A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
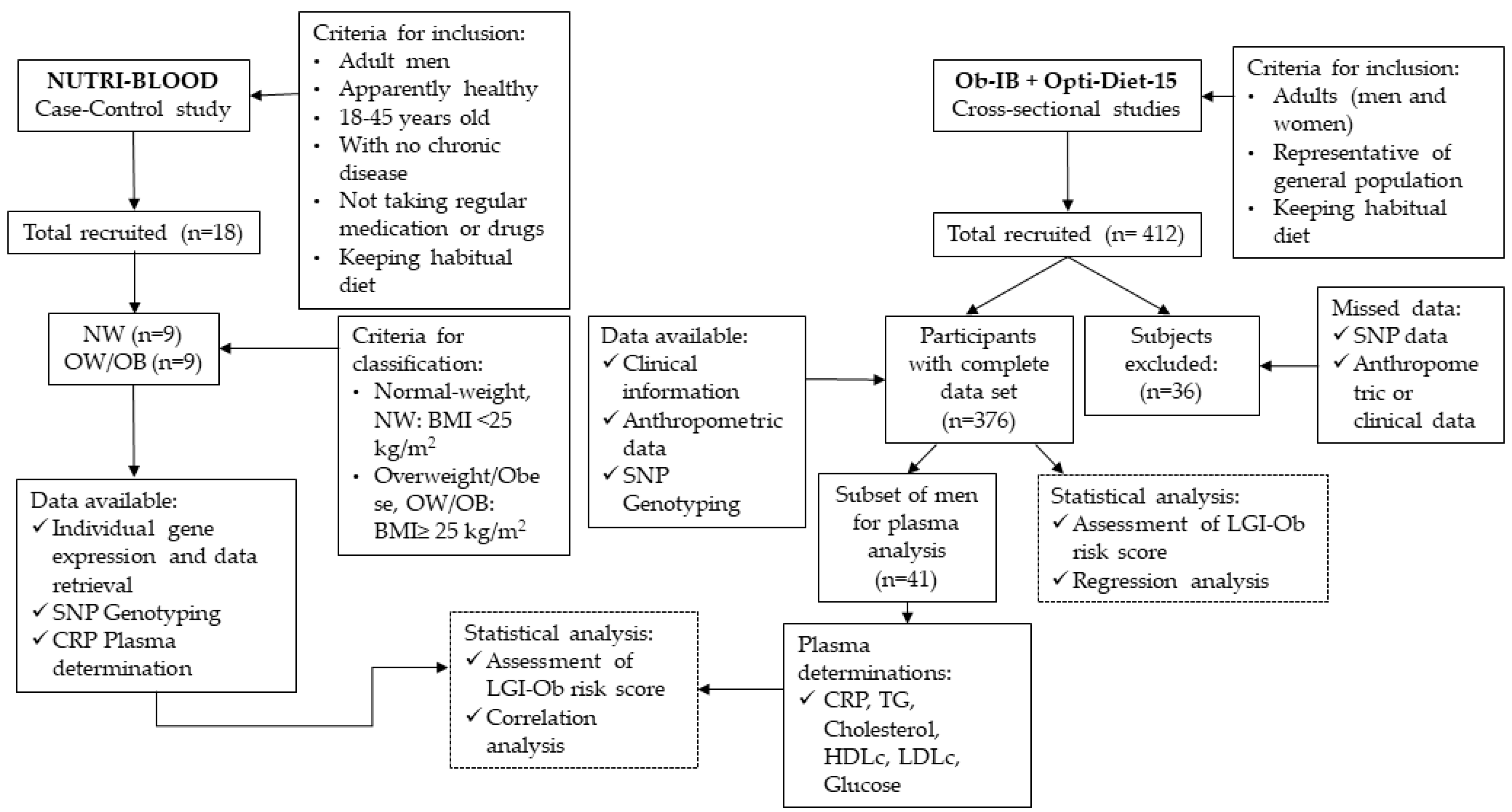
2.1. Study Participants and Design
2.2. Anthropometric Measurements
2.3. Isolation, Culture Conditions and Gene Expression Determination in PBMC
2.4. Quantification of Circulating Parameters
2.5. DNA Isolation and Genotyping
2.6. Elaboration of the Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity (LGI-Ob)
2.7. Assessment of the Metabolic Profile and Health Status
2.8. Statistical Analysis
3. Results
