Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Adiponectin Concentration Using ELISA
2.3. Measurement of Gene Expression Using qPCR Analysis
2.3.1. RNA Extraction
2.3.2. cDNA Synthesis
2.3.3. Real-Time PCR
2.4. Measurements of Inflammatory Markers Concentration in Blood Plasma
2.5. Statistics
3. Results
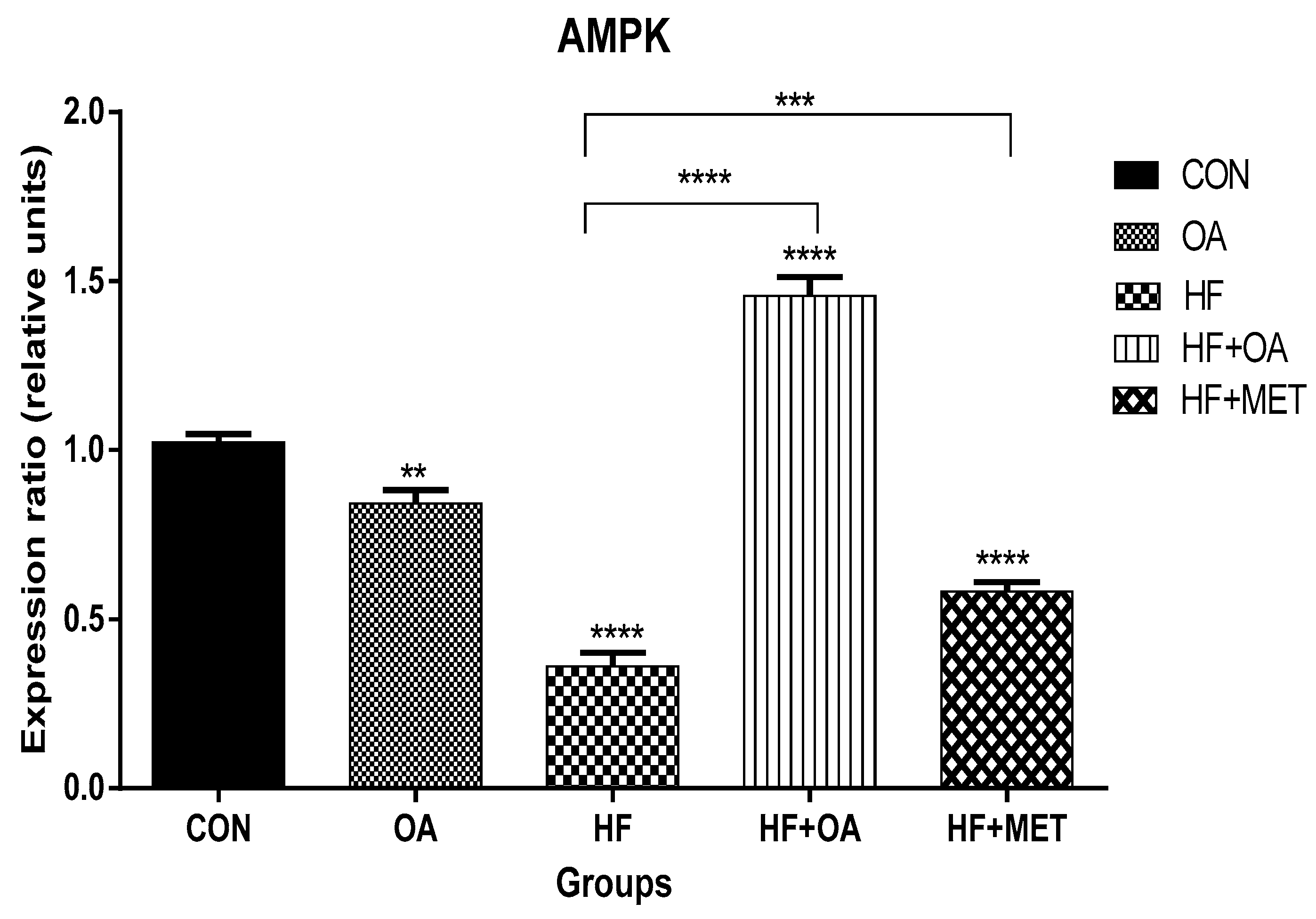
3.1. Gene Expression of AMPK
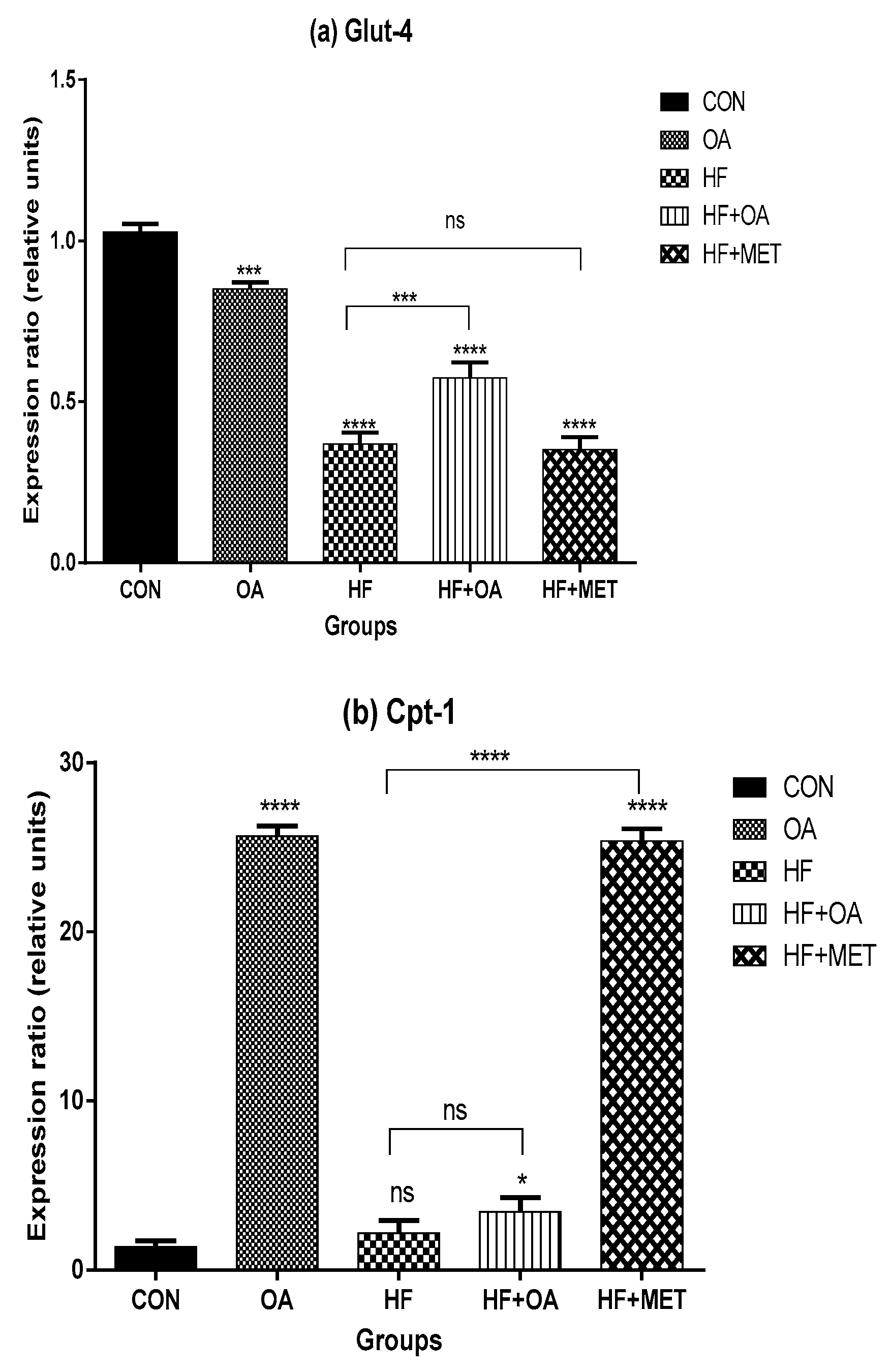
3.2. Gene Expression of Glut-4 and Cpt-1
3.3. Adiponectin Concentration
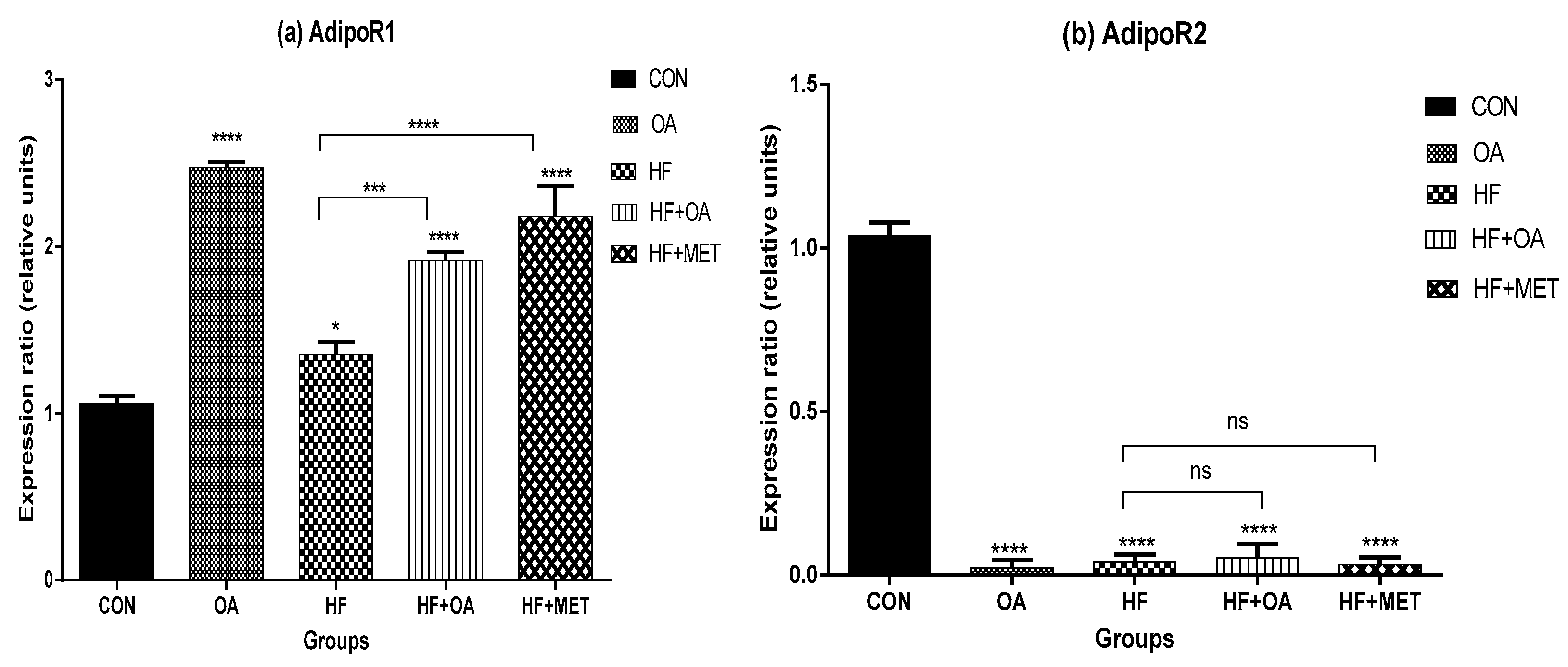
3.4. Gene Expression of Adiponectin Receptors
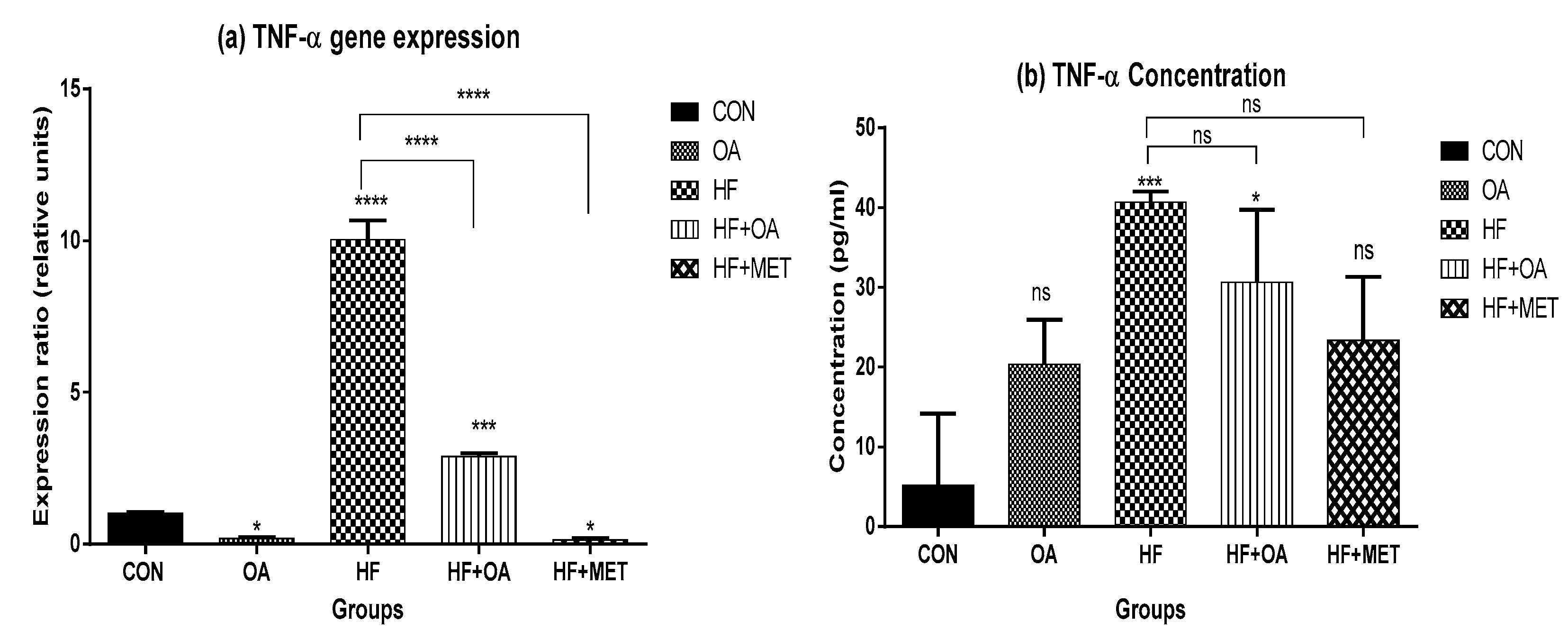
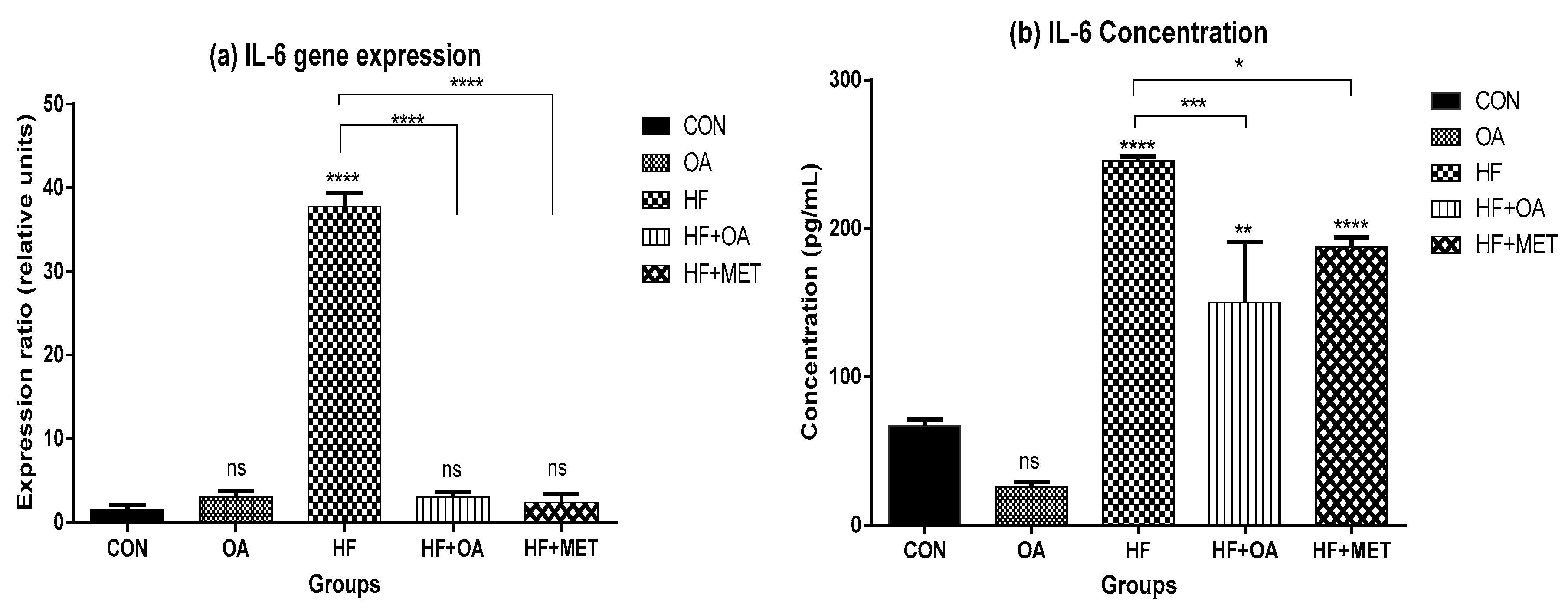
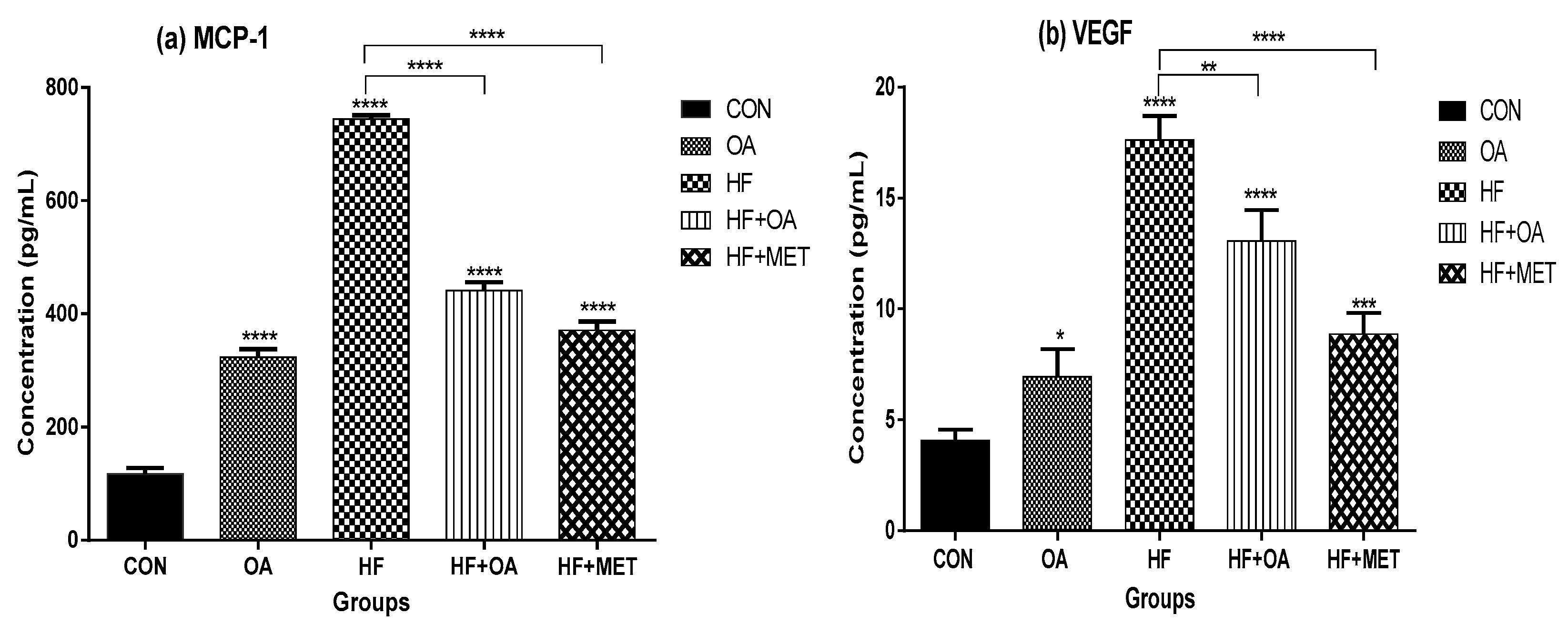
3.5. Inflammatory Biomarkers Concentration and Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghoshal, K.; Bhattacharyya, M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J. Diabetes 2015, 6, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Beck Jorgensen, S. The AMP-activated protein kinase: Role in regulation of skeletal muscle metabolism and insulin sensitivity. MiniRev. Med. Chem. 2007, 7, 521–528. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, C.-H.; Lin, W.-L.; Wu, J.-B. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J. Ethnopharmacol. 2009, 123, 82–90. [Google Scholar] [CrossRef]
