1. Introduction
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer in adults, and it has high malignancy and poor prognosis [
1]. HCC treatment options and prognosis depend on many factors such as tumor size and stage of tumor severity. Surgical treatment for HCC does not give significant outcomes because the cancer can be removed through surgery in only 10%–20% of patients [
2]. Chemotherapy is one of the options that has been used to cure HCC. Thus, the current HCC research focus is on identifying alternative therapeutic drugs with high pharmacological effects and low normal cell toxicity [
3].
Flavonoids are natural polyphenolic compounds that are known for their significant biological beneficial properties (including anti-cancer, anti-allergic, and anti-inflammatory activities), and it is also reported that they have low toxicity. The anti-cancer activities of flavonoids relate to their role in cell cycle arrest and apoptosis, preventing cell invasion and adhesion. The safety and efficacy of flavonoids as anti-cancer agents have been identified in human clinical studies [
4].
Scutellaria baicalensis is one of the fundamental herbs used in traditional Chinese medicine, and it has a wide range of biological activities, such as anti-inflammation and anti-diarrheal effects [
5,
6]. The leaves and flowers of
Scutellaria baicalensis contain flavonoid glycosides such as scutellarein (5,6,7,4′-tetrahydroxyflavone; SCU), a flavone found in the perennial herb that has a range of biological activities. Previous studies demonstrated that the whole flavonoid extract of SCU along with the monomer compound, have a broad scale of biological activities such as anti-oxidant, anti-inflammatory, and anticancer effects through the induction of apoptosis [
7].
Cell cycle arrest and induction of apoptosis are important strategies in cancer therapeutic strategies [
8]. The ability to modulate life or death of a cell is known for therapeutic potential in treating cancer cells. Thus, the focus of research has been directed toward the cell cycle and programmed cell death mechanisms [
9]. The regulating factors of cell cycle processes are frequently modified in human cancer cells. The cyclin-dependent kinases (CDKs) are central players that control the initiation, progression, and completion of the cell cycle. Inhibiting CDK activity is expected to obstruct cell cycle events and lead to cell cycle arrest. Many compounds operate as anti-cancer agents at multiple steps in the cell cycle [
10].
Apoptosis is generally defined as programmed cell death, and it plays important roles in developing and maintaining tissue homeostasis and cancer chemoprevention. Apoptosis is characterized by several distinct morphological features such as cell membrane blebbing, cell shrinkage, chromatin condensation, and DNA fragmentation, followed by the engulfment of macrophages [
11].
The mechanism of apoptosis follows two distinct pathways: the extrinsic death receptor-mediated pathway and the intrinsic mitochondria-mediated pathway. Caspases are the central effectors of apoptosis and the two pathways that lead to other proteases and nucleases to cause apoptosis [
12]. In the extrinsic apoptosis pathway, the Fas ligand (FasL) is upregulated when the cell-surface death receptor, Fas, is activated. The activation of the Fas leads to sequential activation of caspase-8, caspase-3, and polymeric adenosine diphosphate ribose (PARP). In the intrinsic apoptosis pathway, the release of diverse apoptotic stimuli from intrinsic signals including those from DNA damage and oxidative stress converge to the mitochondria and then lead to the release of cytochrome c from the mitochondria to cytoplasm, initiating the caspase cascades [
13].
In this study, we identified the anti-cancer effect of SCU in human hepatoma Hep3B cells. We found evidence that SCU prevented cell proliferation via cell cycle arrest in the G2/M phase and induction of the extrinsic apoptosis pathway in Hep3B cells. These findings suggest that SCU can be used for developing potent anti-cancer agents for HCC treatment.
2. Materials and Methods
2.1. Chemicals and Reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Duchefa Biochemie (Haarlem, the Netherlands). Antibodies to caspase-3, -8, and -9, cleaved caspase-3, -8, and -9, polymeric adenosine diphosphate ribose (PARP), cleaved PARP, Fas, FasL, Cyclin B1, Cdc25C, and Bcl-xL were purchased from Cell Signaling Technology (Danvers, MA, USA). Death receptor 4 (DR4) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies cdk1, Bax, and β-actin were purchased from Millipore (Temecula, CA, USA).
2.2. Cell Culture and Scutellarein (SCU) Treatment
Human hepatocarcinoma cell line Hep3B was obtained from the Korea Cell Line Bank (Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), and antibiotics penicillin/streptomycin (P/S) were purchased from Gibco (BRL Life Technologies, Grand Island, NY, USA). Mycoplasma free Hep3B cells were cultured in DMEM supplemented with 10% FBS and 1% P/S at 37 °C in a humidified atmosphere of 5% CO2. To confirm mycoplasma contamination, we used the e-Myco™ Mycoplasma PCR Detection kit (iNtRON Biotechnology, Seoul, Korea). We cultured Hep3B cells for no more than 15 passages or 2 months. Scutellarein (SCU) was purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, Sichuan, China). Cells grown to 80% confluence were untreated (DMSO) or treated with indicated concentration of SCU for 24 h in complete media.