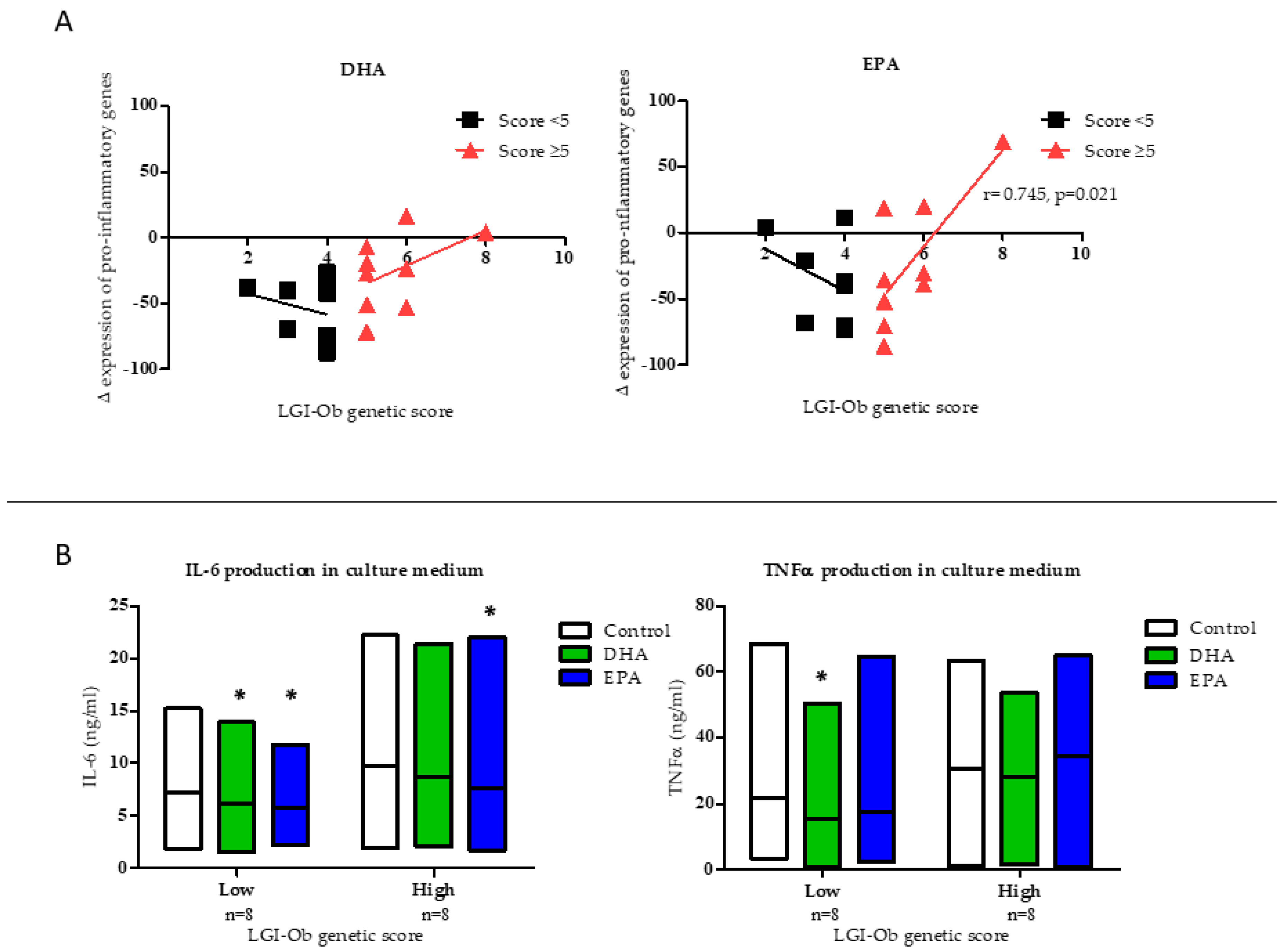
3.1. Anti-inflammatory Potential of EPA and DHA is Influenced by LGI-Ob Genetic Score
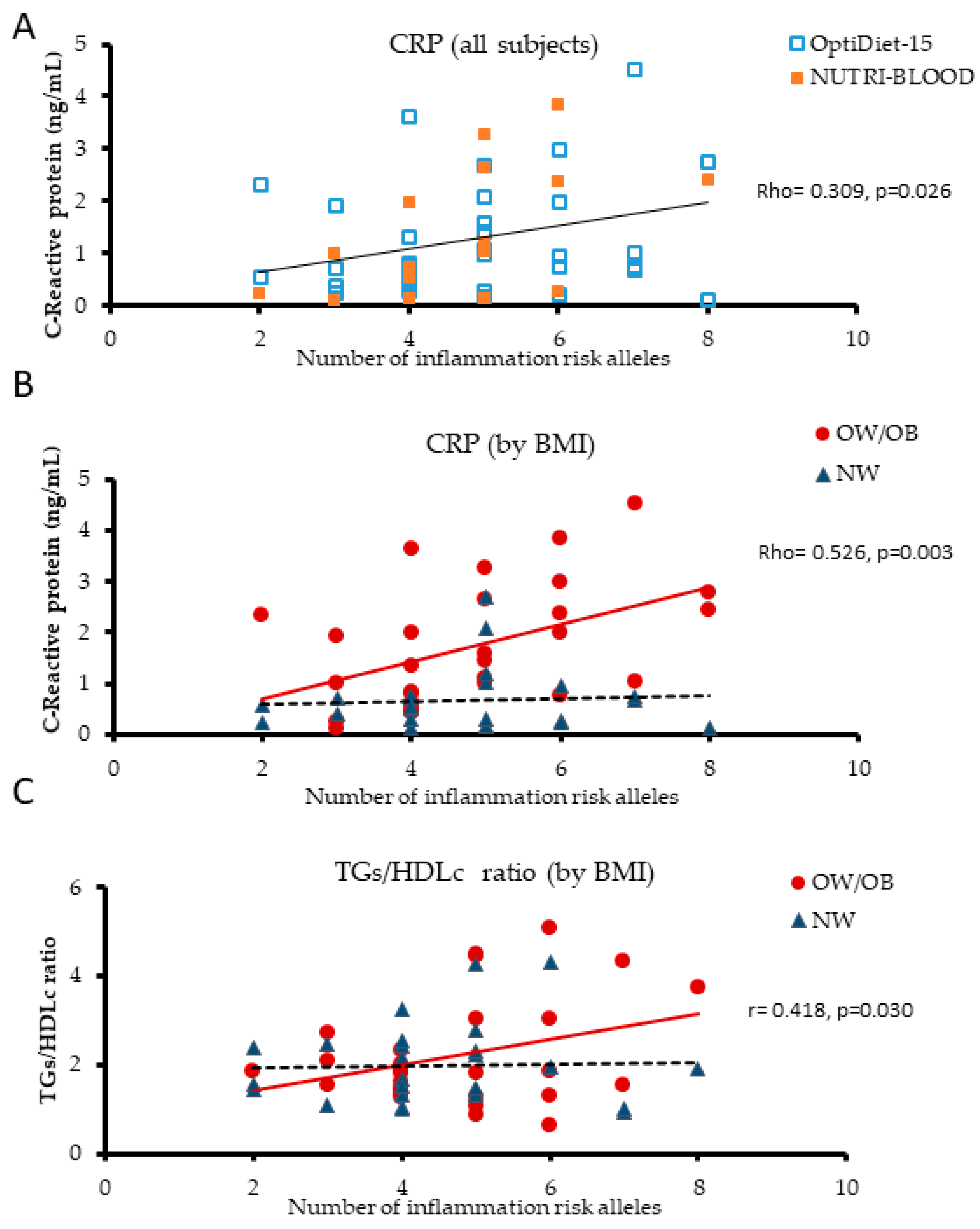
3.2. Plasma Biomarkers of Inflammation are Influenced by LGI-Ob Genetic Score
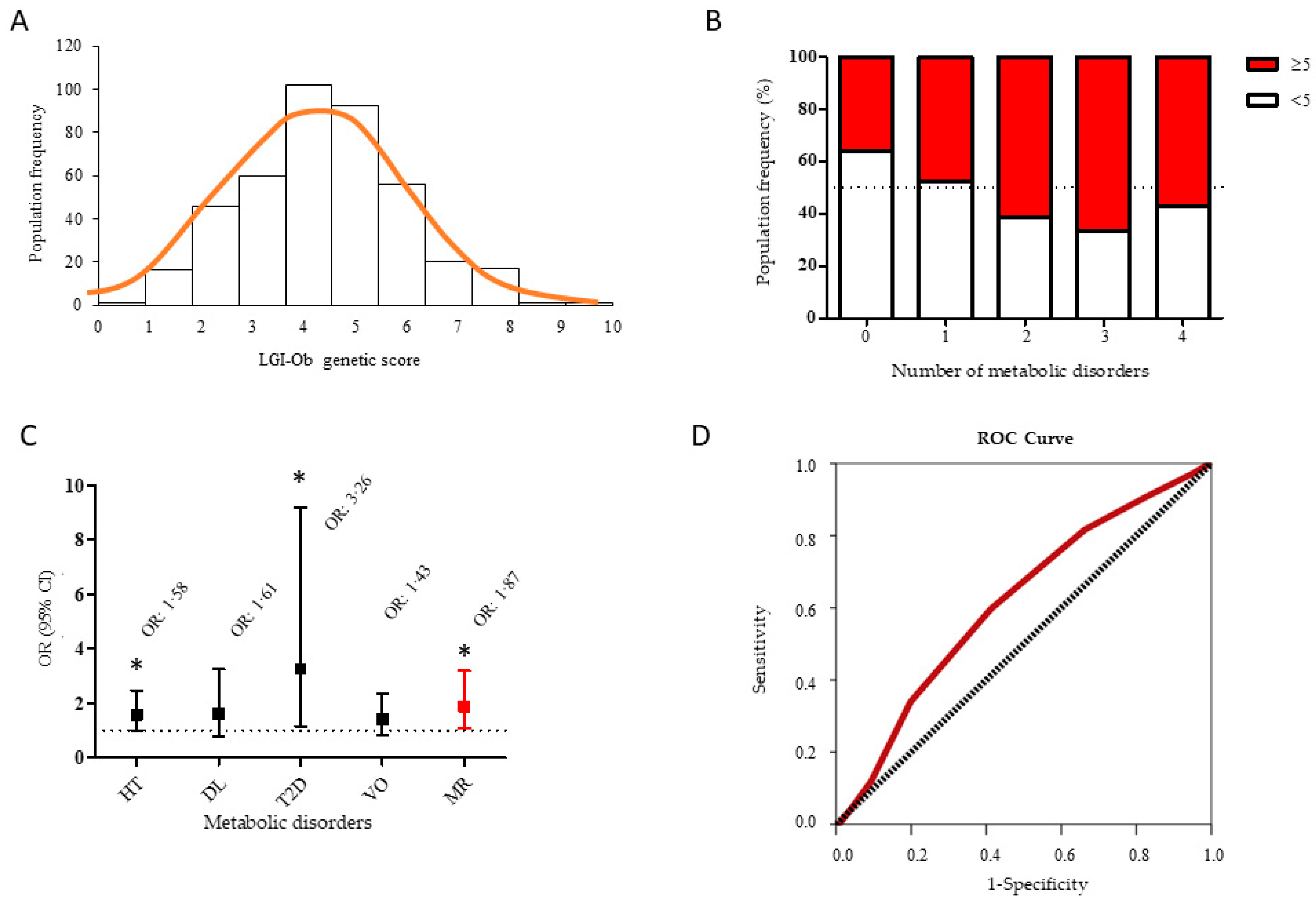
3.3. LGI-Ob Genetic Score May Contribute to Identify Subjects at Higher Risk to Develop Metabolic Syndrome
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Ohman, M.K.; Shen, Y.; Obimba, C.I.; Wright, A.P.; Warnock, M.; Lawrence, D.A.; Eitzman, D.T. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 2008, 117, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes 2009, 117, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.K.; White, C.M. The emerging role of inflammation in cardiovascular disease. Ann. Pharmacother. 2018, 52, 801–809. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Aspects Med. 2018. [Google Scholar] [CrossRef]
- OECD. Obesity Update 2017. Available online: www.oecd.org/health/obesity-update.htm (accessed on 15 May 2018).