- Cree-Green, M.; Gupta, A.; Coe, G.V.; Baumgartner, A.D.; Pyle, L.; Reusch, J.E.; Brown, M.S.; Newcomer, B.R.; Nadeau, K.J. Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J. Diabetes Complicat. 2017, 31, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Winder, W.; Hardie, D. AMP-activated protein kinase, a metabolic master switch: Possible roles in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 1999, 277, E1–E10. [Google Scholar] [CrossRef]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Moehlecke, M.; Canani, L.H.; Trindade, M.R.M.; Friedman, R.; Leitão, C.B. Determinants of body weight regulation in humans. Arch. Endocrinol. Metab. 2016, 60, 152–162. [Google Scholar] [CrossRef]
- Haluzik, M.; Parizkova, J.; Haluzik, M. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol. Res. 2004, 53, 123–130. [Google Scholar] [PubMed]
- Beylot, M.; Pinteur, C.; Peroni, O. Expression of the adiponectin receptors AdipoR1 and AdipoR2 in lean rats and in obese Zucker rats. Metabolism 2006, 55, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ziemke, F.; Mantzoros, C.S. Adiponectin in insulin resistance: Lessons from translational research. Am. J. Clin. Nutr. 2010, 91, 258S–261S. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Chusney, G.D.; Thomas, S.M.; Burt, D. Plasma interleukin-6, tumour necrosis factor α and blood cytokine production in type 2 diabetes. Life Sci. 2000, 67, 291–300. [Google Scholar] [CrossRef]
