Associations between the Level of Trace Elements and Minerals and Folate in Maternal Serum and Amniotic Fluid and Congenital Abnormalities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Biospecimen Collection
2.3. Folate and Element Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Latos-Bielenska, A.; Materna-Kiryluk, A. Polish Registry of Congenital Malformations—Aims and organization of the registry monitoring 300,000 births a year. J. Appl. Genet. 2005, 46, 341–348. [Google Scholar] [PubMed]
- Feldkamp, M.L.; Carey, J.C.; Byrne, J.L.; Krikov, S.; Botto, L.D. Etiology and clinical presentation of birth defects: Population based study. BMJ 2017, 357, j2249. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Biesalski, H.K.; Gärtner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in pregnancy: Current knowledge and unresolved questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, L.; García-Vicent, C.; López, J.; Torró, M.I.; Lurbe, E. Assessment of ten trace elements in umbilical cord blood and maternal blood: Association with birth weight. J. Transl. Med. 2015, 13, 291. [Google Scholar] [CrossRef]
- Suliburska, J.; Kocyłowski, R.; Komorowicz, I.; Grzesiak, M.; Bogdański, P.; Barałkiewicz, D. Concentrations of mineral in amniotic fluid and their relations to selected maternal and fetal parameters. Biol. Trace Elem. Res. 2016, 172, 37–45. [Google Scholar] [CrossRef]
- Čabarkapa, V.; Bogavac, M.; Jakovljević, A.; Pezo, L.; Nikolić, A.; Belopavlović, Z.; Mirjana, D. Serum magnesium level in the first trimester of pregnancy as a predictor of pre-eclampsia—A pilot study. Hypertens. Pregnancy 2018, 37, 144–153. [Google Scholar] [CrossRef]
- Schlegel, R.N.; Cuffe, J.S.; Moritz, K.M.; Paravicini, T.M. Maternal hypomagnesemia causes placental abnormalities and fetal and postnatal mortality. Placenta 2015, 36, 750–758. [Google Scholar] [CrossRef]
- Yokoyama, K.; Takahashi, N.; Yada, Y.; Koike, Y.; Kawamata, R.; Uehara, R.; Kono, Y.; Honma, Y.; Momoi, M.Y. Prolonged maternal magnesium administration and bone metabolism in neonates. Early Hum. Dev. 2010, 86, 187–191. [Google Scholar] [CrossRef]
- Li, Z.; Liang, C.; Huang, K.; Yan, S.; Tao, R.; Sheng, J.; Pan, W.; Xia, X.; Tao, Y.; Xiang, H.; et al. Umbilical Serum Copper Status and Neonatal Birth Outcomes: A Prospective Cohort Study. Biol. Trace Elem. Res. 2018, 183, 200–208. [Google Scholar] [CrossRef]
- Ou, Y.; Bloom, M.S.; Nie, Z.; Han, F.; Mai, J.; Chen, J.; Lin, S.; Liu, X.; Zhuang, J. Associations between toxic and essential trace elements in maternal blood and fetal congenital heart defects. Environ. Int. 2017, 106, 127–134. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Y.; Zhang, B.; Zhou, A.; Zheng, T.; Qian, Z.; Du, X.; Zhou, Y.; Pan, X.; Hu, J.; et al. A nested case-control study of prenatal vanadium exposure and low birthweight. Hum. Reprod. 2016, 31, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Ettinger, A.S.; Shapiro, G.D.; Fisher, M.; Monnier, P.; Morisset, A.S.; Fraser, W.D.; Bouchard, M.F. Maternal and cord blood manganese (Mn) levels and birth weight: The MIREC birth cohort study. Int. J. Hyg. Environ. Health 2018, 221, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, F.; Wang, L.; Ye, W.; Zhang, Q.; Xie, S. Maternal Cadmium Levels during Pregnancy and the Relationship with Preeclampsia and Fetal Biometric Parameters. Biol. Trace Elem. Res. 2018, 186, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, H.; Tian, Y.; Wu, Y.; Luo, L. Genetic variation in folate metabolism is associated with the risk of conotruncal heart defects in a Chinese population. BMC Pediatr. 2018, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Qiu, J.; Zhao, N.; Shao, Y.; Dai, W.; He, X.; Cui, H.; Lin, X.; Lv, L.; Tang, Z.; et al. Maternal folic acid supplementation and dietary folate intake and congenital heart defects. PLoS ONE 2017, 12, e0187996. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.; Xiao, H.Y.; Tay, V.; Karakochuk, C.D.; Liu, Y.A.; Harvey, S.; Lamers, Y.; Houghton, L.A.; Kitts, D.D.; Green, T.J. Folic acid fortified milk increases blood folate to concentrations associated with a very low risk of neural tube defects in Singaporean women of childbearing age. Asia Pac. J. Clin. Nutr. 2016, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.Y.; Malinger, G.; Munoz, H.; et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2011, 37, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.neonatologia.edu.pl/index.php?option=com_content&view=article&id=38&Itemid=248 (accessed on 20 November 2018).
