Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-κB Kinase Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Bone-Resorbing Activity in Organ Cultures of Mouse Calvariae
2.3. Cultures of Primary Mouse Osteoblastic Cells
2.4. Measurement of the PGE2 Content in the Cultured Medium
2.5. Reverse Transcription-Quantitative PCR
2.6. Dual-Luciferase Reporter Assay
2.7. Inhibitor of NF-κB Kinase (IKK) Activity Assay
2.8. Protein Structure Preparation
2.9. Ligand Structure Preparation
2.10. Molecular Docking Studies
2.11. Structural Visualization and Analyses
2.12. RANKL-Induced Osteoclastogenesis in RAW264.7 Cells
2.13. Statistical Analyses
3. Results
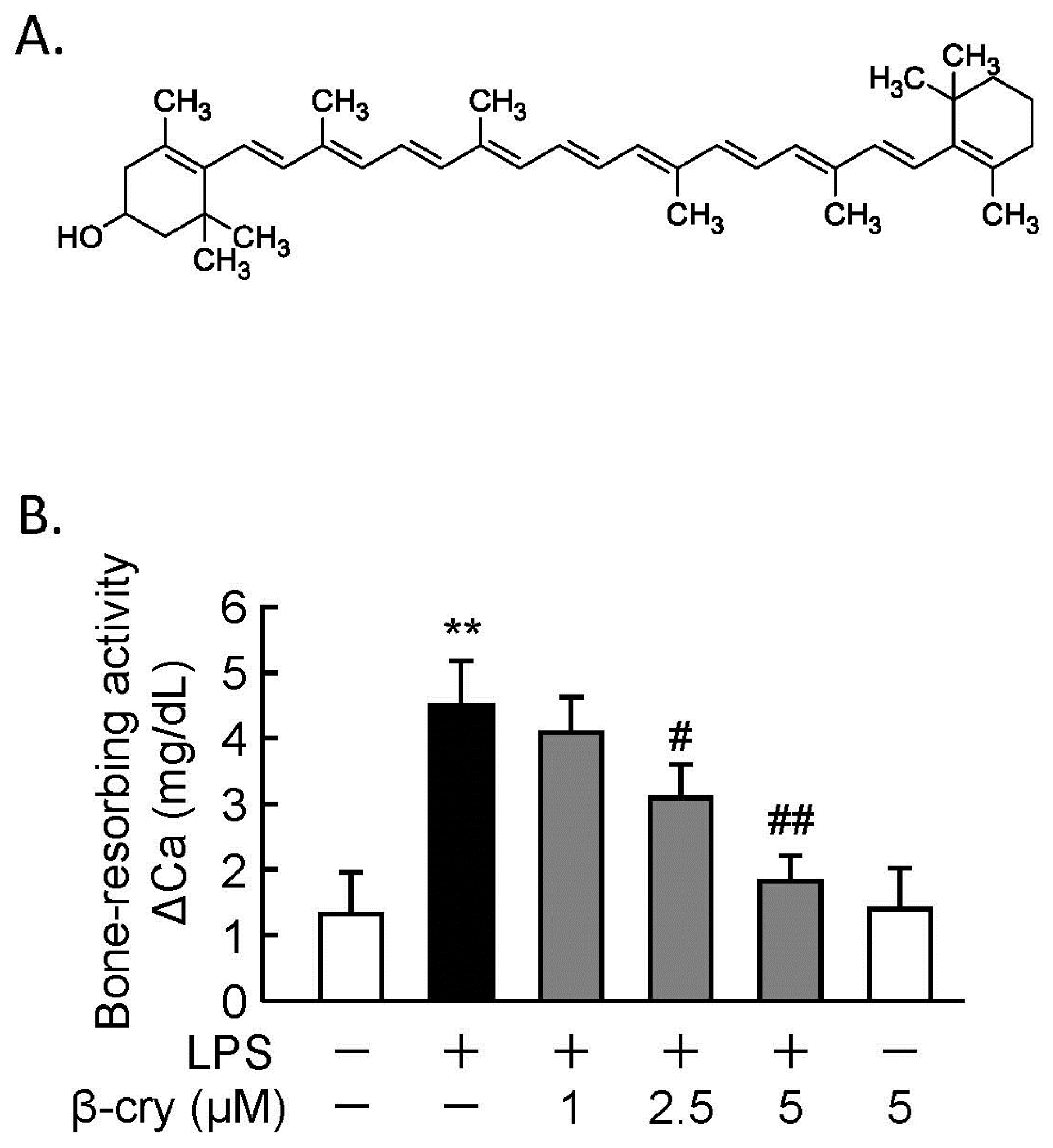
3.1. β-Cry Inhibited LPS-Induced Bone Resorption in Calvarial Organ Culture
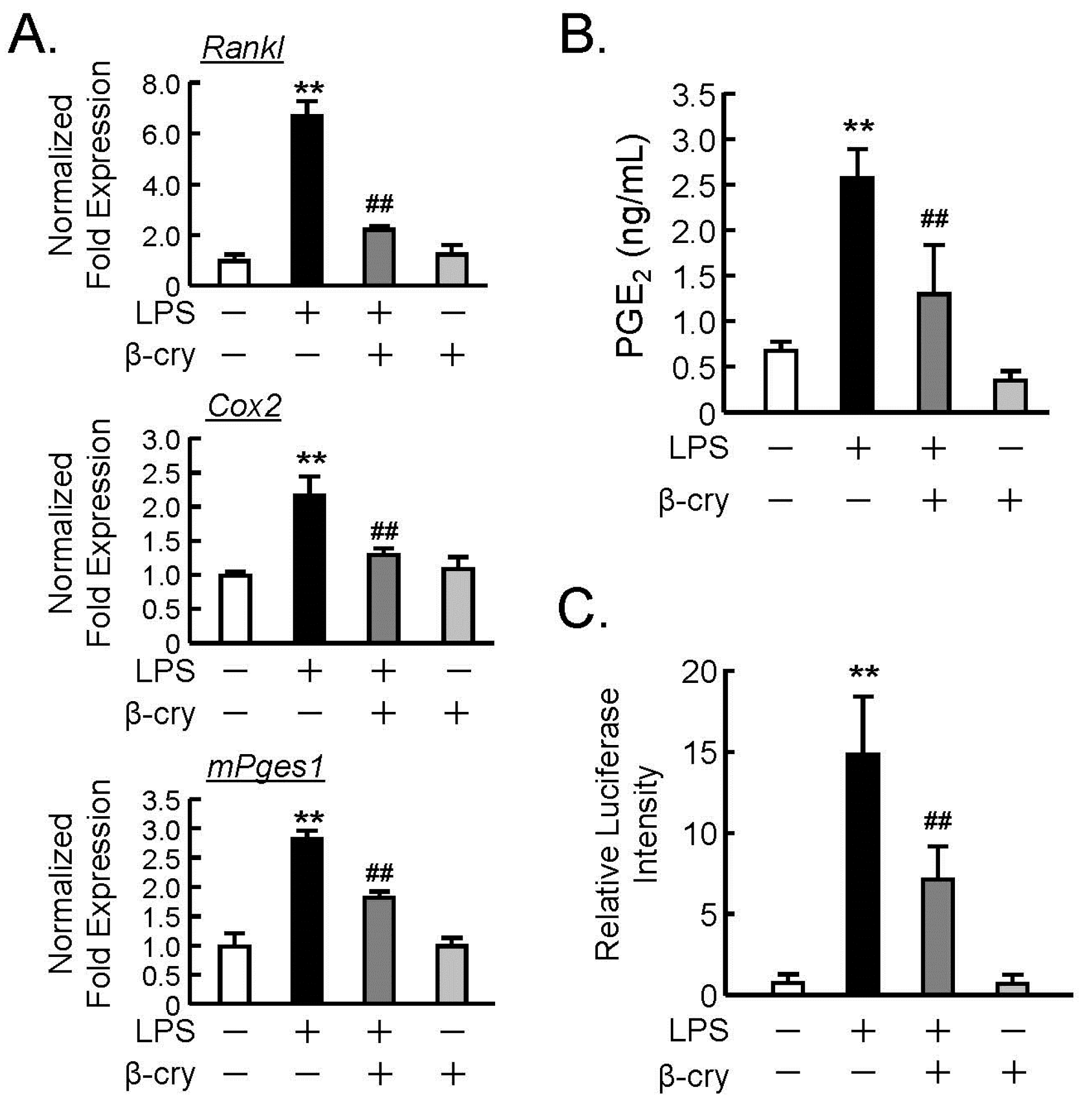
3.2. Effects of β-Cry on the PGE2 Production and NF-κB Signaling in Osteoblasts
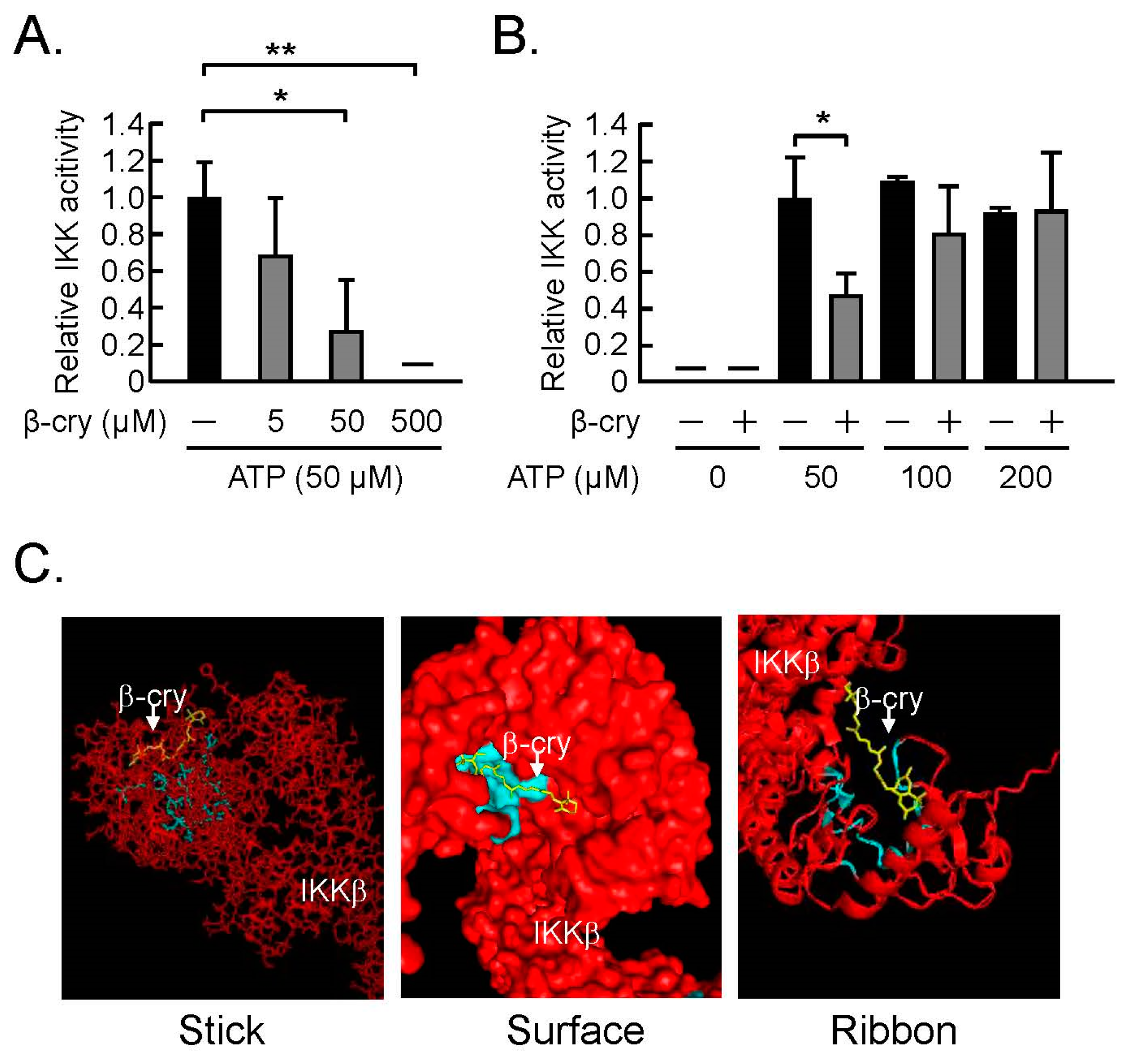
3.3. β-Cry Inhibited IKKβ Activity Via ATP-Competitive Binding
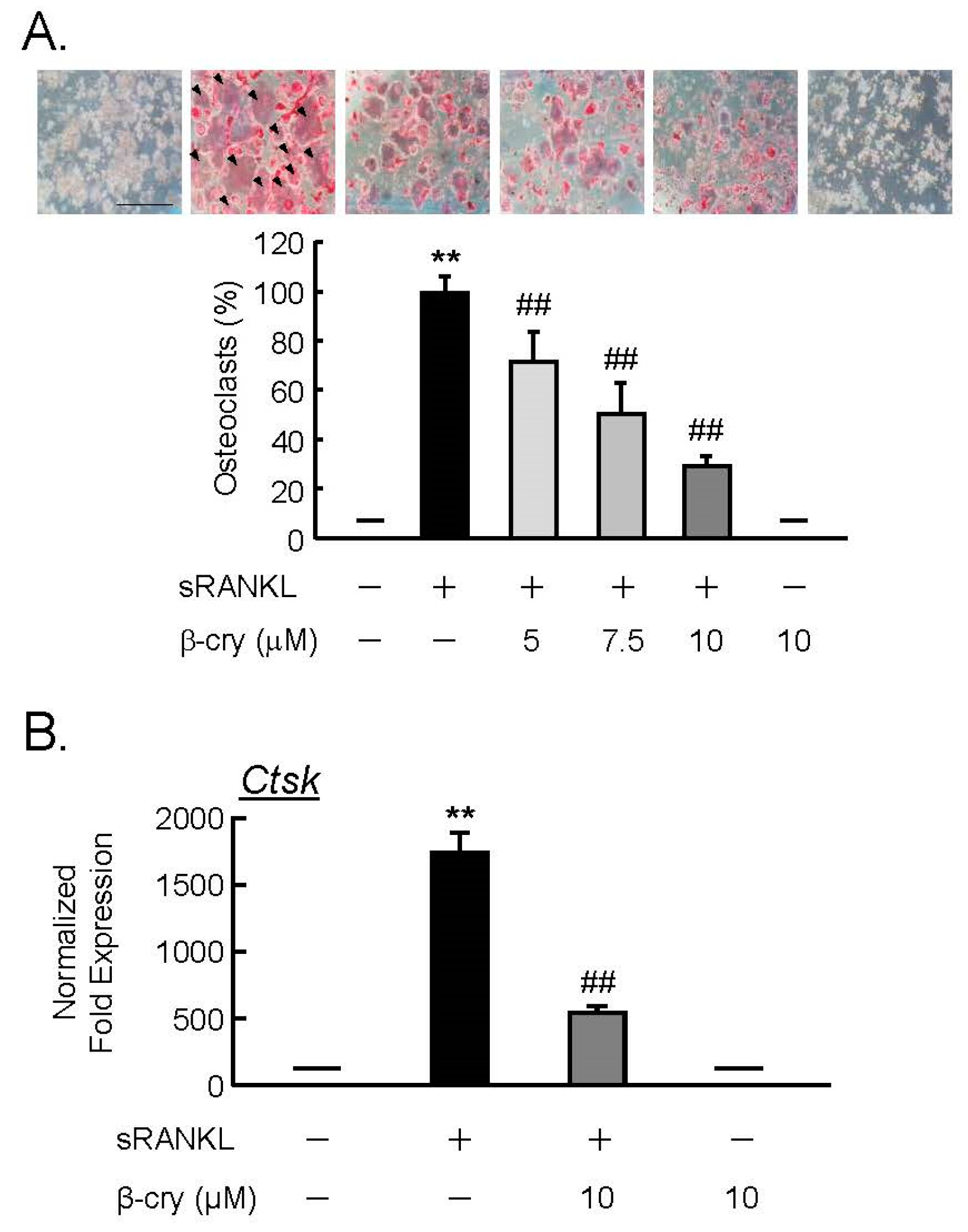
3.4. β-Cry Directly Suppressed sRANKL-Induced Osteoclast Differentiation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, C.; Inada, M.; Suzawa, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J. Biol. Chem. 2000, 275, 19819–19823. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, T.; Miyaura, C.; Inada, M.; Maruyama, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology 2000, 141, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, C.; Inada, M.; Matsumoto, C.; Ohshiba, T.; Uozumi, N.; Shimizu, T.; Ito, A. An Essential Role of Cytosolic