2.3. Cell Viability Assay
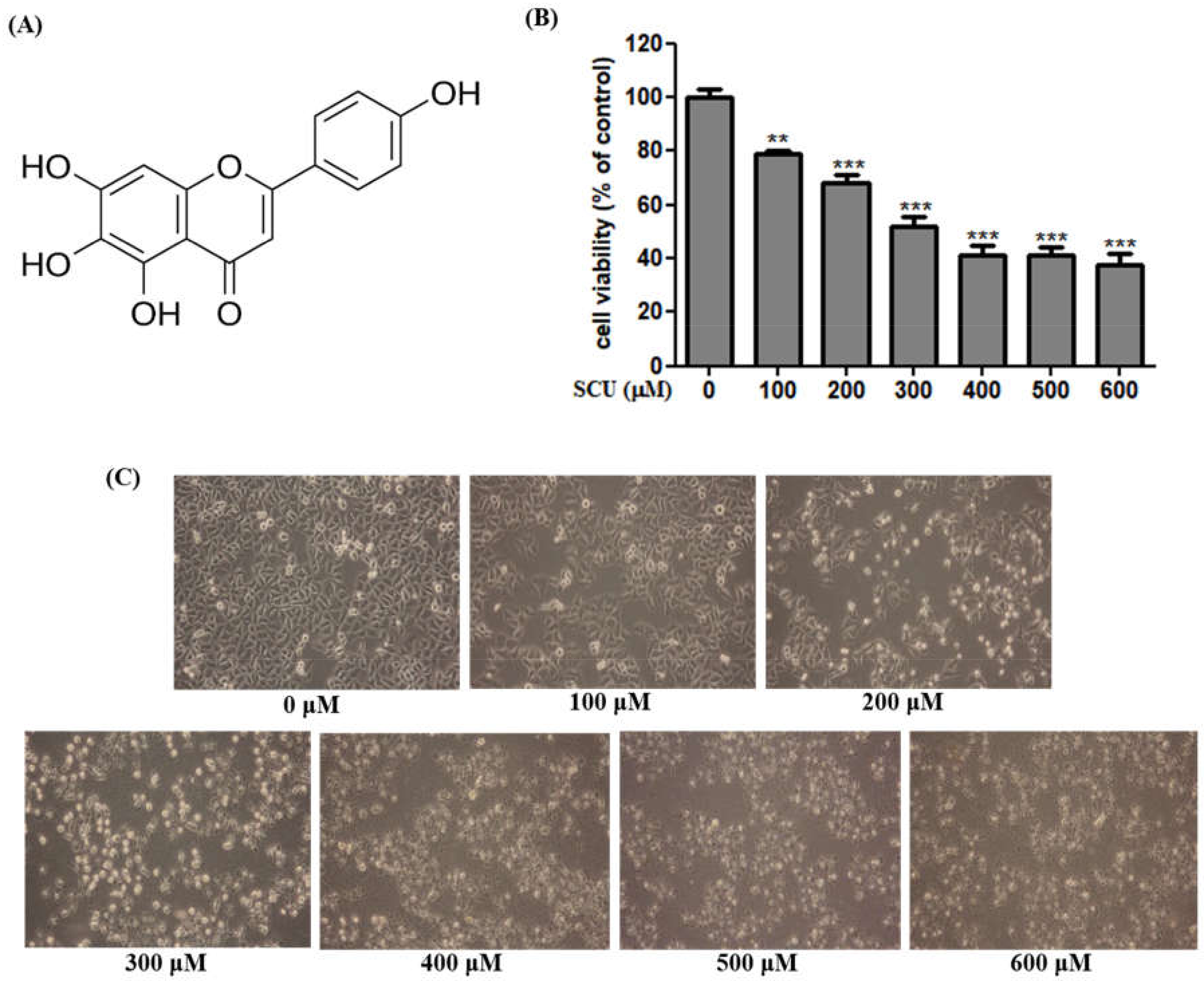
Cell viability was measured using MTT assay. Cells were seeded at 5 × 104 cells in a 48-well plate and incubated overnight, followed by treatment with SCU at the concentrations of 0-, 100-, 200-, 300-, 400-, 500-, and 600-μM for 24 h. After incubation, 50 μL of MTT (0.5 mg/mL) solution was added to each well and incubated for about 3 h at 37 °C. The formazan precipitate formed after incubation was dissolved in 300 μL of DMSO and the absorbance of converted dye was measured at a wavelength of 540 nm with a micro-plate reader (BioTek, Winooski, VT, USA). Cell viability was expressed as a percentage of proliferation versus SCU untreated group.