- Suliburska, J.; Kocyłowski, R.; Grzesiak, M.; Gaj, Z.; Chan, B.; von Kaisenberg, C.; Lamers, Y. Evaluation of folate concentration in amniotic fluid and maternal and umbilical cord blood during labor. Arch. Med. Sci. 2018. [Google Scholar] [CrossRef]
- Markiewicz, B.; Sajnóg, A.; Lorenc, W.; Hanć, A.; Komorowicz, I.; Suliburska, J.; Kocyłowski, R.; Barałkiewicz, D. Multielemental analysis of 18 essential and toxic elements in amniotic fluid samples by ICP-MS: Full procedure validation and estimation of measurement uncertainty. Talanta 2017, 174, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kocyłowski, R.; Grzesiak, M.; Gaj, Z.; Lorenc, W.; Bakinowska, E.; Barałkiewicz, D.; von Kaisenberg, C.S.; Suliburska, J. Evaluation of Essential and Toxic Elements in Amniotic Fluid and Maternal Serum at Birth. Biol. Trace Elem. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nowiński, W.; Bakinowska, E. A logistic model study of endogenous and exogenous factors affecting polish SMES’ internationalization speed. Argum. Oecon. 2012, 28, 155–179. [Google Scholar]
- Urso, C.; Brucculeri, S.; Caimi, G. Acid-base and electrolyte abnormalities in heart failure: Pathophysiology and implications. Heart Fail. Rev. 2015, 20, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Su, L.T.; Chen, H.C.; Habas, R.; Runnels, L.W. Magnesium and embryonic development. Magnes. Res. 2014, 27, 1–8. [Google Scholar] [PubMed]
- Watanabe, M.; Shinohara, A.; Matsukawa, T.; Chiba, M.; Wu, J.; Iesaki, T.; Okada, T. Chronic magnesium deficiency decreases tolerance to hypoxia/reoxygenation injury in mouse heart. Life Sci. 2011, 88, 658–663. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, Y.; Tian, X.; Li, J.; Chen, X.; Yang, J.; Li, X.; Deng, Y.; Li, N.; Liang, J.; et al. Association between maternal aluminum exposure and the risk of congenital heart defects in offspring. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 95–103. [Google Scholar] [CrossRef]