Phospholipase A2α in Prostaglandin E2–mediated Bone Resorption Associated with Inflammation. J. Exp. Med. 2003, 197, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Matsumoto, C.; Uematsu, S.; Akira, S.; Miyaura, C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J. Immunol. 2006, 177, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Kobayashi, M.; Takita, M.; Matsumoto, C.; Miyaura, C.; Inada, M. Hyaluronan inhibits bone resorption by suppressing prostaglandin E synthesis in osteoblasts treated with interleukin-1. Biochem. Biophys. Res. Commun. 2009, 381, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Laulederkind, S.; Kirtikara, K.; Raghow, R.; Ballou, L. The regulation of PGE(2) biosynthesis in MG-63 osteosarcoma cells by IL-1 and FGF is cell density-dependent. Exp. Cell. Res. 2000, 258, 409–416. [Google Scholar] [CrossRef]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.; Miyaura, C.; Inada, M. Epigallocatechin gallate (EGCG) suppresses lipopolysaccharide-induced inflammatory bone resorption, and protects against alveolar bone loss in mice. FEBS Open Bio 2015, 5, 522–527. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Miao, B.; Li, X.; Hu, K.-Q.; Liu, C.; Wang, X.-D. β-Cryptoxanthin Reduced Lung Tumor Multiplicity and Inhibited Lung Cancer Cell Motility by Downregulating Nicotinic Acetylcholine Receptor α7 Signaling. Cancer Prev. Res. 2016, 9, 875–886. [Google Scholar] [CrossRef]
