B.; Lauckner, C. Diabetes management via mobile phones: A systematic review. Telemed. J. E-Health 2012, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Graffigna, G.; Barello, S.; Bonanomi, A.; Menichetti, J. The Motivating Function of Healthcare Professional in eHealth and mHealth Interventions for Type 2 Diabetes Patients and the Mediating Role of Patient Engagement. J. Diabetes Res. 2016, 2016, 2974521. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nilsen, W.J.; Abernethy, A.; Atienza, A.; Patrick, K.; Pavel, M.; Riley, W.T.; Shar, A.; Spring, B.; Spruijt-Metz, D.; et al. Mobile health technology evaluation: The mHealth evidence workshop. Am. J. Prev. Med. 2013, 45, 228–236. [Google Scholar] [CrossRef]
- Forster, H.; Walsh, M.C.; Gibney, M.J.; Brennan, L.; Gibney, E.R. Personalised nutrition: The role of new dietary assessment methods. Proc. Nutr. Soc. 2016, 75, 96–105. [Google Scholar] [CrossRef]
- Webb, T.L.; Joseph, J.; Yardley, L.; Michie, S. Using the internet to promote health behavior change: A systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J. Med. Internet Res. 2010, 12, e4. [Google Scholar] [CrossRef]
- Widya, I.; Bults, R.; de Wijk, R.; Loke, B.; Koenderink, N.; Batista, R.; Jones, V.; Hermens, H. Requirements for a Nutrition Education Demonstrator; Springer: Berlin/Heidelberg, Germany, 2011; pp. 48–53. [Google Scholar]
- Jackson, M. The meaning of requirements. Ann. Softw. Eng. 1997, 3, 5–21. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (RIVM). Dutch Food Composition Database—NEVO Online Version 2016/5.0; RIVM: Bilthoven, The Netherlands, 2016. [Google Scholar]
- Oosterwijk, M.M. Dietary Assessment and Carbohydrate Variability in Patients with Type 2 Diabetes Mellitus. Master’s Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2018. [Google Scholar]
- Nelson, M.B.; Kaminsky, L.A.; Dickin, D.C.; Montoye, A.H. Validity of Consumer-Based Physical Activity Monitors for Specific Activity Types. Med. Sci. Sports Exerc. 2016, 48, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, T.J.; Dontje, M.L.; Sprenger, S.R.; Krijnen, W.P.; van der Schans, C.P.; de Groot, M. Reliability and validity of ten consumer activity trackers. BMC Sports Sci. Med. Rehabil. 2015, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.M.; Krupka, D.J.; Chang, M.J.; Peacock, J.; Ma, Y.; Goldsmith, J.; Schwartz, J.E.; Davidson, K.W. Fitbit(R): An accurate and reliable device for wireless physical activity tracking. Int. J. Cardiol. 2015, 185, 138–140. [Google Scholar] [CrossRef]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Darby, A.; Strum, M.W.; Holmes, E.; Gatwood, J. A Review of Nutritional Tracking Mobile Applications for Diabetes Patient Use. Diabetes Technol. Ther. 2016, 18, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J. SUS: A “quick and dirty” usability scale. In Usability Evaluation in Industry; Jordan, P.W., Thomas, B., Weerdmeester, B.A., McClelland, A.L., Eds.; Taylor and Francis: London, UK, 1996; pp. 189–194. [Google Scholar]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An Empirical Evaluation of the System Usability Scale. Int. J. Hum. Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. J. Usability Stud. 2009, 4, 114–123. [Google Scholar]
- Nobbenhuis, R. Type 2 Diabetes Mellitus and Lifestyle; Insufficient Adherence to the Dutch Standards of Healthy Physical Activity and Healthy Nutrition: Oppurtunities for Improvement with mHealth? Bachelor’s Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2017. [Google Scholar]
- Lankheet, M.H.T. Development of a Coaching Technology for Diabetes Type 2 Patients to Motivate Them into Exercise and Nutrition (Lifestyle) Changes. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2017. [Google Scholar]