- Novaes, R.D.; Mouro, V.G.S.; Gonçalves, R.V.; Mendonça, A.A.S.; Santos, E.C.; Fialho, M.C.Q.; Machado-Neves, M. Aluminum: A potentially toxic metal with dose-dependent effects on cardiac bioaccumulation; mineral distribution; DNA oxidation and microstructural remodeling. Environ. Pollut. 2018, 242 Pt A, 814–826. [Google Scholar] [CrossRef]
- Liu, Z.; He, C.; Chen, M.; Yang, S.; Li, J.; Lin, Y.; Deng, Y.; Li, N.; Guo, Y.; Yu, P.; et al. The effects of lead and aluminum exposure on congenital heart disease and the mechanism of oxidative stress. Reprod Toxicol. 2018, 81, 93–98. [Google Scholar] [CrossRef]
- Sanders, A.P.; Desrosiers, T.A.; Warren, J.L.; Herring, A.H.; Enright, D.; Olshan, A.F.; Meyer, R.E.; Fry, R.C. Association between arsenic; cadmium; manganese; and lead levels in private wells and birth defects prevalence in North Carolina: A semi-ecologic study. BMC Public Health 2014, 14, 955. [Google Scholar] [CrossRef]
- Wier, P.J.; Miller, R.K.; Maulik, D.; Di Sant’Agnese, P.A. Toxicity of cadmium in the perfused human placenta. Toxicol. Appl. Pharmacol. 1990, 105, 156–171. [Google Scholar] [CrossRef]
- Zhang, G.B.; Wang, H.; Hu, J.; Guo, M.Y.; Wang, Y.; Zhou, Y.; Yu, Z.; Fu, L.; Chen, Y.H.; Xu, D.X. Cadmium-induced neural tube defects and fetal growth restriction: Association with disturbance of placental folate transport. Toxicol. Appl. Pharmacol. 2016, 306, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Bo, Q.L.; Ji, Y.L.; Liu, L.; Hu, Y.F.; Chen, Y.H.; Zhang, J.; Zhao, L.L.; Xu, D.X. Maternal cadmium exposure reduces placental zinc transport and induces fetal growth restriction in mice. Reprod. Toxicol. 2016, 63, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public Health 2017, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Ben, M.S.; Boughammoura, S.; Chemek, M.; Haouas, Z.; Banni, M.; Messaoudi, I. Disruption of the zinc metabolism in rat fœtal brain after prenatal exposure to cadmium. Chem. Biol. Interact. 2018, 286, 88–95. [Google Scholar] [CrossRef]