2.4. Morphological Change and DAPI Fluorescent Staining
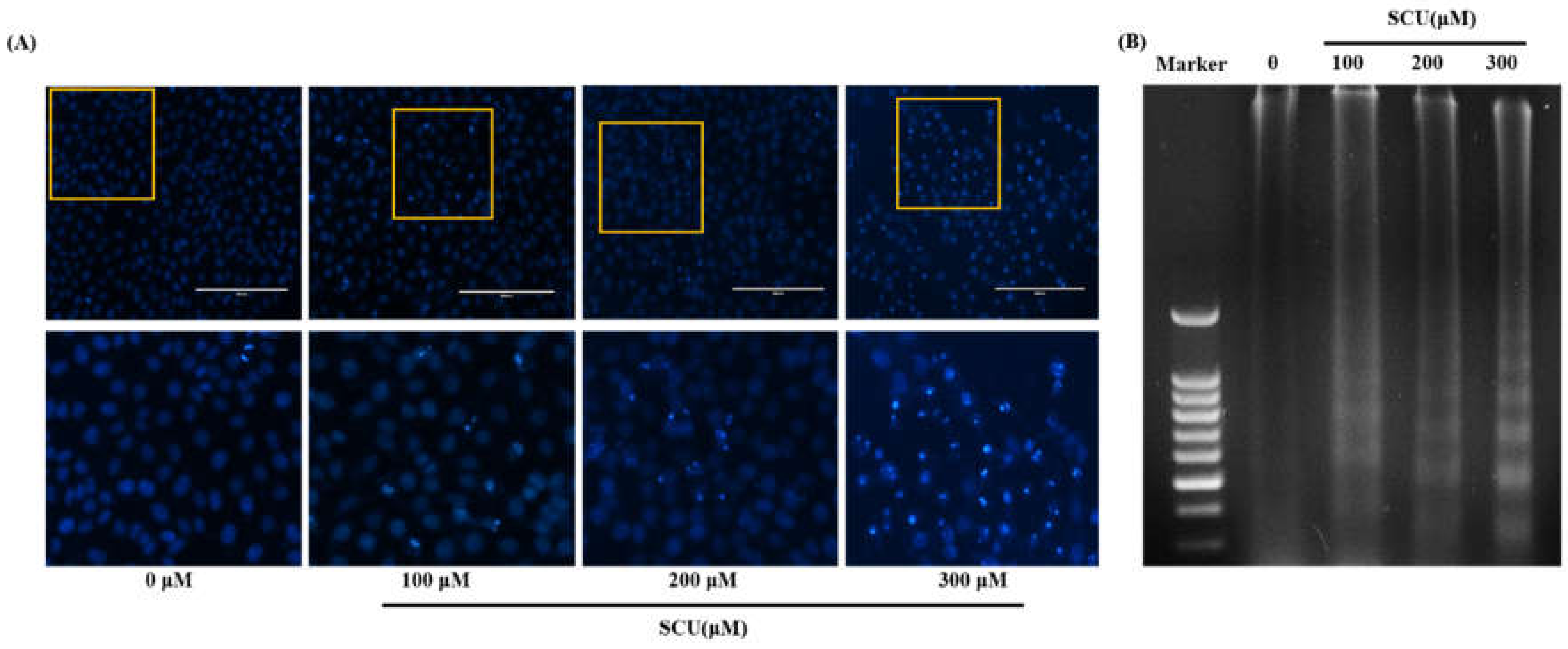
For nuclear morphological analysis, Hep3B cells were plated on 12-well plates at 1 × 105 cells after treatment with various concentrations of SCU (0-, 100-, 200-, and 300-μM) at 37 °C for 24 h. The cells were washed with ice-cold PBS and then fixed with 37% formaldehyde (1:4 dilutions with PBS) for 15 min at room temperature. Subsequently, the fixed cells were washed with PBS and stained with a 4′,6-diamidino-2-phenylindole (DAPI; Vectashield H-1500; Vector Laboratories, Burlingame, CA, USA). The nuclear morphology of the cells was examined by fluorescence microscopy (EVOS®, Life Technologies, Darmstadt, Germany).
2.5. DNA Fragmentation Assay
Hep3B cells were plated on 60-mm plates at 4 × 105 cells after treatment with indicated concentrations of SCU (0-, 100-, 200-, and 300-μM) for 24 h and DNA was isolated from the cells harvested and lysed using lysis buffer containing 1% NP-40, 20 mM ethylenediaminetetraacetate (EDTA), 50 mM Tris-HCl, and pH 7.5 for 30min. The lysed cells were centrifuged at 3,000 rpm for 5 min and the supernatant was collected. The collected supernatant was incubated with 10 μL of 10% sodium dodecyl sulfate (SDS) solution and 5 μL of 100 mg/mL RNase A for 2 h at 56 °C in a water bath. Further, protein digestion was carried out by adding 10 μL of 25 mg/mL proteinase K enzyme and incubated for 2 h at 37 °C, followed by the addition of 65 μL of 5 M NaCl and 500 μL of ice-cold ethanol, and the contents were mixed thoroughly. The mixture was further subjected to incubation for 2 h at −80 °C. It was then centrifuged for 20 min at 12,000 rpm followed by washing the pellet with 1 mL of 80% ice-cold ethanol and air-dried for 10 min at room temperature. The pellet was dissolved with 20 μL of Tris-EDTA (TE) buffer. The total DNA sample was then subjected to 1.5% agarose gel electrophoresis and DNA bands were visualized in UV light absorbance.