- Vollenbroek-Hutten, M.M.; Lankheet, M.H.T.; Goolkate, T.; van Beijnum, B.J.; Hegeman, H.H. Insufficient Behavioral Change Skill Hampers Adoption of Ehealth Services. In Proceedings of the 4th International Conference on Information and Communication Technologies for Ageing Well and e-Health, Funchal, Madeira, Portugal, 22–23 March 2018. [Google Scholar] [CrossRef]
- Palaniappan, U.; Cue, R.I.; Payette, H.; Gray-Donald, K. Implications of day-to-day variability on measurements of usual food and nutrient intakes. J. Nutr. 2003, 133, 232–235. [Google Scholar] [CrossRef]
- Rollo, M.E.; Aguiar, E.J.; Williams, R.L.; Wynne, K.; Kriss, M.; Callister, R.; Collins, C.E. eHealth technologies to support nutrition and physical activity behaviors in diabetes self-management. Diabetes Metab. Syndr. Obes. 2016, 9, 381–390. [Google Scholar] [CrossRef]
- Hoppe, C.D.; Cade, J.E.; Carter, M. An evaluation of diabetes targeted apps for Android smartphone in relation to behaviour change techniques. J. Hum. Nutr. Diet. 2017, 30, 326–338. [Google Scholar] [CrossRef] [PubMed]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Chomutare, T.; Fernandez-Luque, L.; Arsand, E.; Hartvigsen, G. Features of mobile diabetes applications: Review of the literature and analysis of current applications compared against evidence-based guidelines. J. Med. Internet Res. 2011, 13, e65. [Google Scholar] [CrossRef]
- Krebs, P.; Duncan, D.T. Health App Use Among US Mobile Phone Owners: A National Survey. JMIR mHealth uHealth 2015, 3, e101. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Dack, C.; Ross, J.; Michie, S.; May, C.; Stevenson, F.; Farmer, A.; Yardley, L.; Barnard, M.; Murray, E. Digital Health Interventions for Adults with Type 2 Diabetes: Qualitative Study of Patient Perspectives on Diabetes Self-Management Education and Support. J. Med. Internet Res. 2018, 20, e40. [Google Scholar] [CrossRef] [PubMed]
- Rhyner, D.; Loher, H.; Dehais, J.; Anthimopoulos, M.; Shevchik, S.; Botwey, R.H.; Duke, D.; Stettler, C.; Diem, P.; Mougiakakou, S. Carbohydrate Estimation by a Mobile Phone-Based System Versus Self-Estimations of Individuals with Type 1 Diabetes Mellitus: A Comparative Study. J. Med. Internet Res. 2016, 18, e101. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Sisson, S.B.; Collova, T.; Lee, S.M.; Swan, P.D. Pedometer-Determined Step Count Guidelines for Classifying Walking Intensity in a Young Ostensibly Healthy Population. Can. J. Appl. Physiol. 2005, 30, 666–676. [Google Scholar] [CrossRef]
- Marshall, S.J.; Levy, S.S.; Tudor-Locke, C.E.; Kolkhorst, F.W.; Wooten, K.M.; Ji, M.; Macera, C.A.; Ainsworth, B.E. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am. J. Prev. Med. 2009, 36, 410–415. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care 2017, 40, S4–S5. [CrossRef]
- Hagedoorn, I. What is the Actual Daily Movement in Patients with Complicated Type 2 Diabetes Mellitus? Master’s Thesis, University of Groningen, Groningen, The Netherlands, 2017. [Google Scholar]
- Wijndaele, K.; Brage, S.; Besson, H.; Khaw, K.T.; Sharp, S.J.; Luben, R.; Wareham, N.J.; Ekelund, U. Television viewing time independently predicts all-cause and cardiovascular mortality: The EPIC Norfolk study. Int. J. Epidemiol. 2011, 40, 150–159. [Google Scholar] [CrossRef]