- Vettor, R.; Milan, G.; Rossato, M.; Federspil, G. Review article: Adipocytokines and insulin resistance. Aliment. Pharmacol. Ther. 2005, 22, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef]
- Daniele, G.; Mendoza, R.G.; Winnier, D.; Fiorentino, T.; Pengou, Z.; Cornell, J.; Cornell, J.; Andreozzi, F.; Jenkinson, C.; Cersosimo, E. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol. 2014, 51, 123–131. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef]
- Kakizawa, H.; Itoh, M.; Itoh, Y.; Imamura, S.; Ishiwata, Y.; Matsumoto, T.; Yamamoto, K.; Kato, T.; Ono, Y.; Nagata, M.; et al. The relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism 2004, 53, 550–555. [Google Scholar] [CrossRef]
- Murata, T.; Nagai, R.; Ishibashi, T.; Inomata, H.; Ikeda, K.; Horiuchi, S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia 1997, 40, 764–769. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.L.; Wu, H.; Liu, J.Z.; Hu, J.X.; Liao, N.; Peng, J.; Cao, P.P.; Hai, C.X. Antidiabetic effect of oleanolic acid: A promising use of a traditional pharmacological agent. Phytother. Res. 2011, 25, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, T.; Mukwevho, E.; Nkomozepi, P.; Erlwanger, K.H. Neonatal intake of oleanolic acid attenuates the subsequent development of high fructose diet-induced non-alcoholic fatty liver disease in rats. J. Dev. Orig. Health Dis. 2018, 9, 500–510. [Google Scholar] [CrossRef]
- Molepo, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Mukwevho, E. A Study on Neonatal Intake of Oleanolic Acid and Metformin in Rats (Rattus norvegicus) with Metabolic Dysfunction: Implications on Lipid Metabolism and Glucose Transport. Molecules 2018, 23, 2528. [Google Scholar] [CrossRef] [PubMed]
- Musabayane, C.; Bwititi, P.; Ojewole, J. Effects of oral administration of some herbal extracts on food consumption and blood glucose levels in normal and streptozotocin-treated diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 223–228. [Google Scholar] [CrossRef]
- Nyakudya, T.T.; Mukwevho, E.; Erlwanger, K.H. The protective effect of neonatal oral administration of oleanolic acid against the subsequent development of fructose-induced metabolic dysfunction in male and female rats. Nutr. Metab. 2018, 15, 82. [Google Scholar] [CrossRef]
- Patel, S.; Choksi, A.; Pant, R.; Alam, A.; Chattopadhyay, S. Nutritional Programming of Metabolic Syndrome: Role of Nutrients in Shaping the Epigenetics. In Handbook of Nutrition, Diet, and Epigenetics; Springer: Berlin, Germany, 2017; pp. 1–25. [Google Scholar]