2.6. Annexin V Propidium Iodide Apoptosis Detection
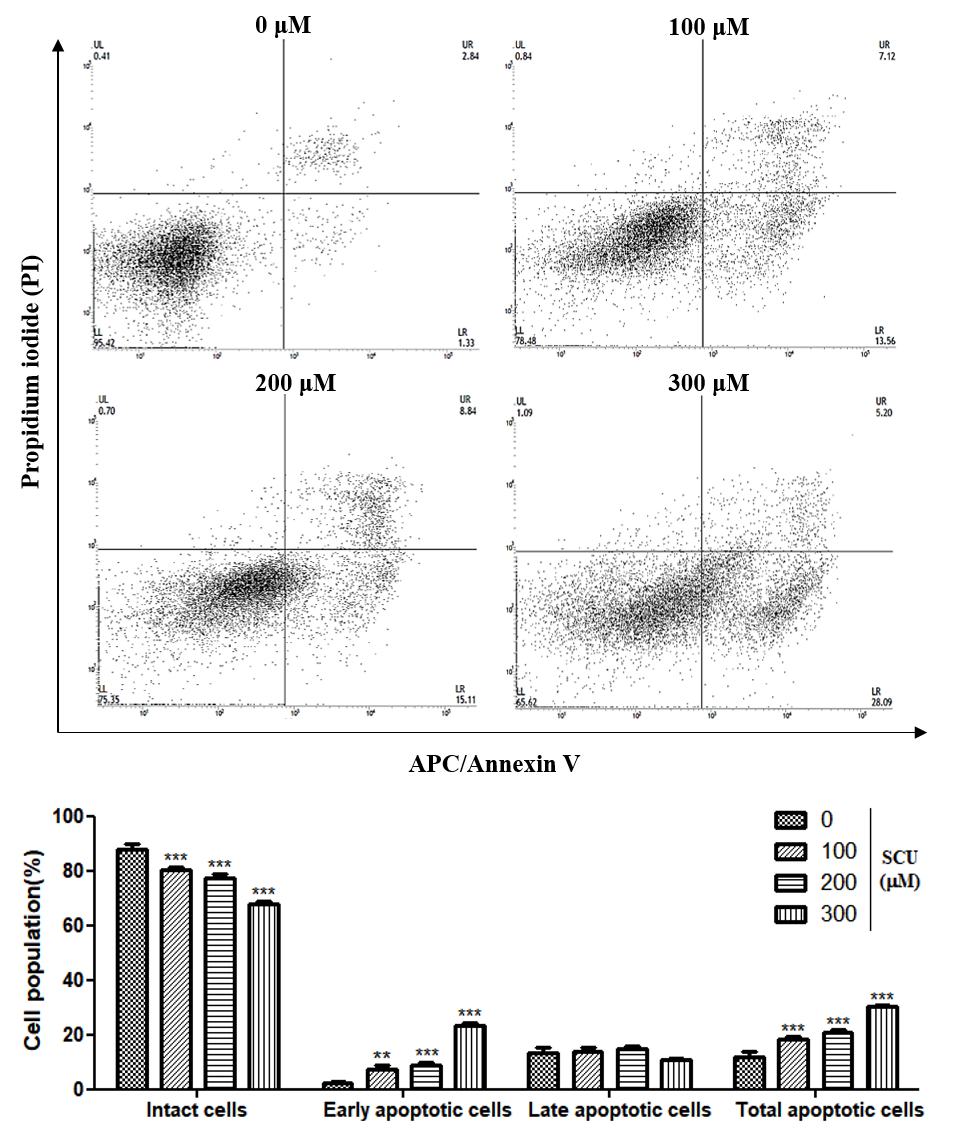
Apoptotic cells were detected by using allophycocyanin (APC)/Annexin V apoptosis detection kit according to the manufacturer’s protocol (BD Biosciences, San Diego, CA, USA). Briefly, cells were plated on 60-mm plates at 4 × 105 cells and then incubated with various concentrations of SCU (0-, 100-, 200-, and 300-μM), for 24 h. The cells were collected and washed with PBS re-suspended in binding buffer. The cells were stained with APC/Annexin V and propidium iodide (PI) for 15 min at room temperature in the dark, prior to the addition of binding buffer. Flow cytometry analysis was performed on the cell suspensions and the data obtained were analyzed using a fluorescence-activated cell sorting machine (FACSVerseTM flow cytometer; BD Biosciences, Franklin Lakes, New Jersey, USA). In total, 10,000 events per sample were sorted and the data were analyzed using BD FACSuiteTM software (BD Biosciences, Becton & Dickson, Mountain View, CA, USA).
