A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. PMM Composition
2.2. PMM Preparation
2.3. Cell Culture
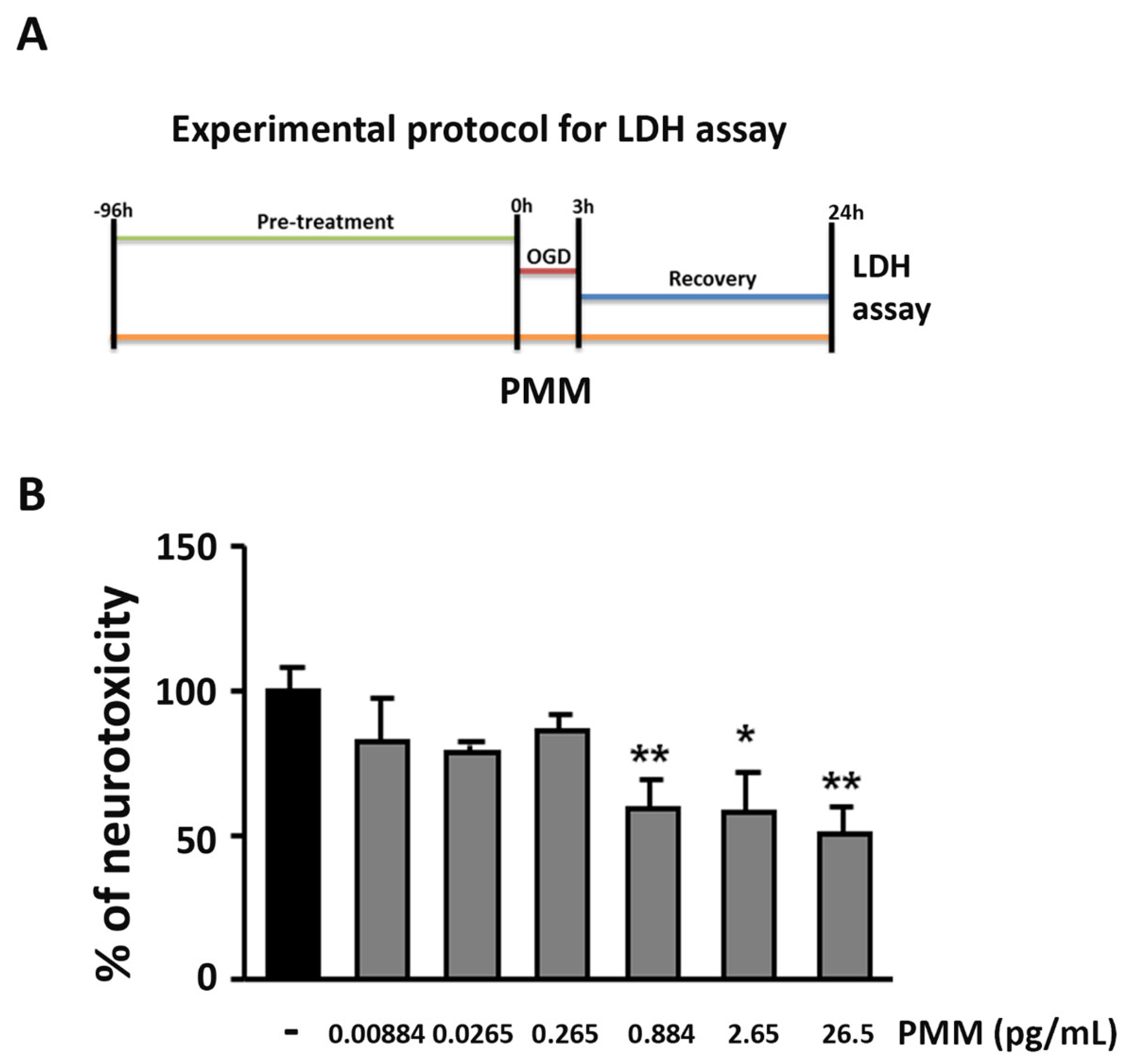
2.4. OGD and Measurement of Lactate Dehydrogenase (LDH) Release
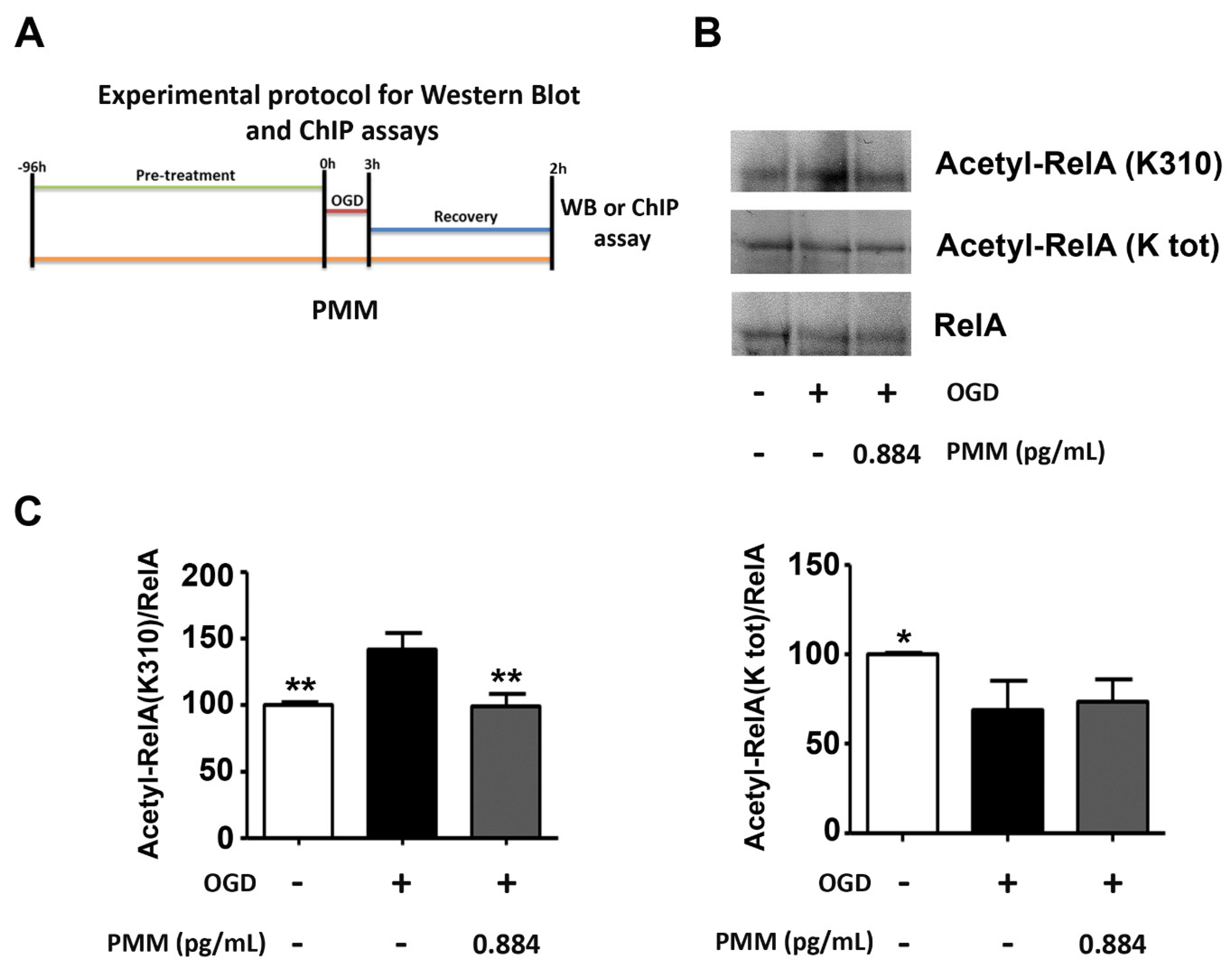
2.5. WB and Co-Immunoprecipitation (co-IP) Assays
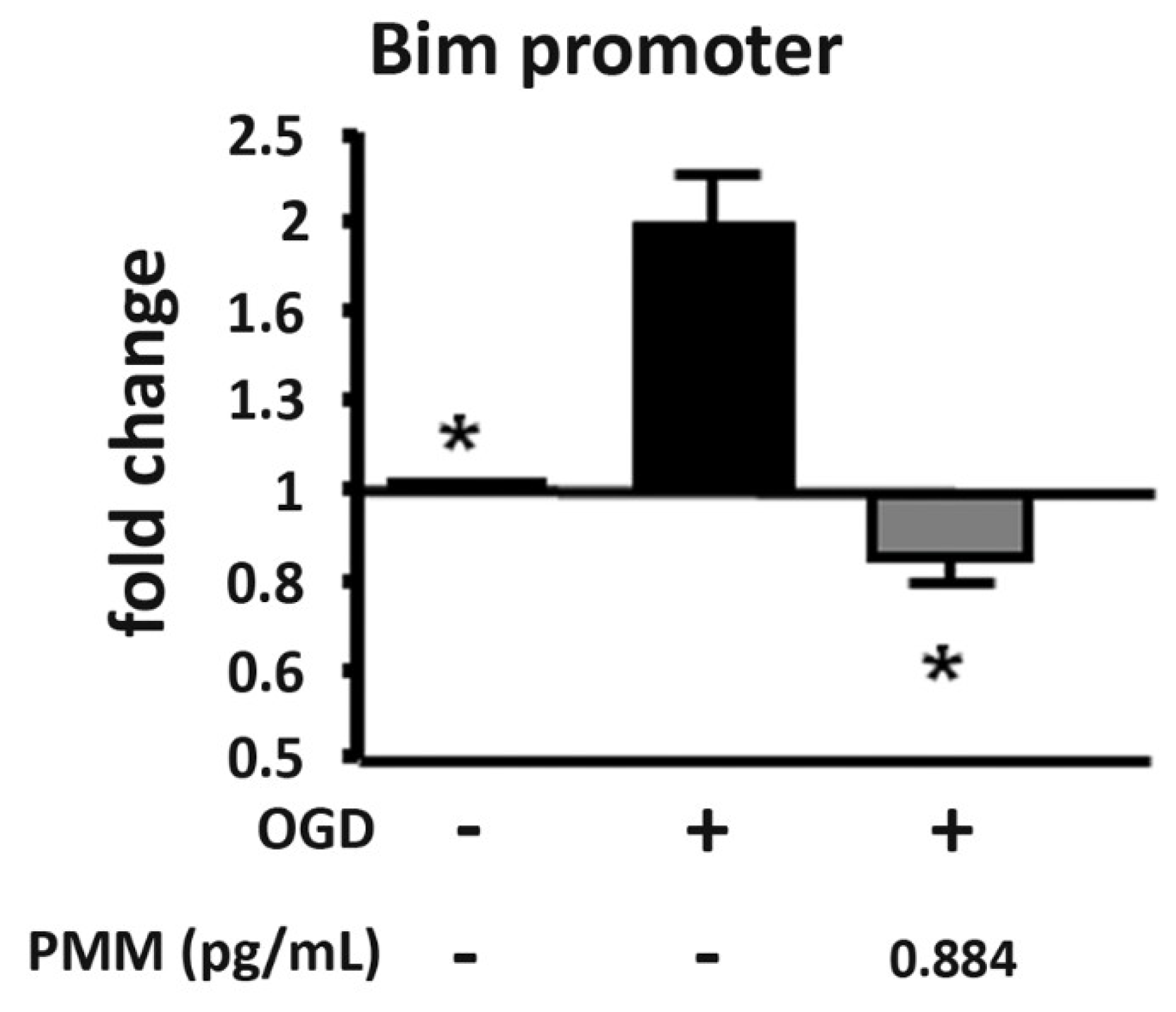
2.6. ChIP Assay
- forward, 5-CTGGATGCAGGTTGGGTAG-3
- reverse, 5 GGGAATGAGAAAGTTAGCTGGA-3.
2.7. Statistical Analysis
3. Results
3.1. Pre-Treatment with PMM Elicits Neuroprotection in Cortical Neurons Exposed to OGD
3.2. Pre-Treatment with PMM Prevents the Derangement of RelA Acetylation in Cortical Neurons Subjected to OGD
3.3. Pre-Treatment with PMM Reduces H3 Histone Acetylation at the Bim Promoter in Neurons Exposed to OGD
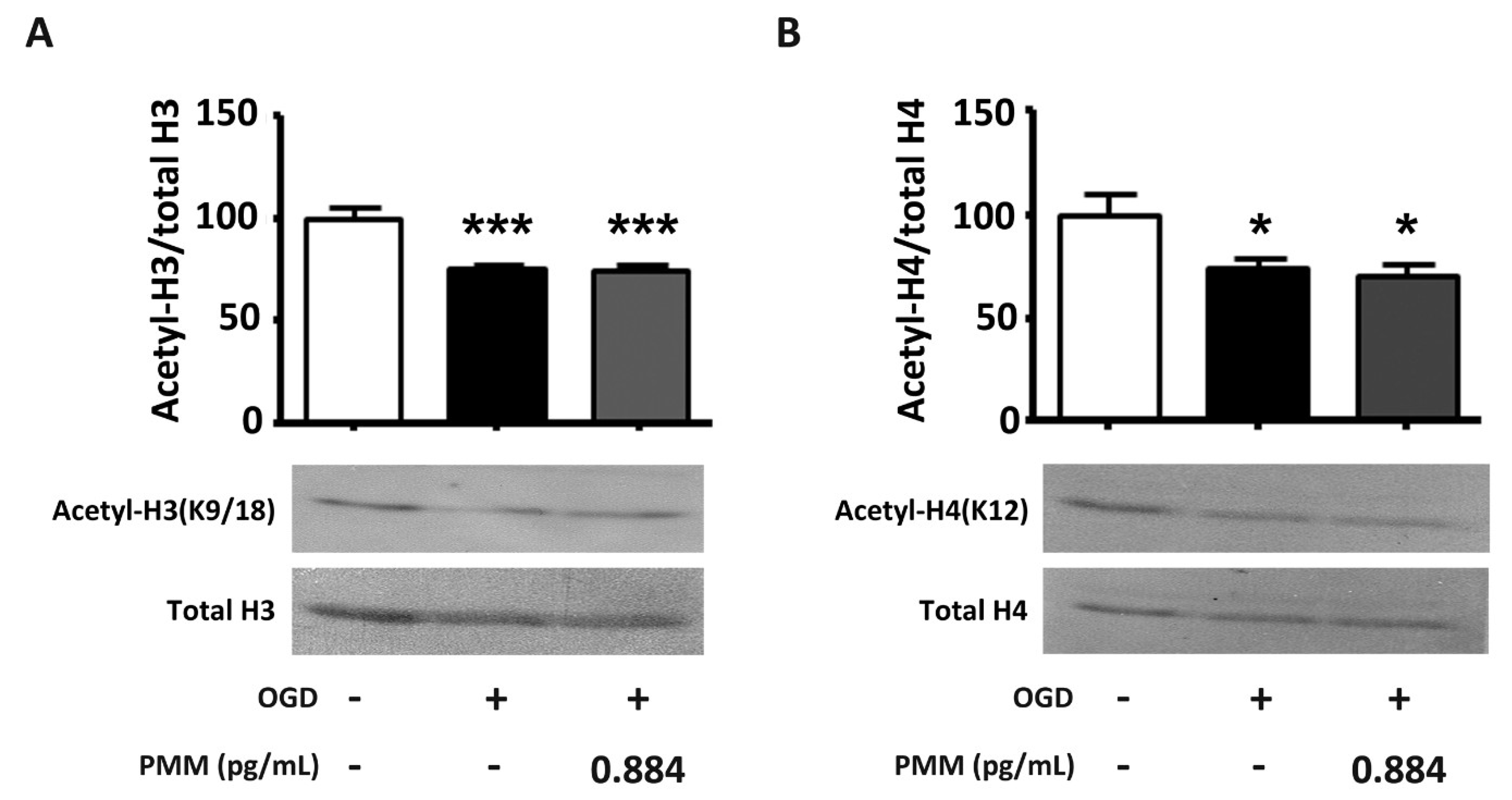
3.4. Pre-Treatment with PMM Does Not Restore the Histone Acetylation Status in Cortical Neurons Subjected to OGD
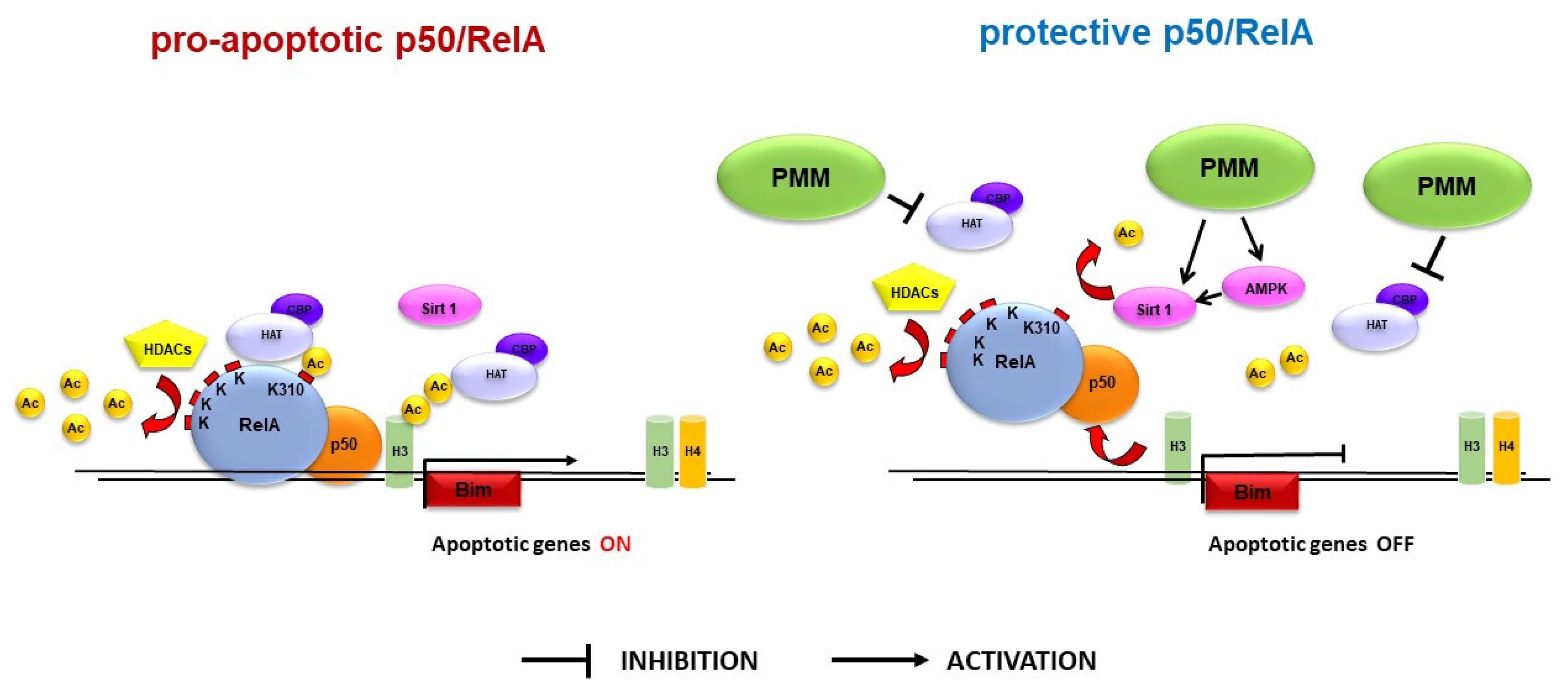
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ayuso, M.I.; Gonzalo-Gobernado, R.; Montaner, J. Neuroprotective diets for stroke. Neurochem. Int. 2017, 107, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C. Dietary Approaches For Stroke Prevention. Stroke 2017, 48, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Kalea, A.Z.; Drosatos, K.; Buxton, J.L. Nutriepigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care. 2018, 21, 252–259. [Google Scholar] [CrossRef]
- Choi, S.W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoo, Y.; Park, Y.J. Epigenetics: Linking nutrition to molecular mechanisms in aging. Prev. Nutr. Food Sci. 2017, 22, 81. [Google Scholar] [CrossRef]
- Sweatt, J.D. Experience-dependent epigenetic modifications in the central nervous system. Biol. Psychiatry 2009, 65, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.; Gensert, J.M.; Beal, M.F.; Ratan, R.R. Remodeling chromatin and stress resistance in the central nervous system: Histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug Targets CNS Neurol. Disord. 2005, 1, 41–50. [Google Scholar] [CrossRef]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32. [Google Scholar] [CrossRef]
- Crampton, S.J.; O’Keeffe, G.W. NF-κB: Emerging roles in hippocampal development and function. Int. J. Biochem. Cell Biol. 2013, 45, 1821–1824. [Google Scholar] [CrossRef]