- Schork, N.J.; Goetz, L.H. Single-subject studies in translational nutrition research. Annu. Rev. Nutr. 2017, 37, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Allayee, H.; Roth, N.; Hodis, H.N. Polyunsaturated fatty acids and cardiovascular disease: Implications for nutrigenetics. J. Nutrigenet. Nutrigenomics 2009, 2, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, A.; Zeyda, M.; Mascher, D.; Itariu, B.K.; Murano, I.; Leitner, L.; Hochbrugger, E.E.; Fraisl, P.; Cinti, S.; Serhan, C.N.; et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 2013, 62, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision nutrition and omega-3 polyunsaturated fatty acids: A case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients 2017. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; López-Vicario, C.; Rius, B.; Titos, E. Pro-resolving actions of SPM in adipose tissue biology. Mol. Aspects Med. 2017, 58, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, C.; Kloiber, S.; Nieters, A.; Seiler, H.; Himmerich, H.; Kohli, M.A.; Lucae, S.; Wolfram, G.; Gieger, C.; Wichmann, H.-E.; et al. Gene-PUFA interactions and obesity risk. Br. J. Nutr. 2011, 106, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Bodhini, D.; Gaal, S.; Shatwan, I.; Ramya, K.; Ellahi, B.; Surendran, S.; Sudha, V.; Anjana, M.R.; Mohan, V.; Lovegrove, J.A.; et al. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS ONE 2017, 12, e0188382. [Google Scholar] [CrossRef] [PubMed]
- Warodomwichit, D.; Arnett, D.K.; Kabagambe, E.K.; Tsai, M.Y.; Hixson, J.E.; Straka, R.J.; Province, M.; An, P.; Lai, C.-Q.; Borecki, I.; et al. Polyunsaturated fatty acids modulate the effect of TCF7L2 gene variants on postprandial lipemia. J. Nutr. 2009, 139, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Q.; Corella, D.; Demissie, S.; Cupples, L.A.; Adiconis, X.; Zhu, Y.; Parnell, L.D.; Tucker, K.L.; Ordovas, J.M. Dietary intake of n-6 fatty acids modulates effect of apolipoprotein a5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: The framingham heart study. Circulation 2006, 113, 2062–2070. [Google Scholar] [CrossRef]
- Noumi, Y.; Kawamura, R.; Tabara, Y.; Maruyama, K.; Takata, Y.; Nishida, W.; Okamoto, A.; Nishimiya, T.; Onuma, H.; Saito, I.; et al. An inverse association between serum resistin levels and n-3 polyunsaturated fatty acids intake was strongest in the SNP-420 G/G genotype in the Japanese cohort: The toon genome study. Clin. Endocrinol. 2018, 88, 51–57. [Google Scholar] [CrossRef]
- Martinelli, N.; Girelli, D.; Malerba, G.; Guarini, P.; Illig, T.; Trabetti, E.; Sandri, M.; Friso, S.; Pizzolo, F.; Schaeffer, L.; et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 2008, 88, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Cifre, M.; Díaz-Rua, R.; Varela Calviño, R.; Reynés, B.; Pericás-Beltrán, J.; Palou, A.; Oliver, P. Human peripheral blood mononuclear cell in vitro system to test the efficacy of food bioactive compounds: Effects of polyunsaturated fatty acids and their relation with BMI. Mol. Nutr. Food Res. 2016, 201600353, 1–12. [Google Scholar] [CrossRef]
- Reynés, B.; Díaz-Rúa, R.; Cifre, M.; Oliver, P.; Palou, A. Peripheral blood mononuclear cells as a potential source of biomarkers to test the efficacy of weight-loss strategies. Obesity 2014, 23, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Reynés, B.; Priego, T.; Cifre, M.; Oliver, P.; Palou, A. Peripheral blood cells, a transcriptomic tool in nutrigenomic and obesity studies: Current state of the art. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1006–1020. [Google Scholar] [CrossRef]
- He, C.-S.; Fraser, W.D.; Gleeson, M. Influence of vitamin d metabolites on plasma cytokine concentrations in endurance sport athletes and on multiantigen stimulated cytokine production by whole blood and peripheral blood mononuclear cell cultures. ISRN Nutr. 2014, 2014, 820524. [Google Scholar] [CrossRef]
- Stepien, M.; Nugent, A.P.; Brennan, L. Metabolic profiling of human peripheral blood mononuclear cells: Influence of vitamin d status and gender. Metabolites 2014, 4, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sun, Z.; Li, J.; Zhang, R.; Zhang, X.; Liu, S.; Li, J.; Xu, C.; Hu, D.; Sun, Y. Pulse pressure and mean arterial pressure in relation to ischemic stroke among patients with uncontrolled hypertension in rural areas of China. Stroke 2008, 39, 1932–1937. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M. Expression and sequence variants of inflammatory genes; effects on plasma inflammation biomarkers following a 6-week supplementation with fish oil. Int. J. Mol. Sci. 2016, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Alcala-Diaz, J.F.; Garcia-Rios, A.; Delgado-Lista, J.; Ortiz-Morales, A.; Rangel-Zuñiga, O.; Tinahones, F.J.; Gonzalez-Guardia, L.; Malagon, M.M.; Bellido-Muñoz, E.; et al. Polymorphism at the TNF-alpha gene interacts with Mediterranean diet to influence triglyceride metabolism and inflammation status in metabolic syndrome patients: From the CORDIOPREV clinical trial. Mol. Nutr. Food Res. 2014, 58, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Zúñiga, O.A.; Corina, A.; Lucena-Porras, B.; Cruz-Teno, C.; Gómez-Delgado, F.; Jiménez-Lucena, R.; Alcalá-Díaz, J.F.; Haro-Mariscal, C.; Yubero-Serrano, E.M.; Delgado-Lista, J.; et al. TNFA gene variants related to the inflammatory status and its association with cellular aging: From the CORDIOPREV study. Exp. Gerontol. 2016, 83, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Keramat, L.; Sadrzadeh-Yeganeh, H.; Sotoudeh, G.; Zamani, E.; Eshraghian, M.; Mansoori, A.; Koohdani, F. Apolipoprotein A2 -265T/C polymorphism interacts with dietary fatty acids intake to modulate inflammation in type 2diabetes mellitus patients. Nutrition 2017, 37, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Koohdani, F.; Sadrzadeh-Yeganeh, H.; Djalali, M.; Eshraghian, M.; Zamani, E.; Sotoudeh, G.; Mansournia, M.-A.; Keramat, L. APO A2 − 265T/C polymorphism is associated with increased inflammatory responses in patients with type 2 diabetes mellitus. Diabetes Metab. J. 2016, 40, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Jimenez, F.; Tanaka, T.; Perez-Martinez, P.; Jimenez-Gomez, Y.; Marin, C.; Ruano, J.; Parnell, L.; Ordovas, J.M.; Lopez-Miranda, J. An apolipoprotein A-II polymorphism (-265T/C, rs5082) regulates postprandial response to a saturated fat overload in healthy men. J. Nutr. 2007, 137, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Peloso, G.; Arnett, D.K.; Cupples, L.A.; Tucker, K.; Lai, C.; Parnell, L.D.; Coltell, O.; Lee, Y.; Jose, M. APOA2, Dietary fat and body mass index: Replication of a gene-diet interacton in three independent populations. Arch. Intern. Med. 2010, 169, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Arnett, D.K.; Tsai, M.Y.; Kabagambe, E.K.; Peacock, J.M.; Hixson, J.E.; Straka, R.J.; Province, M.; Lai, C.-Q.; Parnell, L.D.; et al. The -256T>C polymorphism in the apolipoprotein a-ii gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin. Chem. 2007, 53, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Zamani, E.; Sadrzadeh-Yeganeh, H.; Sotoudeh, G.; Keramat, L.; Eshraghian, M.; Rafiee, M.; Koohdani, F. The interaction between ApoA2 −265T>C polymorphism and dietary fatty acids intake on oxidative stress in patients with type 2 diabetes mellitus. Eur. J. Nutr. 2017, 56, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Imbert, A.; Igoudjil, A.; Descatoire, V.; Cazanave, S.; Pessayre, D.; Degoul, F. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet. Genomics 2005, 15, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Cruz, I.B.M.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. The MnSOD Ala16Val SNP: Relevance to human diseases and interaction with environmental factors. Free Radic. Res. 2013, 47, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Capeleto, D.; Barbisan, F.; Azzolin, V.; Dornelles, E.B.; Rogalski, F.; Teixeira, C.F.; Machado, A.K.; Cadoná, F.C.; da Silva, T.; Duarte, T.; et al. The anti-inflammatory effects of resveratrol on human peripheral blood mononuclear cells are influenced by a superoxide dismutase 2 gene polymorphism. Biogerontology 2015, 16, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-L.; Kuo, L.-T.; Sung, F.-C.; Yeh, C.-C. Association between Polymorphisms of Antioxidant Gene (MnSOD, CAT, and GPx1) and Risk of Coronary Artery Disease. Biomed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Tao, F.; Wu, X.X.; Tan, Y.Z.; He, L.; Lu, H. Polymorphic variations in manganese superoxide dismutase (MnSOD) and endothelial nitric oxide synthase (eNOS) genes contribute to the development of type 2 diabetes mellitus in the Chinese Han population. Genet. Mol. Res. 2015, 14, 12993–13002. [Google Scholar] [CrossRef] [PubMed]