2.7. Analysis of Cell Cycle Distribution or DNA Content by Flow Cytometry
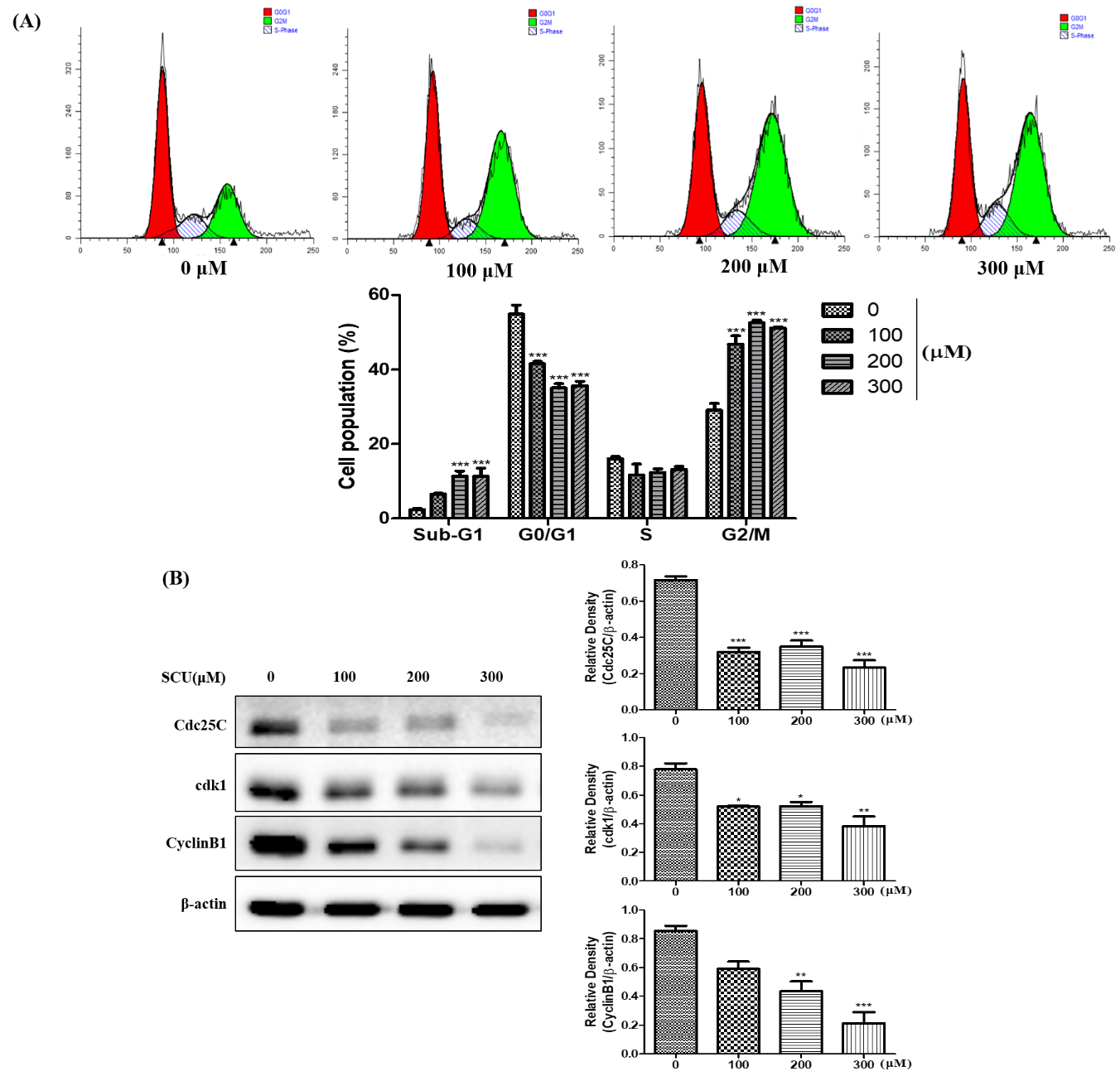
Flow cytometry was performed to analyze the cell cycle distribution and cell death. Hep3B cells were treated with various concentrations of SCU (0-, 100-, 200- and 300-μM) for 24 h at 37 °C. The cells were washed with ice-cold PBS. After incubation, trypsinized cells were collected in a 15-mL conical tube, and the pellet was obtained by centrifugation at 1200 rpm for 4 min. The pellets were washed twice with ice-cold PBS and fixed in 70% ice-cold ethanol for 1 h at −20 °C. The cell suspension was centrifuged further and the cells were washed in PBS and re-suspended in 400 μL of PBS containing 50 μg/mL PI (Sigma-Aldrich, St. Louis, MO, USA) and 50 μg/mL RNase A, followed by incubation in dark conditions for 15 min at room temperature. After incubation, flow cytometry analysis was performed on the cell suspensions and the data obtained were analyzed using FACSVerseTM flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The acquired FACS data was analyzed using ModFit LT software (Verity Software House, Topsham, ME, USA).