- Katsuura, S.; Imamura, T.; Bando, N.; Yamanishi, R. β-Carotene and β-cryptoxanthin but not lutein evoke redox and immune changes in RAW264 murine macrophages. Mol. Nutr. Food Res. 2009, 53, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yılmaz, I.; Juturu, V. β-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Horie, T.; Fukasawa, K.; Ozaki, K.; Onishi, Y.; Kanayama, T.; Iezaki, T.; Kaneda, K.; Sugiura, M.; Hinoi, E. Amelioration of the Development of Osteoarthritis by Daily Intake of β-Cryptoxanthin. Biol. Pharm. Bull. 2017, 40, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Okamoto, M.; Fukasawa, K.; Iezaki, T.; Onishi, Y.; Yoneda, Y.; Sugiura, M.; Hinoi, E. Daily intake of β-cryptoxanthin prevents bone loss by preferential disturbance of osteoclastic activation in ovariectomized mice. J. Pharmacol. Sci. 2015, 129, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yamaguchi, M. beta-Cryptoxanthin stimulates cell proliferation and transcriptional activity in osteoblastic MC3T3-E1 cells. Int. J. Mol. Med. 2005, 15, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yamaguchi, M. β-cryptoxanthin stimulates cell differentiation and mineralization in osteoblastic MC3T3-E1 cells. J. Cell. Biochem. 2005, 95, 1224–1234. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M. The bone anabolic carotenoid beta-cryptoxanthin enhances transforming growth factor-beta1-induced SMAD activation in MC3T3 preosteoblasts. Int. J. Mol. Med. 2009, 24, 671–675. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M. The bone anabolic carotenoids p-hydroxycinnamic acid and β-cryptoxanthin antagonize NF-κB activation in MC3T3 preosteoblasts. Mol. Med. Rep. 2009, 2, 641–644. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J. Biomed. Sci. 2012, 19, 36. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Inhibitory effect of beta-cryptoxanthin on osteoclast-like cell formation in mouse marrow cultures. Biochem. Pharmacol. 2004, 67, 1297–1305. [Google Scholar] [CrossRef]