- Paludo, F.J.O.; Bristot, I.J.; Alho, C.S.; Gelain, D.P.; Moreira, J.C.F. Effects of 47C allele (rs4880) of the SOD2 gene in the production of intracellular reactive species in peripheral blood mononuclear cells with and without lipopolysaccharides induction. Free Radic. Res. 2014, 48, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Fang, S.; Du, X.; Jia, C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: A meta-analysis. Diabetologia 2011, 54, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.; da Cruz, I.B.M.; Barbisan, F.; Capelleto, D.; Moresco, R.N.; Duarte, M.M.M.F. The effects of rosuvastatin on lipid-lowering, inflammatory, antioxidant and fibrinolytics blood biomarkers are influenced by Val16Ala superoxide dismutase manganese-dependent gene polymorphism. Pharmacogenomics J. 2016, 16, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kakkoura, M.G.; Demetriou, C.A.; Loizidou, M.A.; Loucaides, G.; Neophytou, I.; Malas, S.; Kyriacou, K.; Hadjisavvas, A. MnSOD and CAT polymorphisms modulate the effect of the Mediterranean diet on breast cancer risk among Greek-Cypriot women. Eur. J. Nutr. 2016, 55, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Middelberg, R.P.S.; Ferreira, M.A.R.; Henders, A.K.; Heath, A.C.; Madden, P.A.F.; Montgomery, G.W.; Martin, N.G.; Whitfield, J.B. Genetic variants in LPL, OASL and TOMM40/APOE-C1-C2-C4 genes are associated with multiple cardiovascular-related traits. BMC Med. Genet. 2011, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.T.; Wang, J.; Lanktree, M.B.; Cao, H.; McIntyre, A.D.; Ban, M.R.; Martins, R.A.; Kennedy, B.A.; Hassell, R.G.; Visser, M.E.; et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 2010, 42, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Chasman, D.I.; Paré, G.; Mora, S.; Hopewell, J.C.; Peloso, G.; Clarke, R.; Cupples, L.A.; Hamsten, A.; Kathiresan, S.; Mälarstig, A.; et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009, 5, e1000730. [Google Scholar] [CrossRef] [PubMed]
- Surakka, I.; Horikoshi, M.; Mägi, R.; Sarin, A.-P.; Mahajan, A.; Lagou, V.; Marullo, L.; Ferreira, T.; Miraglio, B.; Timonen, S.; et al. The impact of low-frequency and rare variants on lipid levels. Nat. Genet. 2015, 47, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Pollin, T.I.; Jablonski, K.A.; McAteer, J.B.; Saxena, R.; Kathiresan, S.; Kahn, S.E.; Goldberg, R.B.; Altshuler, D.; Florez, J.C. Diabetes prevention program research group, for the D. P. P. R. triglyceride response to an intensive lifestyle intervention is enhanced in carriers of the GCKR Pro446Leu polymorphism. J. Clin. Endocrinol. Metab. 2011, 96, E1142–E1147. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, J.; Duhamel, A.; Dumont, J.; Dallongeville, J.; Molnar, D.; Widhalm, K.; Manios, Y.; Sjöström, M.; Kafatos, A.; Breidenassel, C.; et al. The n-3 long-chain PUFAs modulate the impact of the GCKR Pro446Leu polymorphism on triglycerides in adolescents. J. Lipid Res. 2015, 56, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, P.; Delgado-Lista, J.; Garcia-Rios, A.; Mc Monagle, J.; Gulseth, H.L.; Ordovas, J.M.; Shaw, D.I.; Karlström, B.; Kiec-Wilk, B.; Blaak, E.E.; et al. Glucokinase regulatory protein genetic variant interacts with omega-3 pufa to influence insulin resistance and inflammation in metabolic syndrome. PLoS ONE 2011, 6, e20555. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Skogstrand, K.; Hougaard, D.M.; Astrup, A.; Hansen, T.; Pedersen, O.; Sørensen, T.I.A.; Jess, T. Influences of the common FTO rs9939609 variant on inflammatory markers throughout a broad range of body mass index. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A Common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Schulze, M.B.; Stefan, N.; Häring, H.-U.; Döring, F.; Joost, H.-G.; Al-Hasani, H.; Boeing, H.; Pischon, T. Association of the FTO rs9939609 single nucleotide polymorphism with c-reactive protein levels. Obesity 2009, 17, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Kesse-Guyot, E.; McManus, R.; Hercberg, S.; Lairon, D.; Planells, R.; Roche, H.M. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 2012, 142, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Mehlig, K.; Leander, K.; Berg, C.; Tognon, G.; Strandhagen, E.; Björck, L.; Rosengren, A.; Lissner, L.; Nyberg, F. FTO gene variation, macronutrient intake and coronary heart disease risk: A gene-diet interaction analysis. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Arnett, D.K.; Tucker, K.L.; Kabagambe, E.K.; Tsai, M.; Parnell, L.D.; Lai, C.-Q.; Lee, Y.-C.; Warodomwichit, D.; Hopkins, P.N.; et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J. Nutr. 2011, 141, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, S.; Ahmad, M.K.; Singh, R.; Kant Kumar, S.; Pradhan, A.; Chandra, S.; Kumar, S. Promoter variants of TNF-α rs1800629 and IL-10 rs1800871 are independently associated with the susceptibility of coronary artery disease in north Indian. Cytokine 2018, 110, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ansari, W.M.; Humphries, S.E.; Naveed, A.K.; Khan, O.J.; Khan, D.A. Influence of cytokine gene polymorphisms on proinflammatory/anti-inflammatory cytokine imbalance in premature coronary artery disease. Postgrad. Med. J. 2017, 93, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Misra, S.; Kumar, A.; Pandit, A.K.; Chakravarty, K.; Prasad, K. Association between tumor necrosis factor-α (-238G/A and -308G/A) gene polymorphisms