2.8. Analysis of Mitochondrial Membrane Potential Using JC-1 Staining
Flow cytometry analysis was performed to detect the mitochondrial membrane potential in Hep3B cells. The cells were treated with various concentrations of SCU (0-, 100-, 200- and 300-μM) for 24 h at 37 °C. After treatment, the cells were stained with 10 μM the fluorescent dye 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1 dye; R&D Systems, Minneapolis, MN, USA) and incubated for about 10 min at 37 °C. The cells were harvested by centrifucation at 1200 rpm for 5 min. The cell pellet obtained was suspended in serum free DMEM media. Flow cytometry analysis was performed on the cell suspensions using FACSVerseTM flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The acquired FACS data was analyzed using BD FACSuiteTM software (BD Biosciences, Becton & Dickson, Mountain View, CA, USA).
2.9. Western Blotting Analysis
Hep3B cells were seeded in 60-mm plates at 4 × 105 per well and treated with indicated concentrations of SCU (0-, 100-, 200-, and 300-μM) for 24 h at 37 °C. Cells were lysed using radioimmuno-precipitation assay (RIPA) buffer (iNtRON Biotechnology, Seoul, Korea) containing phosphatase and protease inhibitor cocktail (Thermo Scientific, Rockford, IL, USA) for 30 min in ice. Protein lysates were centrifuged at 10,000 rpm for 10 min at 4 °C and the protein concentrations were determined using a Pierce™ BCA assay (Thermo Fisher Scientific, Rockford, IL, USA). The protein extract was mixed with required volume of 5X sample buffer and kept at 100 °C for 5 min. Protein samples were resolved on 8%–15% SDS polyacrylamide gels and subjected to electrophoresis. The separated proteins were electro transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% (bovine serum albumin (BSA) in Tris-buffered saline containing 1% Tween 20 (TBS-T, pH 7.4) at room temperature for 1 h, and incubated overnight at 4 °C with β-actin (1:10,000), Cdc25C (1:1000), cdk1 (1:1000), Cyclin B1 (1:1000), Bax (1:1000), Bcl-xL (1:1000), caspase-9 (1:1000), cleaved caspase-9 (1:500), DR4 (1:500), Fas (1:1000), FasL (1:500), caspase-8 (1:1000), cleaved caspase-8 (1:500), caspase-3 (1:1000), cleaved caspase-3 (1:500), and PARP (1:1000) primary antibody. The membranes were washed with TBS-T buffer for every 10 min in five repetitions at room temperature. Furthermore, they were incubated with 1:1000 dilution of horseradish peroxidase (HRP)-conjugated secondary antibody for 3 h at room temperature. The obtained protein blots were developed under an electrochemiluminescence (ECL) detection system (Bio-Rad Laboratory, Hercules, CA, USA). Protein quantification was analyzed using ImageJ software program (U.S. National Institutes of Health, Bethesda, MD, USA). The densitometry readings of the protein bands were normalized by comparing with the expression of β-actin.
2.10. Statistical Analysis
All the experimental results were expressed as the mean ± standard error of the mean (SEM) of triplicate samples. Significant differences between groups were calculated by one-way factorial analysis of variance (ANOVA) followed by a Bonferroni’s test and p < 0.05 was considered statistically significant. * p < 0.05 vs. Hep3B cell control, ** p < 0.01 vs. Hep3B cell control, *** p < 0.001 vs. Hep3B cell control.
4. Discussion
Hepatocellular carcinoma (HCC) has increased threefold during the last 15 years and tops the mortality statistics for cancers (23.2%) [
15]. More than 500,000 individuals suffer from this disease annually [
16]. The preferred treatment for HCC is liver transplantation or resection, but despite surgical and regional therapies, prognosis remains unsatisfactory because of high tumor recurrence and tumor progression [
17]. Recent interests in herbal therapeutic agents for treating diseases have gained attention due to their potential benefits and availability [
18]. Flavonoids, as vital secondary metabolites, possess high-yield benefits in treating cancer with increased efficacy [
19]. Growing evidence indicates that herbal extracts and their compounds, especially flavonoids, can inhibit cell growth and promote cancer cell death through different mechanisms including cell cycle arrest, autophagy, and apoptosis [
20,
21].
Compounds isolated from
Scutellaria baicalensis exhibit pharmacological activities and have been recognized as valuable resources for the development of therapeutics. Scutellarein (SCU) is a major active flavonoid extracted from
Scutellaria baicalensis with a wide range of biological activities such as anti-oxidant, anti-inflammatory and also anti-cancer effects by inducing apoptosis [
22]. Accumulating research evidence has highlighted the potential role of (SCU) and its main metabolite SCU in the treatment of cancer [
23].
In the present study, we examined the flavonoid SCU for its anti-cancer effects in hepatocellular carcinoma cells, Hep3B. Cell viability analysis revealed that SCU effectively inhibits cancer progression in Hep3B cell in a dose-dependent manner. We also investigated the mechanism by which SCU induces apoptosis in Hep3B cells.