- Uchiyama, S.; Yamaguchi, M. Beta-cryptoxanthin stimulates apoptotic cell death and suppresses cell function in osteoclastic cells: Change in their related gene expression. J. Cell. Biochem. 2006, 98, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Ashida, N.; Yokoyama, S.; Tominari, T.; Hirata, M.; Ogawa, K.; Sugiura, M.; Yano, M.; Inada, M.; Miyaura, C. The Protective Effects of β-Cryptoxanthin on Inflammatory Bone Resorption in a Mouse Experimental Model of Periodontitis. Biosci. Biotechnol. Biochem. 2013, 77, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Misquitta, Y.R.; Olland, A.; Johnson, M.A.; Kelleher, K.S.; Kriz, R.; Lin, L.L.; Stahl, M.; Mosyak, L. Crystal structure of a human IκB kinase b asymmetric dimer. J. Biol. Chem. 2013, 288, 22758–22767. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2011, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jobin, C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 2005, 115, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Kato, M.; Matsumoto, H.; Nagao, A.; Yano, M. Serum concentration of β-cryptoxanthin in Japan reflects the frequency of Satsuma mandarin (Citrus Unshiu Marc.) consumption. J. Health Sci. 2002, 48, 350–353. [Google Scholar] [CrossRef]
- Sugiura, M.; Ogawa, K.; Yano, M. Absorption, storage and distribution of β-cryptoxanthin in rat after chronic administration of Satsuma mandarin (Citrus unshiu MARC.) juice. Biol. Pharm. Bull. 2013, 36, 147–151. [Google Scholar] [CrossRef]