and risk of ischemic stroke: A meta-analysis. Pulse 2016, 3, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Oki, E.; Norde, M.N.; Carioca, A.A.F.; Souza, J.M.P.; Castro, I.A.; Marchioni, D.M.L.; Fisberg, R.M.; Rogero, M.M. Polymorphisms of the TNF-α gene interact with plasma fatty acids on inflammatory biomarker profile: A population-based, cross-sectional study in São Paulo, Brazil. Br. J. Nutr. 2017, 117, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- West, M.; Greason, E.; Kolmakova, A.; Jahangiri, A.; Asztalos, B.; Pollin, T.I.; Rodriguez, A. Scavenger receptor class B type I protein as an independent predictor of high-density lipoprotein cholesterol levels in subjects with hyperalphalipoproteinemia. J. Clin. Endocrinol. Metab. 2009, 94, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.R.B.; Voight, B.F.; Yengo, L.; Amin, N.; Dupuis, J.; Ganser, M.; Grallert, H.; Navarro, P.; Li, M.; Qi, L.; et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, H.; Pan, A.; Patel, B.; Xiang, G.; Qi, L.; Kaplan, R.C.; Hu, F.; Wylie-Rosett, J.; Qi, Q. FTO genotype and weight loss in diet and lifestyle interventions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1162–1170. [Google Scholar] [CrossRef]
- Whitworth, J.A.; World Health Organization, international society of hypertension writing group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens. 2003, 21, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Welty, F.K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef]
- Giannini, C.; Santoro, N.; Caprio, S.; Kim, G.; Lartaud, D.; Shaw, M.; Pierpont, B.; Weiss, R. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011, 34, 1869–1874. [Google Scholar] [CrossRef]
- Kim-Dorner, S.-J.; Deuster, P.A.; Zeno, S.A.; Remaley, A.T.; Poth, M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism 2010, 59, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.A.; Davies, M.J.; Morris, D.H.; Yates, T.; Srinivasan, B.T.; Webb, D.; Brady, E.; Khunti, K. The association of the triglyceride-to-HDL cholesterol ratio with insulin resistance in white european and south asian men and women. PLoS ONE 2012, 7, e50931. [Google Scholar] [CrossRef] [PubMed]
- Miralles, C.S.W.; Wollinger, L.M.; Marin, D.; Genro, J.P.; Contini, V.; Dal Bosco, S.M. Waist-to-height ratio (WHtR) and triglyceride to HDL-c ratio (TG/HDL-c) as predictors of cardiometabolic risk. Nutr. Hosp. 2015, 31, 2115–2121. [Google Scholar] [CrossRef]
- Jayanthi, R.; Srinivasan, A.R.; Hanifah, M.; Maran, A.L. Associations among Insulin Resistance, Triacylglycerol/High Density Lipoprotein (TAG/HDL ratio) and Thyroid hormone levels—A study on Type 2 diabetes mellitus in obese and overweight subjects. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S121–S126. [Google Scholar] [CrossRef]
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Loos, R.J. The genetics of adiposity. Curr. Opin. Genet. Dev. 2018, 50, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Nauroth, J.M.; Liu, Y.C.; Van Elswyk, M.; Bell, R.; Hall, E.B.; Chung, G.; Arterburn, L.M. Docosahexaenoic Acid (DHA) and Docosapentaenoic Acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema In Vivo. Lipids 2010, 45, 375–384. [Google Scholar] [CrossRef]
- Walker, C.; West, A.; Browning, L.; Madden, J.; Gambell, J.; Jebb, S.; Calder, P. The pattern of fatty acids displaced by EPA and DHA following 12 months supplementation varies between blood cell and plasma fractions. Nutrients 2015, 7, 6281–6293. [Google Scholar] [CrossRef]
- Huang, C.-W.; Chien, Y.-S.; Chen, Y.-J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.-T. Role of n-3 polyunsaturated fatty acids in ameliorating the obesity-induced metabolic syndrome in animal models and humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef]
- Marigorta, U.M.; Gibson, G. A simulation study of gene-by-environment interactions in GWAS implies ample hidden effects. Front. Genet. 2014, 5, 225. [Google Scholar] [CrossRef]
- Loos, R.J.F.F. Genetic determinants of common obesity and their value in prediction. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 211–226. [Google Scholar] [CrossRef] [PubMed]
- de Toro-Martín, J.; Arsenault, B.; Després, J.-P.; Vohl, M.-C. Precision nutrition: A review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rúa, R.; Keijer, J.; Caimari, A.; van Schothorst, E.M.; Palou, A.; Oliver, P. Peripheral blood mononuclear cells as a source to detect markers of homeostatic alterations caused by the intake of diets with an unbalanced macronutrient composition. J. Nutr. Biochem. 2015, 26, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, G.; Kang, M.; Yoo, H.J.; Jeong, T.; Lee, S.; Lee, J.H. Replacing carbohydrate with protein and fat in prediabetes or type2 diabetes: Greater effect on metabolites in PBMC than plasma. Nutr. Metab. 2016. [Google Scholar] [CrossRef] [PubMed]