- Lanzillotta, A.; Porrini, V.; Bellucci, A.; Benarese, M.; Branca, C.; Parrella, E.; Spano, P.F.; Pizzi, M. NF-κB in Innate Neuroprotection and Age-Related Neurodegenerative Diseases. Front. Neurol. 2015, 6, 98. [Google Scholar] [CrossRef]
- Sarnico, I.; Branca, C.; Lanzillotta, A.; Porrini, V.; Benarese, M.; Spano, P.F.; Pizzi, M. NF-κB and epigenetic mechanisms as integrative regulators of brain resilience to anoxic stress. Brain Res. 2012, 1476, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, A.; Sarnico, I.; Ingrassia, R.; Boroni, F.; Branca, C.; Benarese, M.; Faraco, G.; Blasi, F.; Chiarugi, A.; Spano, P.; et al. The acetylation of RelA in Lys310 dictates the NF-κB-dependent response in post-ischemic injury. Cell Death Dis. 2010, 1, e96. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, R.; Lanzillotta, A.; Sarnico, I.; Benarese, M.; Blasi, F.; Borgese, L.; Bilo, F.; Depero, L.; Chiarugi, A.; Spano, P.F.; et al. 1B/(-)IRE DMT1 expression during brain ischemia contributes to cell death mediated by NF-κB/RelA acetylation at Lys310. PLoS ONE 2012, 7, e38019. [Google Scholar] [CrossRef] [PubMed]
- Inta, I.; Paxian, S.; Maegele, I.; Zhang, W.; Pizzi, M.; Spano, P.; Sarnico, I.; Muhammad, S.; Herrmann, O.; Inta, D.; et al. Bim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemia. J. Neurosci. 2006, 26, 12896–12903. [Google Scholar] [CrossRef] [PubMed]
- Sarnico, I.; Lanzillotta, A.; Boroni, F.; Benarese, M.; Alghisi, M.; Schwaninger, M.; Inta, I.; Battistin, L.; Spano, P.; Pizzi, M. NF-kappaB p50/RelA and c-Rel-containing dimers: Opposite regulators of neuron vulnerability to ischaemia. J. Neurochem. 2009, 108, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, A.; Pignataro, G.; Branca, C.; Cuomo, O.; Sarnico, I.; Benarese, M.; Annunziato, L.; Spano, P.; Pizzi, M. Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol. Dis. 2013, 49, 177–189. [Google Scholar] [CrossRef]