- Thorp, A.A.; Owen, N.; Neuhaus, M.; Dunstan, D.W. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. Am. J. Prev. Med. 2011, 41, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Grontved, A.; Hu, F.B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A meta-analysis. JAMA 2011, 305, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Barr, E.L.; Healy, G.N.; Salmon, J.; Shaw, J.E.; Balkau, B.; Magliano, D.J.; Cameron, A.J.; Zimmet, P.Z.; Owen, N. Television viewing time and mortality: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 2010, 121, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Arsand, E.; Tatara, N.; Ostengen, G.; Hartvigsen, G. Mobile phone-based self-management tools for type 2 diabetes: The few touch application. J. Diabetes Sci. Technol. 2010, 4, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Schoeppe, S.; Alley, S.; Rebar, A.L.; Hayman, M.; Bray, N.A.; Van Lippevelde, W.; Gnam, J.-P.; Bachert, P.; Direito, A.; Vandelanotte, C. Apps to improve diet, physical activity and sedentary behaviour in children and adolescents: A review of quality, features and behaviour change techniques. Int. J. Behav. Nutr. Phys. Act. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Edelman, S.V.; Argento, N.B.; Pettus, J.; Hirsch, I.B. Clinical Implications of Real-time and Intermittently Scanned Continuous Glucose Monitoring. Diabetes Care 2018, 41, 2265–2274. [Google Scholar] [CrossRef]
- Goyal, S.; Cafazzo, J.A. Mobile phone health apps for diabetes management: Current evidence and future developments. QJM 2013, 106, 1067–1069. [Google Scholar] [CrossRef]
- Arnhold, M.; Quade, M.; Kirch, W. Mobile applications for diabetics: A systematic review and expert-based usability evaluation considering the special requirements of diabetes patients age 50 years or older. J. Med. Internet Res. 2014, 16, e104. [Google Scholar] [CrossRef]
- Van Kerkhof, L.; van de Laar, K.; Schooneveldt, B.; Hegger, I. e-Medication Met Behulp van Apps: Gebruik en Gebruikerservaringen; Rijksinstituut voor Volksgezondheid en Milieu (RIVM): Bilthoven, The Netherlands, 2015. [Google Scholar]
- El-Gayar, O.; Timsina, P.; Nawar, N.; Eid, W. Mobile applications for diabetes self-management: Status and potential. J. Diabetes Sci. Technol. 2013, 7, 247–262. [Google Scholar] [CrossRef]
- McMillan, K.A.; Kirk, A.; Hewitt, A.; MacRury, S. A Systematic and Integrated Review of Mobile-Based Technology to Promote Active Lifestyles in People with Type 2 Diabetes. J. Diabetes Sci. Technol. 2017, 11, 299–307. [Google Scholar] [CrossRef] [PubMed]
| Diet (n) | PA and SB (n) | Glucose Values (n) | Shared (n) | Total (n) | |
|---|---|---|---|---|---|
| Function & events | 17 | 5 | 4 | 3 | 29 |
| Interaction & usability | 7 | 5 | 5 | 7 | 24 |
| Content & structure | 8 | 4 | 2 | 4 | 18 |
| Style & aesthetics | 5 | 1 | 1 | 3 | 10 |
| 37 | 15 | 12 | 17 | 81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
den Braber, N.; Vollenbroek-Hutten, M.M.R.; Oosterwijk, M.M.; Gant, C.M.; Hagedoorn, I.J.M.; van Beijnum, B.-J.F.; Hermens, H.J.; Laverman, G.D. Requirements of an Application to Monitor Diet, Physical Activity and Glucose Values in Patients with Type 2 Diabetes: The Diameter. Nutrients 2019, 11, 409. https://doi.org/10.3390/nu11020409
den Braber N, Vollenbroek-Hutten MMR, Oosterwijk MM, Gant CM, Hagedoorn IJM, van Beijnum B-JF, Hermens HJ, Laverman GD. Requirements of an Application to Monitor Diet, Physical Activity and Glucose Values in Patients with Type 2 Diabetes: The Diameter. Nutrients. 2019; 11(2):409. https://doi.org/10.3390/nu11020409
Chicago/Turabian Styleden Braber, Niala, Miriam M. R. Vollenbroek-Hutten, Milou M. Oosterwijk, Christina M. Gant, Ilse J. M. Hagedoorn, Bert-Jan F. van Beijnum, Hermie J. Hermens, and Gozewijn D. Laverman. 2019. "Requirements of an Application to Monitor Diet, Physical Activity and Glucose Values in Patients with Type 2 Diabetes: The Diameter" Nutrients 11, no. 2: 409. https://doi.org/10.3390/nu11020409
APA Styleden Braber, N., Vollenbroek-Hutten, M. M. R., Oosterwijk, M. M., Gant, C. M., Hagedoorn, I. J. M., van Beijnum, B.-J. F., Hermens, H. J., & Laverman, G. D. (2019). Requirements of an Application to Monitor Diet, Physical Activity and Glucose Values in Patients with Type 2 Diabetes: The Diameter. Nutrients, 11(2), 409. https://doi.org/10.3390/nu11020409