- Article, O. Effects of n−3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018. [Google Scholar] [CrossRef]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef]
| Genotype | Associated Risk 2 | Biomarkers/risk associated with risk allele | Interactions described concerning the risk allele | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs code | Gene | a | b | c | a | b | c | ||
| rs1800629 | TNFα | GG | GA | AA | 0 | 2 | 2 | TNFα, CRP [31] CAD risk [62] IL18, IL18:IL-0 and TNFα:IL10 ratios [63] Ischemic Stroke [64] Disturbed plasma levels of PUFA [65] | Higher CRP levels after fish oil supplementation [31] Resistance to improve in inflammatory CRP plasmatic profile after dietary intervention [32] |
| rs5082 | APOA2 | CC | TC | TT | 0 | 1 | 2 | CRP [34,35] Obesity and Insulin Resistance [37,38] | More prone to high CRP levels in obesity [35] Protective effect against T2D, but not in case of obesity [35] Higher intakes of omega 3 are not associated with increases of SOD activity [39] Higher intakes of omega 6 are associated with increased oxidative biomarkers [39] |
| rs4880 | SOD2 | CC | CT | TT | 0 | 1 | 2 | IL6, TNFα, IL1β [42] CAD risk [43] T2D risk [44] DNA damage [45] | Attenuated response to statin drugs regarding lipid and inflammatory profile [47] Less risk of Breast Cancer in women when the consumption of fish or vegetables are high [48] |
| rs1260326 | GCKR | CC | CT | TT | 0 | 1 | 2 | CRP [49,66] TAGs [49,50,51] TC [52] T2D risk [53] | Omega 3 PUFAs interaction regarding triglycerides levels [54] Lack of lowering effect of omega 3 PUFA on fasting insulin, HOMA-IR and CRP [55] |
| rs9939609 | FTO | TT | TA | AA | 0 | 1 | 2 | BMI, BF [56] BMI [57] T2DM risk [67] CRP [56,58] | Lower PUFA:SFA ratio are associated with higher obesity risk than in TT subjects [59] Lower effect of diet/lifestyle interventions [68] |
| Metabolic Condition | No | Yes |
|---|---|---|
| HT |
|
|
| DL |
|
|
| T2D |
|
|
| VO |
|
|
| MR |
|
|
| Subset Population (n = 59) | BMI < 25 (kg/m2) (n = 28) | BMI ≥ 25 (kg/m2) (n = 31) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Age (years) | 33.4 | 13.8 | 28.0 | 9.6 | 38.3 | 15.2 | 0.002 |
| Anthropometric measures | |||||||
| Height (m) | 1.80 | 0.07 | 1.80 | 0.07 | 1.80 | 0.07 | 0.696 |
| Weight (kg) | 81.7 | 18.2 | 68.8 | 8.7 | 93.4 | 16.5 | <0.0001 |
| BMI (kg/m2) | 26.4 | 5.5 | 22.1 | 2.0 | 30.4 | 4.7 | <0.0001 |
| Body fat percentage (%) | 23.6 | 7.7 | 18.1 | 5.7 | 28.6 | 5.6 | <0.0001 |
| Cardiovascular health indicators | |||||||
| SBP (mmHg) | 132.5 | 15.6 | 128.6 | 14.7 | 136.0 | 15.8 | 0.056 |
| DBP (mmHg) | 80.3 | 11.5 | 77.3 | 9.3 | 83.0 | 12.8 | 0.055 |
| MAP | 97.7 | 11.7 | 94.4 | 10.1 | 100.7 | 12.3 | 0.037 |
| Circulating parameters | |||||||
| Glucose (mg/dL) | 96.10 | 28.35 | 91.93 | 25.08 | 100.00 | 31.01 | 0.195 |
| Total cholesterol (mg/dL) | 190.39 | 45.74 | 184.71 | 36.26 | 195.86 | 53.42 | 0.313 |
| LDLc (mg/dL) | 106.90 | 39.30 | 104.43 | 34.12 | 109.29 | 44.21 | 0.645 |
| HDLc (mg/dL) | 60.57 | 18.65 | 61.61 | 18.25 | 59.64 | 19.26 | 0.695 |
| Triglycerides (mg/dL) | 120.30 | 58.69 | 101.71 | 30.82 | 138.24 | 72.77 | 0.016 |
| CRP (ng/mL) | 1.26 | 1.10 | 0.67 | 0.63 | 1.73 | 1.18 | <0.0001 |
| General Adult Population | ||
|---|---|---|
| Mean | SD | |
| Demographic Descriptors | ||
| Gender (% female) | 63.0 | |
| Age (years) | 36.0 | 15.0 |
| European (%) | 86.4 | |
| Overweight/obese (%) | 41.0 | |
| Anthropometric measures | ||
| Height (m) | 1.67 | 0.90 |
| Weight (kg) | 69.7 | 16.3 |
| Hips (cm) | 95.6 | 11.2 |
| Waist (cm) | 84.1 | 15.8 |
| Waist to Height Ratio | 0.50 | 0.10 |
| BMI (kg/m2) | 24.9 | 5.2 |
| Body fat percentage (%) | 29.3 | 8.9 |
| Cardiovascular health indicators | ||
| SBP (mmHg) | 125.4 | 15.6 |
| DBP (mmHg) | 73.1 | 9.9 |
| MAP | 72.1 | 33.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmés, S.; Cifre, M.; Palou, A.; Oliver, P.; Serra, F. A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome. Nutrients 2019, 11, 298. https://doi.org/10.3390/nu11020298
Galmés S, Cifre M, Palou A, Oliver P, Serra F. A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome. Nutrients. 2019; 11(2):298. https://doi.org/10.3390/nu11020298
Chicago/Turabian StyleGalmés, Sebastià, Margalida Cifre, Andreu Palou, Paula Oliver, and Francisca Serra. 2019. "A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome" Nutrients 11, no. 2: 298. https://doi.org/10.3390/nu11020298
APA StyleGalmés, S., Cifre, M., Palou, A., Oliver, P., & Serra, F. (2019). A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome. Nutrients, 11(2), 298. https://doi.org/10.3390/nu11020298