- Choi, Y.B.; Kim, Y.I.; Lee, K.S.; Kim, B.S.; Kim, D.J. Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 2004, 1019, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gundimeda, U.M.; McNeill, T.H.; Elhiani, A.A.; Schiffman, J.E.; Hinton, D.R.; Gopalakrishna, R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cε. J. Biol. Chem. 2012, 287, 34694–34708. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, M.; Jing, X.; Shi, H.; Ren, M.; Lou, H. (-)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem. Res. 2014, 39, 1292–1299. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, Y.H.; Kim, J.M.; Lee, H.; Park, W.K.; Lim, M.B.; Chu, Y.K.; Lo, E.H.; Lee, S.R. Green tea polyphenol (-)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J. Neurosci. Res. 2009, 87, 567–575. [Google Scholar] [CrossRef]
- Zhang, F.; Li, N.; Jiang, L.; Chen, L.; Huang, M. Neuroprotective effects of (-)-epigallocatechin-3-gallate against focal cerebral ischemia/reperfusion injury in rats through attenuation of inflammation. Neurochem. Res. 2015, 40, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Parrella, E.; Porrini, V.; Iorio, R.; Benarese, M.; Lanzillotta, A.; Mota, M.; Fusco, M.; Tonin, P.; Spano, P.; Pizzi, M. PEA and luteolin synergistically reduce mast cell-mediated toxicity and elicit neuroprotection in cell-based models of brain ischemia. Brain Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Porrini, V.; Sarnico, I.; Benarese, M.; Branca, C.; Mota, M.; Lanzillotta, A.; Bellucci, A.; Parrella, E.; Faggi, L.; Spano, P.; et al. Neuroprotective and anti-apoptotic effects of csp-1103 in primary cortical neurons exposed to oxygen and glucose deprivation. Int. J. Mol. Sci. 2017, 18, 184. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; Dossena, M.; Bertolotti, P.; Boroni, F.; Sarnico, I.; Faraco, G.; Chiarugi, A.; Frontini, A.; Giordano, A.; Liou, H.C.; et al. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke 2009, 40, 610–617. [Google Scholar] [CrossRef]
- Yildirim, F.; Ji, S.; Kronenberg, G.; Barco, A.; Olivares, R.; Benito, E.; Dirnagl, U.; Gertz, K.; Endres, M.; Harms, C.; et al. Histone acetylation and CREB binding protein are required for neuronal resistance against ischemic injury. PLoS ONE 2014, 9, e95465. [Google Scholar] [CrossRef] [PubMed]
- Faggi, L.; Pignataro, G.; Parrella, E.; Porrini, V.; Vinciguerra, A.; Cepparulo, P.; Cuomo, O.; Lanzillotta, A.; Mota, M.; Benarese, M.; et al. Synergistic association of valproate and resveratrol reduces brain injury in ischemic stroke. Int. J. Mol. Sci. 2018, 19, 172. [Google Scholar] [CrossRef]