- Hu, J.; Peng, Y.; Zheng, T.; Zhang, B.; Liu, W.; Wu, C.; Jiang, M.; Braun, J.M.; Liu, S.; Buka, S.L.; et al. Effects of trimester-specific exposure to vanadium on ultrasound measures of fetal growth and birth size: A longitudinal prospective prenatal cohort study. Lancet Planet. Health 2018, 2, e427–e437. [Google Scholar] [CrossRef]
- Hu, J.; Xia, W.; Pan, X.; Zheng, T.; Zhang, B.; Zhou, A.; Buka, S.L.; Bassig, B.A.; Liu, W.; Wu, C.; et al. Association of adverse birth outcomes with prenatal exposure to vanadium: A population-based cohort study. Lancet Planet. Health 2017, 1, e230–e241. [Google Scholar] [CrossRef]
- Pedersen, M.L.; Lind, O.; Abelsen, T.; Olesen, J.; Jørgensen, M.E. Gestational diabetes and macrosomia among Greenlanders. Time to change diagnostic strategy? Int. J. Circumpolar Health 2018, 77, 1528126. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V.; Grabeklis, A.R.; Serebryansky, E.P.; Demidov, V.A.; Tinkov, A.A. Hair Trace Elements in Overweight and Obese Adults in Association with Metabolic Parameters. Biol. Trace Elem. Res. 2018, 186, 12–20. [Google Scholar] [CrossRef]
- Tascilar, M.E.; Ozgen, I.T.; Abaci, A.; Serdar, M.; Aykut, O. Trace elements in obese Turkish children. Biol. Trace Elem. Res. 2011, 143, 188–195. [Google Scholar] [CrossRef]
- Mikelson, C.K.; Troisi, J.; LaLonde, A.; Symes, S.J.K.; Thurston, S.W.; DiRe, L.M.; David, A.C.; Miller, R.K.; Richards, S.M. Placental concentrations of essential; toxic; and understudied metals and relationships with birth outcomes in Chattanooga; TN. Environ. Res. 2018, 168, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Reza-López, S.A.; Aguirre-Chacón, E.O.; Sánchez-Ramírez, B.; Guerrero-Salgado, F.; Chávez-Corral, D.V.; Levario-Carrillo, M. Folate transporter expression in placenta from pregnancies complicated with birth defects. Birth Defects Res. 2018, 110, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Schön, C.; Wilhelm, M.; Pietrzik, K.; Pilz, S. Dietary and lifestyle predictors of folate insufficiency in non-supplemented German women. Int. J. Food Sci. Nutr. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Surmiak, P.; Baumert, M.; Paprotny, M. Abnormal Biomarkers of Homocysteine Metabolism in Neonates with Conotruncal Heart Defects. BioMed Res. Int. 2017, 2017, 7404397. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hanaoka, T.; Tamura, N.; Sasaki, S.; Miyashita, C.; Araki, A.; Ito, S.; Minakami, H.; Cho, K.; Endo, T.; et al. Association Between Maternal Serum Folate Concentrations in the First Trimester and the Risk of Birth Defects: The Hokkaido Study of Environment and Children’s Health. J. Epidemiol. 2018. [Google Scholar] [CrossRef] [PubMed]
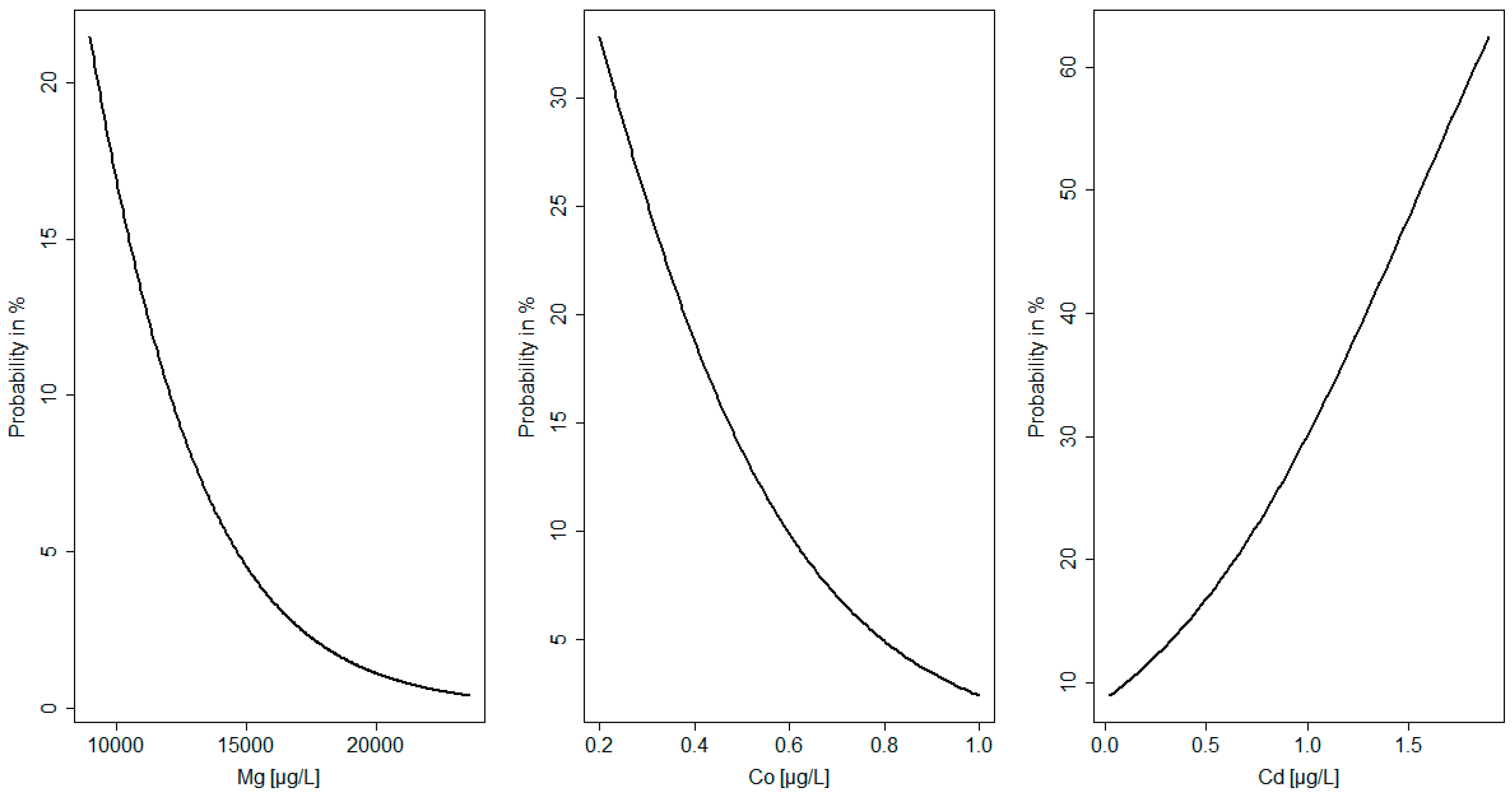
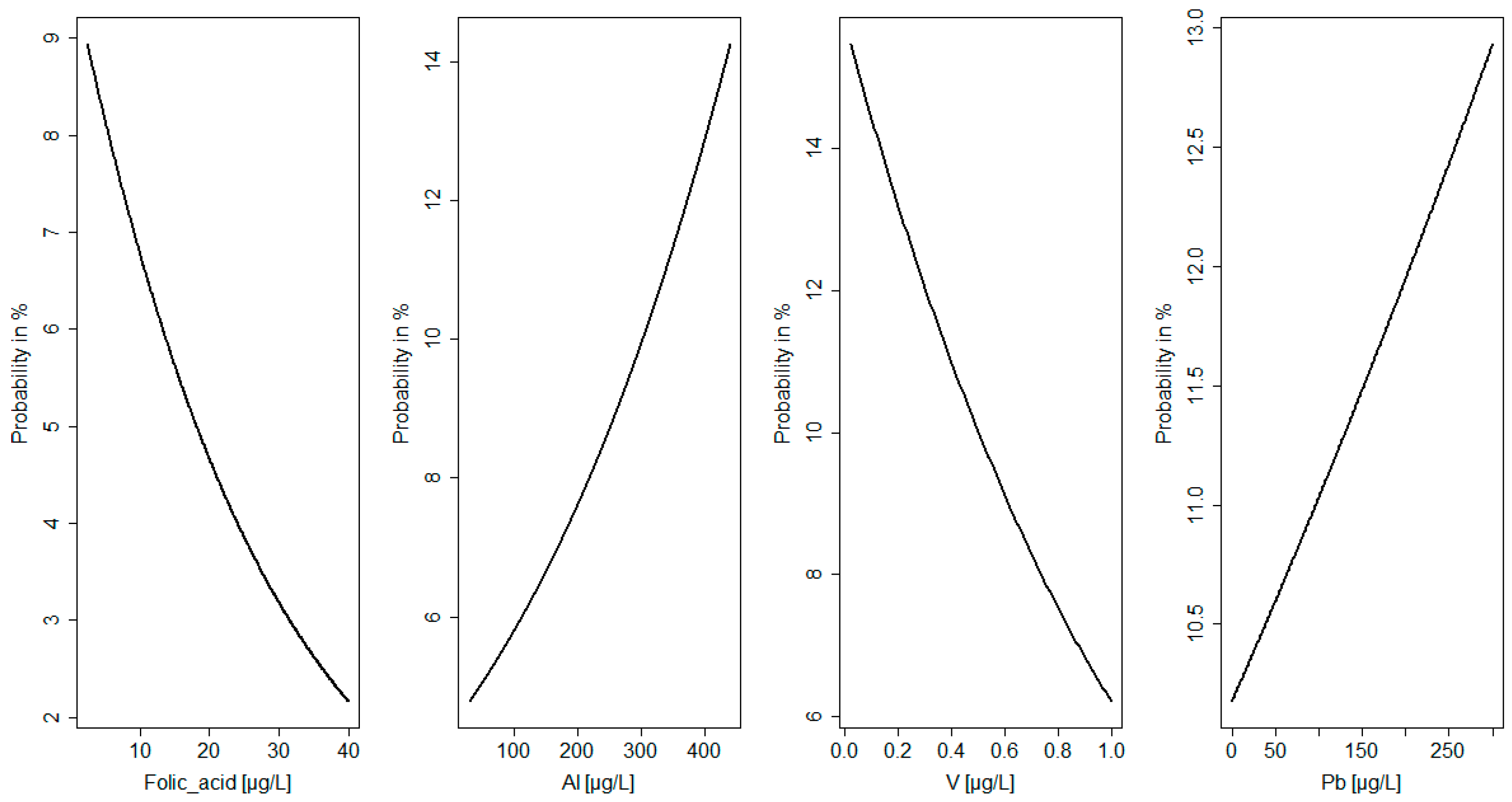
| Maternal Parameters | Newborn Parameters | ||
|---|---|---|---|
| Parameter | Value | Parameter | Value |
| Number of women | 258 | Gender | |
| F/M | 144/114 | ||
| Age of mother (y) | 29.4 ± 4.8 | Birth weight (g) | 3228.5 ± 595.2 |
| 29 | 3300 | ||
| 18–43 | 1200–5000 | ||
| Weight of mother in pregnancy (kg) | 77.4 ± 12.8 76 46–124 | Vaginal delivery (%) Cesarean section (%) | 72% 28% |
| BMI of mother before pregnancy (kg/m2) | 22.6 ± 3.9 | Birth defects (%) | 5.4 |
| 21.7 | |||
| 16.3–40.8 | |||
| Week of gestation | 38.3 ± 2.2 | Macrosomia (%) | 8.1 |
| 39 | |||
| 27–42 | |||
| Gravidity/Parity (%) | Hypotrophy (%) | 10.9 | |
| 0 | 51/57 | ||
| 1 | 31/35 | ||
| 2 | 12/6 | ||
| 3 | 3/1 | ||
| ≥4 | 3/1 | ||
| Smoking | Newborn disorders (%) | 8.9 | |
| Before pregnancy (%) | 18.2 | ||
| During pregnancy (%) | 5 | ||
| Disorders during pregnancy (%) | |||
| Hypertension | 10 | ||
| Diabetes | 5 | ||
| Inflammation | 4 | ||
| Education (%) | |||
| Primary school | 2 | ||
| Vocational school | 5 | ||
| High school | 25 | ||
| University | 68 | ||
| Socioeconomic status (%) | |||
| Poor | 4 | ||
| Moderate | 21 | ||
| Good | 69 | ||
| Very good | 6 | ||