- Imada, K.; Tsuchida, A.; Ogawa, K.; Sofat, N.; Nagase, H.; Ito, A.; Sato, T. Anti-arthritic actions of β-cryptoxanthin against the degradation of articular cartilage in vivo and in vitro. Biochem. Biophys. Res. Commun. 2016, 476, 352–358. [Google Scholar] [CrossRef]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary beta-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [CrossRef]
- D’Acquisto, F.; Iuvone, T.; Rombolà, L.; Sautebin, L.; Di Rosa, M.; Carnuccio, R. Involvement of NF-kappaB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997, 418, 175–178. [Google Scholar] [CrossRef]
- Díaz-Muñoz, M.D.; Osma-García, I.C.; Cacheiro-Llaguno, C.; Fresno, M.; Iñiguez, M.A. Coordinated up-regulation of cyclooxygenase-2 and microsomal prostaglandin E synthase 1 transcription by nuclear factor kappa B and early growth response-1 in macrophages. Cell Signal. 2010, 22, 1427–1436. [Google Scholar] [CrossRef]
- Takeda, H.; Tominari, T.; Ichimaru, R.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Inada, M.; Miyaura, C. Lutein, a carotenoid, inhibits lipopolysaccharide-induced alveolar bone loss associated with inflammation in a mouse model of periodontitis. Curr. Top. Biochem. Res. 2016, 17, 71–76. [Google Scholar]
- Jacobs, M.D.; Harrison, S.C. Structure of an IkappaBalpha/NF-kappaB complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef]