- Faraco, G.; Pancani, T.; Formentini, L.; Mascagni, P.; Fossati, G.; Leoni, F.; Moroni, F.; Chiarugi, A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol. Pharmacol. 2006, 70, 1876–1884. [Google Scholar] [CrossRef]
- Choi, K.C.; Jung, M.G.; Lee, Y.H.; Yoon, J.C.; Kwon, S.H.; Kang, H.B.; Kim, M.J.; Cha, J.H.; Kim, Y.J.; Jun, W.J.; et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef]
- Chang, X.; Rong, C.; Chen, Y.; Yang, C.; Hu, Q.; Mo, Y.; Zhang, L.; He, W.; Cheng, S.; Hou, X.; et al. (-)-Epigallocatechin-3-gallate attenuates cognitive deterioration in Alzheimer’s disease model mice by upregulating neprilysin expression. Exp. Cell Res. 2015, 334, 136–145. [Google Scholar] [CrossRef]
- Rajendran, P.; Ho, E.; Williams, D.E.; Dashwood, R.H. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin. Epigenetics 2011, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Park, E.Y.; Kwak, S.; Heo, S.H.; Ryu, J.W.; Park, J.H.; Choi, K.C.; Lee, S.W. Protective effect of α-lipoic acid against radiation-induced fibrosis in mice. Oncotarget 2016, 7, 15554. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.J.; Kwon, Y.S.; Park, S.M.; Shin, T.; Park, J.H.; Kim, H.C.; Kwon, M.S.; Wie, M.B. Quercetin attenuates oxygen-glucose deprivation- and excitotoxin-induced neurotoxicity in primary cortical cell cultures. Biol. Pharm. Bull. 2003, 26, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Shah, F.A.; Koh, P.O. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Yang, M.; Qi, X.; Shen, X.; Chen, X.; Zhang, F. Quercetin ameliorates ischemia/reperfusion-induced cognitive deficits by inhibiting ASK1/JNK3/caspase-3 by enhancing the Akt signaling pathway. Biochem. Biophys. Res. Commun. 2016, 478, 199–205. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C. Protective effects of quercetin against brain injury in a rat model of lipopolysaccharide-induced fetal brain injury. Int. J. Dev. Neurosci. 2018, 71, 175–180. [Google Scholar] [CrossRef]
- Gao, X.; Chen, W.; Li, J.; Shen, C.; Zhou, P.; Che, X.; Li, X.; Xie, R. The protective effect of alpha-lipoic acid against brain ischemia and reperfusion injury via mTOR signaling pathway in rats. Neurosci. Lett. 2018, 671, 108–113. [Google Scholar] [CrossRef]