| Birth Defects | Macrosomia | Hypotrophy | Newborn Disorders | |
|---|---|---|---|---|
| Mg | 13,274 ± 3482 * | 14,838 ± 2797 | 15,471 ± 2999 | 14,519 ± 2309 |
| Co | 0.55 ± 0.19 | 0.52 ± 0.13 * | 0.61 ± 0.18 | 0.59 ± 0.14 |
| Cu | 2161.0 ± 389.0 | 2158.1 ± 458.1 | 2077 ± 445.7 | 2271.7 ± 362.3 |
| Zn | 1056.9 ± 408.4 | 913.9 ± 194.2 | 953.8 ± 351.8 | 950.4 ± 359.4 |
| Sr | 53.0 ± 21.7 | 124.4 ± 117.9 | 126.1 ± 125.3 | 114.1 ± 132.4 |
| Cd | 0.18 ± 0.12 | 0.13 ± 0.08 | 0.13 ± 0.10 | 0.17 ± 0.08 |
| Ba | 17.61 ± 11.54 | 17.53 ± 11.4 | 18.73 ± 11.06 | 14.64 ± 11.03 |
| Pb | 1.56 ± 0.80 | 5.95 ± 10.98 | 6.98 ± 12.86 | 2.63 ± 6.99 |
| U | 0.32 ± 0.13 | 0.32 ± 0.07 | 0.35 ± 0.12 | 0.32 ± 0.12 |
| Ca | 96,142 ± 7491 | 97,220 ± 20,009 | 99,184 ± 21,951 | 92,597 ± 15,830 |
| Cr | 4.61 ± 4.07 | 5.49 ± 5.86 | 5.10 ± 4.39 | 3.28 ± 2.95 |
| Al. | 250.3 ± 176.2 | 283.0 ± 176.1 | 369.8 ± 216.6 | 300.6 ± 152 |
| Mn | 14.1 ± 58.12 | 15.41 ± 18.64 | 10.30 ± 4.86 | 13.05 ± 7.20 |
| V | 0.27 ± 0.16 | 0.28 ± 0.1 | 0.33 ± 0.14 | 0.26 ± 0.11 |
| Fe | 1475 ± 569.8 | 1257.7 ± 496.4 | 1218.1 ± 581.8 | 1136.2 ± 507.5 |
| Birth Defects | Macrosomia | Hypotrophy | Newborn Disorders | |
|---|---|---|---|---|
| Mg | 8363 ± 2794 | 9511 ± 2462 | 9948 ± 2959 | 10,107 ± 2521 |
| Co | 0.24 ± 0.05 | 0.26 ± 0.08 | 0.27 ± 0.11 | 0.27 ± 0.09 |
| Cu | 79.87 ± 23.66 | 75.70 ± 25.69 | 78.44 ± 27.69 | 82.31 ± 23.66 |
| Zn | 400.8 ± 218.7 | 579.8 ± 328.4 | 427.6 ± 193.6 | 454.2 ± 260.7 |
| Sr | 67.82 ± 62.34 | 75.82 ± 50.88 | 75.83 ± 65.22 | 72.87 ± 62.85 |
| Cd | 0.09 ± 0.03 | 0.13 ± 0.11 | 0.12 ± 0.09 | 0.12 ± 0.10 |
| Ba | 12.03 ± 4.65 | 12.40 ± 0.42 | 12.90 ± 10.52 | 14.08 ± 8.02 * |
| Pb | 44.20 ± 66.11 | 36.28 ± 49.34 | 49.60 ± 67.82 | 54.96 ± 101.99 |
| U | 0.02 ± 0.01 | 0.06 ± 0.12 | 0.05 ± 0.06 | 0.04 ± 0.06 |
| Ca | 61,084 ± 19,355 | 73,397 ± 23,957 | 76,052 ± 22,226 | 69,302 ± 16,239 |
| Cr | 3.18 ± 2.89 | 2.61 ± 2.38 | 3.05 ± 3.05 | 3.29 ± 3.36 |
| Al. | 144.8 ± 54.1 | 133.1 ± 65.2 | 140.8 ± 58.38 | 165.77 ± 91.17 * |