- Viollet, B.; Andreelli, F.; Jørgensen, S.B.; Perrin, C.; Flamez, D.; Mu, J.; Wojtaszewski, J.F.; Schuit, F.C.; Birnbaum, M.; Richter, E.; et al. Physiological Role of AMP-Activated ProteinKinase(AMPK): Insights from Knockout Mouse Models. Biochem. Soc. Trans. 2003, 31 Pt 1, 216–219. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, C.; Durand, A.; Vidal, H.; Rieusset, J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008, 34, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Goto, A.; Morita, A.; Deura, K.; Sasaki, S.; Aiba, N.; Shimbo, T.; Terauchi, Y.; Miyachi, M.; Noda, M.; et al. Low-molecular-weight adiponectin and high-molecular-weight adiponectin levels in relation to diabetes. Obesity 2014, 22, 401–407. [Google Scholar] [CrossRef]
- Chabrolle, C.; Tosca, L.; Crochet, S.; Tesseraud, S.; Dupont, J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: Potential role in ovarian steroidogenesis. Domest. Anim. Endocrinol. 2007, 33, 480–487. [Google Scholar] [CrossRef]
- Emanuela, F.; Grazia, M.; Marco, D.R.; Maria Paola, L.; Giorgio, F.; Marco, B. Inflammation as a link between obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef]