- Lv, C.; Maharjan, S.; Wang, Q.; Sun, Y.; Han, X.; Wang, S.; Mao, Z.; Xin, Y.; Zhang, B. α-Lipoic acid promotes neurological recovery after ischemic stroke by activating the nrf2/ho-1 pathway to attenuate oxidative damage. Cell Physiol. Biochem. 2017, 43, 1273–1287. [Google Scholar] [CrossRef]
- Xie, R.; Li, X.; Ling, Y.; Shen, C.; Wu, X.; Xu, W.; Gao, X. Alpha-lipoic acid pre- and post-treatments provide protection against in vitro ischemia-reperfusion injury in cerebral endothelial cells via Akt/mTOR signaling. Brain Res. 2012, 1482, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, L.; Bonafede, R.; Scambi, I.; Parrella, E.; Pizzi, M.; Mariotti, R. Acetylation state of RelA modulated by epigenetic drugs prolongs survival and induces a neuroprotective effect on ALS murine model. Sci. Rep. 2018, 8, 12875. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Oliveira, J.L.; Silva, FP.; Carnier, J.; Mennitti, L.V.; Santana, A.A.; de Souza, G.H.; Ribeiro, E.B.; Oller do Nascimento, C.M.; Lira, F.S.; et al. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by ampk activation via lkb1 in mice fed a high-fat diet. PLoS ONE 2015, 10, e0141227. [Google Scholar] [CrossRef] [PubMed]
- Bae, U.J.; Park, J.; Park, I.W.; Chae, B.M.; Oh, M.R.; Jung, S.J.; Ryu, G.S.; Chae, S.W.; Park, B.H. Epigallocatechin-3-gallate-rich green tea extract ameliorates fatty liver and weight gain in mice fed a high fat diet by activating the sirtuin 1 and AMP Activating Protein Kinase Pathway. Am. J. Chin. Med. 2018, 46, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.R.; Choi, H.C. Quercetin-induced amp-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. J. Med. Food. 2018, 2, 146–153. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, Y.; Sun, Y.; Li, Y.; Yao, Q.; Li, J.; Zhang, Q.; Gao, Y.; Gao, L.; et al. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J. Nutr. Biochem. 2014, 25, 1207–1217. [Google Scholar] [CrossRef]
- Valdecantos, M.P.; Pérez-Matute, P.; González-Muniesa, P.; Prieto-Hontoria, P.L.; Moreno-Aliaga, M.J.; Martínez, J.A. Lipoic acid improves mitochondrial function in nonalcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesity 2012, 20, 1974–1983. [Google Scholar] [CrossRef]
- Aşcı, H.; Saygın, M.; Yeşilot, Ş.; Topsakal, Ş.; Cankara, F.N.; Özmen, Ö.; Savran, M. Protective effects of aspirin and vitamin C against corn syrup consumption-induced cardiac damage through sirtuin-1 and HIF-1α pathway. Anatol. J. Cardiol. 2016, 16, 648. [Google Scholar] [CrossRef]