| Mn | 4.41 ± 1.28 | 6.20 ± 3.30 | 5.89 ± 2.98 | 5.66 ± 2.93 |
| V | 0.26 ± 0.13 | 0.27 ± 0.19 | 0.33 ± 0.20 | 0.31 ± 0.18 |
| Fe | 470.3 ± 298.8 | 491.1 ± 191.1 | 479.3 ± 255.0 | 502.8 ± 278.1 |
| Birth Defects | Macrosomia | Hypotrophy | Newborn Disorders | |
|---|---|---|---|---|
| AF | 10.93 ± 7.15 | 11.51 ± 7.32 | 10.91 ± 9.42 | 10.93 ± 8.12 |
| MS | 41.63 ± 23.03 | 46.33 ± 22.28 | 47.20 ± 28.52 | 40.33 ± 24.80 * |
| UCB | 83.62 ± 34.89 | 84.95 ± 35.85 | 74.99 ± 24.37 | 79.51 ± 37.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocylowski, R.; Grzesiak, M.; Gaj, Z.; Lorenc, W.; Bakinowska, E.; Barałkiewicz, D.; von Kaisenberg, C.S.; Lamers, Y.; Suliburska, J. Associations between the Level of Trace Elements and Minerals and Folate in Maternal Serum and Amniotic Fluid and Congenital Abnormalities. Nutrients 2019, 11, 328. https://doi.org/10.3390/nu11020328
Kocylowski R, Grzesiak M, Gaj Z, Lorenc W, Bakinowska E, Barałkiewicz D, von Kaisenberg CS, Lamers Y, Suliburska J. Associations between the Level of Trace Elements and Minerals and Folate in Maternal Serum and Amniotic Fluid and Congenital Abnormalities. Nutrients. 2019; 11(2):328. https://doi.org/10.3390/nu11020328
Chicago/Turabian StyleKocylowski, Rafal, Mariusz Grzesiak, Zuzanna Gaj, Wiktor Lorenc, Ewa Bakinowska, Danuta Barałkiewicz, Constantin S. von Kaisenberg, Yvonne Lamers, and Joanna Suliburska. 2019. "Associations between the Level of Trace Elements and Minerals and Folate in Maternal Serum and Amniotic Fluid and Congenital Abnormalities" Nutrients 11, no. 2: 328. https://doi.org/10.3390/nu11020328
APA StyleKocylowski, R., Grzesiak, M., Gaj, Z., Lorenc, W., Bakinowska, E., Barałkiewicz, D., von Kaisenberg, C. S., Lamers, Y., & Suliburska, J. (2019). Associations between the Level of Trace Elements and Minerals and Folate in Maternal Serum and Amniotic Fluid and Congenital Abnormalities. Nutrients, 11(2), 328. https://doi.org/10.3390/nu11020328