- Adli, M.; Merkhofer, E.; Cogswell, P.; Baldwin, A.S. IKKalpha and IKKbeta each function to regulate NF-kappaB activation in the TNF-induced/canonical pathway. PLoS ONE 2010, 5, e9428. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhou, P.; Kang, W.; Luo, L.; Fan, X.; Yan, J.; Liang, H. The small-molecule inhibitor selectivity between IKKα and IKKβ kinases in NF-κB signaling pathway. J. Recept. Signal Transduct. Res. 2015, 35, 307–318. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirata, N.; Ichimaru, R.; Tominari, T.; Matsumoto, C.; Watanabe, K.; Taniguchi, K.; Hirata, M.; Ma, S.; Suzuki, K.; Grundler, F.M.W.; et al. Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-κB Kinase Activity. Nutrients 2019, 11, 368. https://doi.org/10.3390/nu11020368
Hirata N, Ichimaru R, Tominari T, Matsumoto C, Watanabe K, Taniguchi K, Hirata M, Ma S, Suzuki K, Grundler FMW, et al. Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-κB Kinase Activity. Nutrients. 2019; 11(2):368. https://doi.org/10.3390/nu11020368
Chicago/Turabian StyleHirata, Narumi, Ryota Ichimaru, Tsukasa Tominari, Chiho Matsumoto, Kenta Watanabe, Keita Taniguchi, Michiko Hirata, Sihui Ma, Katsuhiko Suzuki, Florian M.W. Grundler, and et al. 2019. "Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-κB Kinase Activity" Nutrients 11, no. 2: 368. https://doi.org/10.3390/nu11020368
APA StyleHirata, N., Ichimaru, R., Tominari, T., Matsumoto, C., Watanabe, K., Taniguchi, K., Hirata, M., Ma, S., Suzuki, K., Grundler, F. M. W., Miyaura, C., & Inada, M. (2019). Beta-Cryptoxanthin Inhibits Lipopolysaccharide-Induced Osteoclast Differentiation and Bone Resorption via the Suppression of Inhibitor of NF-κB Kinase Activity. Nutrients, 11(2), 368. https://doi.org/10.3390/nu11020368