Apoptosis plays an important role in developing and maintaining of tissue homeostasis. Intensive efforts have been made to explore the molecular mechanisms of the apoptotic signaling pathways from initiation to execution of apoptosis. Intensive efforts have been made to explore the molecular mechanisms of the apoptotic signaling pathways from initiation to execution of apoptosis [
13]. The nuclear morphological analysis performed in this study also significantly depicted the induction of apoptosis [
24]. DNA fragmentation assay revealed differently sized fragments of DNA in terms of apoptotic bodies, which confirmed that apoptotic cell death is induced by SCU in Hep3B cells. Similarly, flow cytometry analysis of SCU-treated Hep3B cells through allophycocyanin (APC)/Annexin V and propidium iodide (PI) double staining showed up-regulation of the apoptotic cell death fraction in the early stage, and the total apoptotic fraction also increased significantly.
In our study, SCU induced G2/M phase cell cycle arrest by inhibiting and the protein expression of Cdc25C, cdk1 and Cyclin B1. Previous studies showed that increases in the number of cells in the G2/M phase were clearly related to apoptosis [
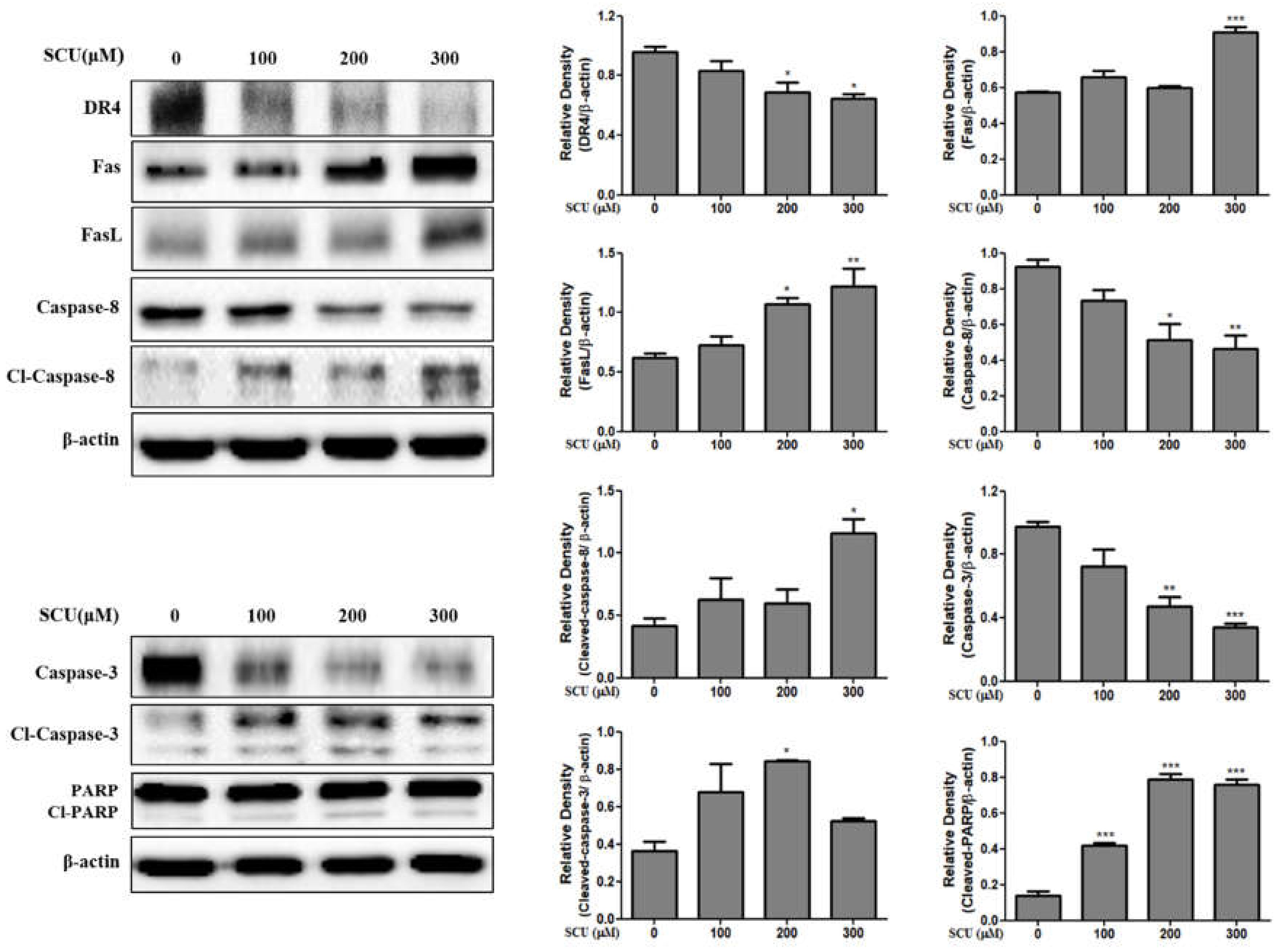
20]. Western blot analysis suggested that SCU induced apoptosis by activating caspases. The present study showed that treatment with SCU increased the expression of Fas ligand (FasL); FasL belongs to the tumor necrosis factor family and binds Fas, which leads to apoptosis in Fas-bearing cells [
25]. The up-regulation of Fas ligand (FasL) can activate the apoptotic factors caspase-8 and caspase-3, leading to the cleavage of polymeric adenosine diphosphate ribose (PARP). In this study, Fas and Fas ligand (FasL) showed significant increases that activated cleaved caspase-8, caspase-3, and PARP, but the expression of the death receptor 4 (DR4) decreased. Thus, our results confirm that SCU induced Fas-mediated extrinsic apoptotic cell death in Hep3B cells.