- Wei, W.; Li, L.; Zhang, Y.; Geriletu; Yang, J.; Zhang, Y.; Xing, Y. Vitamin C protected human retinal pigmented epithelium from oxidant injury depending on regulating SIRT1. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil. Neural. Repair. 2010, 24, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur. J. Neurosci. 2006, 23, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Simon, S.; Rubio, G.A.; Xia, X.; Cai, W.; Choi, R.; Striker, G.E.; Elliot, S.J. Inhibition of advanced glycation end products (AGEs) accumulation by pyridoxamine modulates glomerular and mesangial cell estrogen receptor α expression in aged female mice. PLoS ONE 2016, 11, e0159666. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Duan, Z.; Liu, X.; Yuan, Y. N-acetyl-l-cysteine ameliorates the PM2.5-induced oxidative stress by regulating SIRT-1 in rats. Environ. Toxicol. Pharmacol. 2018, 57, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Soane, L.; Li Dai, W.; Fiskum, G.; Bambrick, L.L. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J. Neurosci. Res. 2010, 88, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Choi, B.R.; Yang, H.; Hwang, Y.; Park, J.H.; LaFerla, F.M.; Han, J.S.; Lee, K.W. Sulforaphane epigenetically enhances neuronal BDNF expression and TrkB signaling pathways. Mol. Nutr. Food Res. 2017, 61, 1600194. [Google Scholar] [CrossRef]
- Agrawal, M.; Kumar, V.; Kashyap, M.P.; Khanna, V.K.; Randhawa, G.S.; Pant, A.B. Ischemic insult induced apoptotic changes in PC12 cells: Protection by trans resveratrol. Eur. J. Pharmacol. 2011, 666, 5–11. [Google Scholar] [CrossRef]
- Ziemińska, E.; Stafiej, A.; Toczyłowska, B.; Lazarewicz, J.W. Synergistic neurotoxicity of oxygen-glucose deprivation and tetrabromobisphenol a in vitro: Role of oxidative stress. Pharmacol. Rep. 2012, 64, 1166–1178. [Google Scholar] [CrossRef]
- Pang, X.; Hou, X. Synergistic protective effect of FTY720 and vitamin E against simulated cerebral ischemia in vitro. Mol. Med. Rep. 2017. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, X.; Zhao, S.; Wei, C.; Yin, Y.; Liu, T.; Jiang, S.; Xie, J.; Wan, X.; Mao, M.; et al. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 2013, 63, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Pawlas, N.; Małecki, A. Neuroprotective effect of N-acetylcysteine in neurons exposed to arachidonic acid during simulated ischemia in vitro. Pharmacol. Rep. 2009, 61, 743–750. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1025–1032. [Google Scholar] [PubMed]