- Lim, H.S.; Lip, G.Y.; Blann, A.D. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: Relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 2005, 180, 113–118. [Google Scholar] [CrossRef]
- Mukwevho, E.; Kohn, T.A.; Lang, D.; Nyatia, E.; Smith, J.; Ojuka, E.O. Caffeine induces hyperacetylation of histones at the MEF2 site on the Glut4 promoter and increases MEF2A binding to the site via a CaMK-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E582–E588. [Google Scholar] [CrossRef] [PubMed]
- Kinfe, H.H.; Belay, Y.H.; Joseph, J.S.; Mukwevho, E. Evaluation of the Influence of thiosemicarbazone–triazole hybrids on genes implicated in lipid oxidation and accumulation as potential anti-obesity agents. Bioorg. Med. Chem. Lett. 2013, 23, 5275–5278. [Google Scholar] [CrossRef] [PubMed]
- Mukwevho, E.; Joseph, J.S. Calmodulin dependent protein kinase II activation by exercise regulates saturated & unsaturated fatty acids and improves some metabolic syndrome markers. Life Sci. 2014, 111, 53–61. [Google Scholar]
- Khoza, B.; Khoza, B.S.; Chimuka, L.; Mukwevho, E.; Steenkamp, P.A.; Madal, N.E. The effect of temperature on pressurised hot water extraction of pharmacologically important metabolites as analysed by UPLC-qTOF-MS and PCA. Evid. Based Complement. Altern. Med. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules 2014, 19, 19376–19389. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, A.B.; Ayeles, A.O.; Mukwevho, E.; Masola, B. Antioxidant strategies in the management of diabetic neuropathy. BioMedRes. Int. 2015, 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.S.; Ayeleso, A.O.; Mukwevho, E. Exercise increases hyper-acetylation of histones on the Cis-element of NRF-1 binding to the Mef2a promoter: Implications on type 2 diabetes. Biochem. Biophys. Res. Commun. 2017, 486, 83–87. [Google Scholar] [CrossRef]
- Joseph, J.S.; Ayeleso, A.O.; Mukwevho, E. Role of exercise-induced calmodulin protein kinase (CaMK) II activation in the regulation of omega-6 fatty acids and lipid metabolism genes in rat skeletal muscle. Physiol. Res. 2017, 66, 969–977. [Google Scholar] [PubMed]
- Ayeleso, T.; Ramachela, K.; Mukwevho, E. Aqueous-Methanol Extracts of Orange-Fleshed Sweet Potato (Ipomoea batatas) Ameliorate Oxidative Stress and Modulate Type 2 Diabetes Associated Genes in Insulin Resistant C2C12 Cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef]

| Genes | Forward Primer (F) and Reverse Prime (R) |
|---|---|
| AdipoR1 | F: 3′-AAGCACCGGCAGACAAGAGC-5′ R: 3′-AGGAAGAACCAGCCCATCTG-5′ |
| AdipoR2 | F: 3′-CTGTGTGCTGGGCATTGCAG-5′ R: 3′-AGCCTATCTGCCCTATGGTG-5′ |
| AMPK-α | F: 5′-GGCAAAGTGAAGATTGGAGAACA-3′ R: 5′-AACTGCCACTTTATGGCCTGT C-3′ |