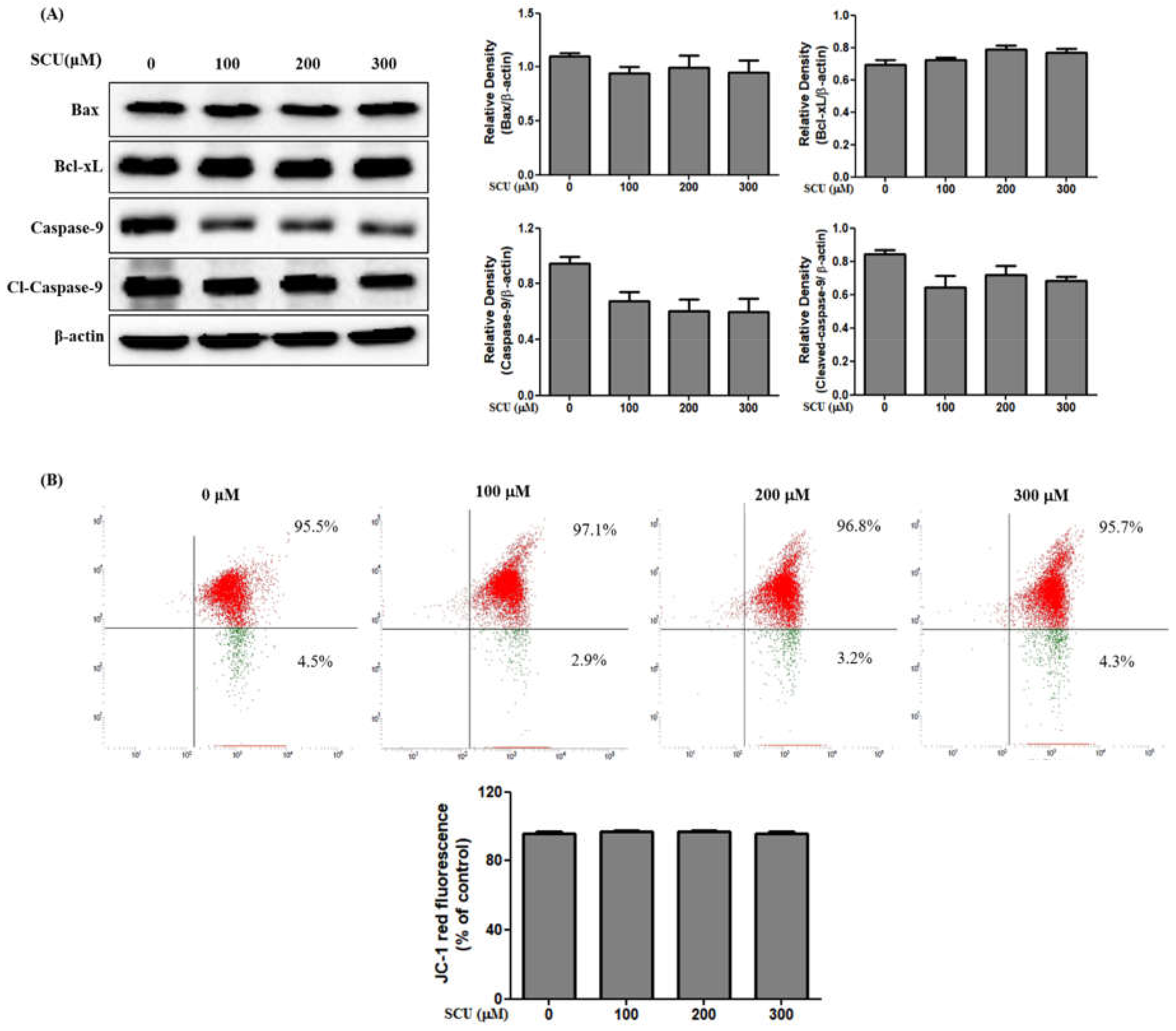
In contrast, the mitochondrial-dependent apoptosis pathway, called the intrinsic pathway, was not activated in Hep3B cells treated with SCU. Overexpression of Bcl-xL, an anti-apoptotic protein, prevents cells from initiating apoptosis, while increase of Bax, a pro-apoptotic protein, induces the intrinsic apoptosis pathway [
26]. In this study, we found that the levels of Bax and Bcl-xL and mitochondrial membrane potential does not change in SCU-treated Hep3B cells. In conclusion, SCU can inhibit the viability of hepatocellular carcinoma Hep3B cells via the Fas-mediated extrinsic apoptotic pathway. Findings in the current study strongly suggest that SCU can be used for developing potent anti-cancer agents for hepatocellular carcinoma Hep3B treatment.