- Nakagawa, K.; Miyazawa, T. Absorption and distribution of tea catechin, (-)-epigallocatechin-3-gallate, in the rat. J. Nutr. Sci. Vitaminol. 1997, 43, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- De Boer, V.C.; Dihal, A.A.; van der Woude, H.; Arts, I.C.; Wolffram, S.; Alink, G.M.; Rietjens, I.M.; Keijer, J.; Hollman, P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718–1725. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Baño, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Ikuta, N.; Okamoto, H.; Furune, T.; Uekaji, Y.; Terao, K.; Uchida, R.; Iwamoto, K.; Miyajima, A.; Hirota, T.; Sakamoto, N. Bioavailability of an r-α-lipoic acid/γ-cyclodextrin complex in healthy volunteers. Int. J. Mol. Sci. 2016, 17, 949. [Google Scholar] [CrossRef]
- Panigrahi, M.; Sadguna, Y.; Shivakumar, B.R.; Kolluri, S.V.R.; Roy, S.; Packer, L.; Ravindranath, V. α-lipoic acid protects against reperfusion injury following cerebral ischemia in rats. Brain Res. 1996, 717, 184–188. [Google Scholar] [CrossRef]
- Katz, M.; Won, S.J.; Park, Y.; Orr, A.; Jones, D.P.; Swanson, R.A.; Glass, G.A. Cerebrospinal fluid concentrations of N-acetylcysteine after oral administration in Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 500–503. [Google Scholar] [CrossRef] [PubMed]
| PMM Components | Amount per Daily Dose (mg) | Content per 100 mg (%) |
|---|---|---|
| Polyphenols and α-lipoic acid | 850.00 | 21.57 |
| green tea extract (40% EGCG) | 500.00 (200.00) | 12.69 (5.08) |
| quercetin | 150.00 | 3.81 |
| α-lipoic acid | 150.00 | 3.81 |
| resveratrol | 50.00 | 1.27 |
| Vitamins | 1272.66 | 32.30 |
| vitamin C (*) | 890.00 | 22.59 |
| vitamin E (**) | 120.00 | 3.05 |
| vitamin B3 (***) | 70.00 | 1.78 |
| vitamin B1 | 50.00 | 1.27 |
| vitamin B6 (****) | 18.00 | 0.46 |
| β-carotene | 14.00 | 0.36 |
| others | 110.66 | 2.81 |
| Amino acids | 1222.50 | 31.03 |
| L-lysine | 525.00 | 13.33 |
| L-glutamine | 150.00 | 3.81 |
| L-proline | 120.00 | 3.05 |
| N-acetyl L-cysteine | 110.00 | 2.79 |