| Glut-4 | F: 5′-GCAGCGAGTGACTGGACCA-3′ R: 5′-CCAGCCACGTTGCATTGTAG-3′ |
| CPT-1 | F: 5′-CGGTTCAAGAATGGCATCATC-3′ R: 5′-TCACACCCACCACCACGAT-3′ |
| TNF-α | F: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ R: 5′-TGGGAGTAGACAAGGTACAACCC-3′ |
| IL-6 | F: 5′-GCCACTGCCTTCCCTACTTCA-3′ R: 5′-GACAGTGCATCATCGCTGTTCA-3′ |
| Actin | F: 5′-GACGAGGCCCAGAGCAAGAGA-3′ R: 5′-GGGTGTTGAAGGTCTCAAACA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matumba, M.G.; Ayeleso, A.O.; Nyakudya, T.; Erlwanger, K.; Chegou, N.N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. https://doi.org/10.3390/nu11020226
Matumba MG, Ayeleso AO, Nyakudya T, Erlwanger K, Chegou NN, Mukwevho E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients. 2019; 11(2):226. https://doi.org/10.3390/nu11020226
Chicago/Turabian StyleMatumba, Mashudu Given, Ademola Olabode Ayeleso, Trevor Nyakudya, Kennedy Erlwanger, Novel N. Chegou, and Emmanuel Mukwevho. 2019. "Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet" Nutrients 11, no. 2: 226. https://doi.org/10.3390/nu11020226
APA StyleMatumba, M. G., Ayeleso, A. O., Nyakudya, T., Erlwanger, K., Chegou, N. N., & Mukwevho, E. (2019). Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients, 11(2), 226. https://doi.org/10.3390/nu11020226