| L-arginine | 105.00 | 2.67 |
| L-methionine | 105.00 | 2.67 |
| glycine | 60.00 | 1.52 |
| others | 47.50 | 1.21 |
| Fruits and Plants extract | 454.00 | 11.52 |
| broccoli extract | 150.00 | 3.81 |
| others | 304.00 | 7.72 |
| Mineral salts | 140.63 | 3.57 |
| calcium (*****) | 50.00 | 1.27 |
| potassium (citrate) | 30.00 | 0.76 |
| zinc (amino acid chelated) | 12.50 | 0.32 |
| magnesium (citrate) | 10.00 | 0.25 |
| manganese (gluconate) | 8.00 | 0.20 |
| phosphorus (******) | 8.00 | 0.20 |
| others | 22.12 | 0.56 |
| Total | 3939.79 | 100.00 |
| Nutrients | Concentrations | |||||
|---|---|---|---|---|---|---|
| PMM (pg/mL) | 0.00884 | 0.0265 | 0.265 | 0.884 | 2.65 | 26.5 |
| EGCG (nM) | 0.0010 | 0.0030 | 0.030 | 0.10 | 0.30 | 3.0 |
| quercetin (nM) | 0.0011 | 0.0033 | 0.033 | 0.11 | 0.33 | 3.3 |
| α-lipoic acid (nM) | 0.0016 | 0.0049 | 0.049 | 0.16 | 0.49 | 4.9 |
| resveratrol (nM) | 0.0005 | 0.0015 | 0.015 | 0.05 | 0.15 | 1.5 |
| vitamin C (nM) | 0.0089 | 0.0268 | 0.268 | 0.89 | 2.68 | 26.8 |
| vitamin E (nM) | 0.0006 | 0.0019 | 0.019 | 0.06 | 0.19 | 1.9 |
| vitamin B6 (nM) | 0.0002 | 0.0005 | 0.005 | 0.02 | 0.05 | 0.5 |
| N-acetyl L-cysteine (nM) | 0.0015 | 0.0045 | 0.045 | 0.15 | 0.45 | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faggi, L.; Porrini, V.; Lanzillotta, A.; Benarese, M.; Mota, M.; Tsoukalas, D.; Parrella, E.; Pizzi, M. A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia. Nutrients 2019, 11, 345. https://doi.org/10.3390/nu11020345
Faggi L, Porrini V, Lanzillotta A, Benarese M, Mota M, Tsoukalas D, Parrella E, Pizzi M. A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia. Nutrients. 2019; 11(2):345. https://doi.org/10.3390/nu11020345
Chicago/Turabian StyleFaggi, Lara, Vanessa Porrini, Annamaria Lanzillotta, Marina Benarese, Mariana Mota, Dimitris Tsoukalas, Edoardo Parrella, and Marina Pizzi. 2019. "A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia" Nutrients 11, no. 2: 345. https://doi.org/10.3390/nu11020345
APA StyleFaggi, L., Porrini, V., Lanzillotta, A., Benarese, M., Mota, M., Tsoukalas, D., Parrella, E., & Pizzi, M. (2019). A Polyphenol-Enriched Supplement Exerts Potent Epigenetic-Protective Activity in a Cell-Based Model of Brain Ischemia. Nutrients, 11(2), 345. https://doi.org/10.3390/nu11020345





