Evaluation of a Novel Tool for Screening Inadequate Food Intake in Age-Related Macular Degeneration Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Baseline Questionnaire
2.3. Food Frequency Questionnaire
2.4. Short Dietary Questionnaire (SDQ-AMD)
2.5. Statistical Analysis
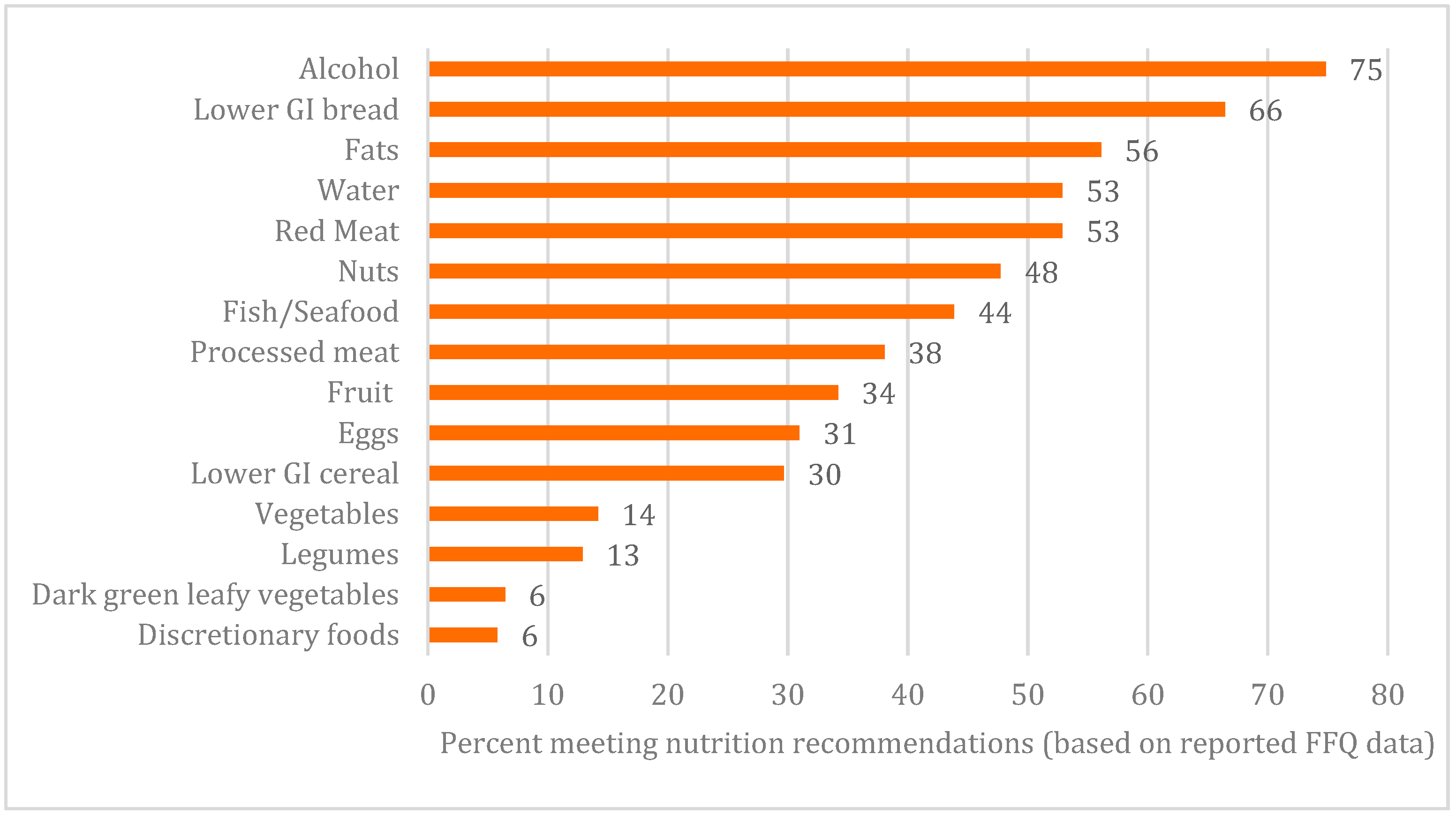
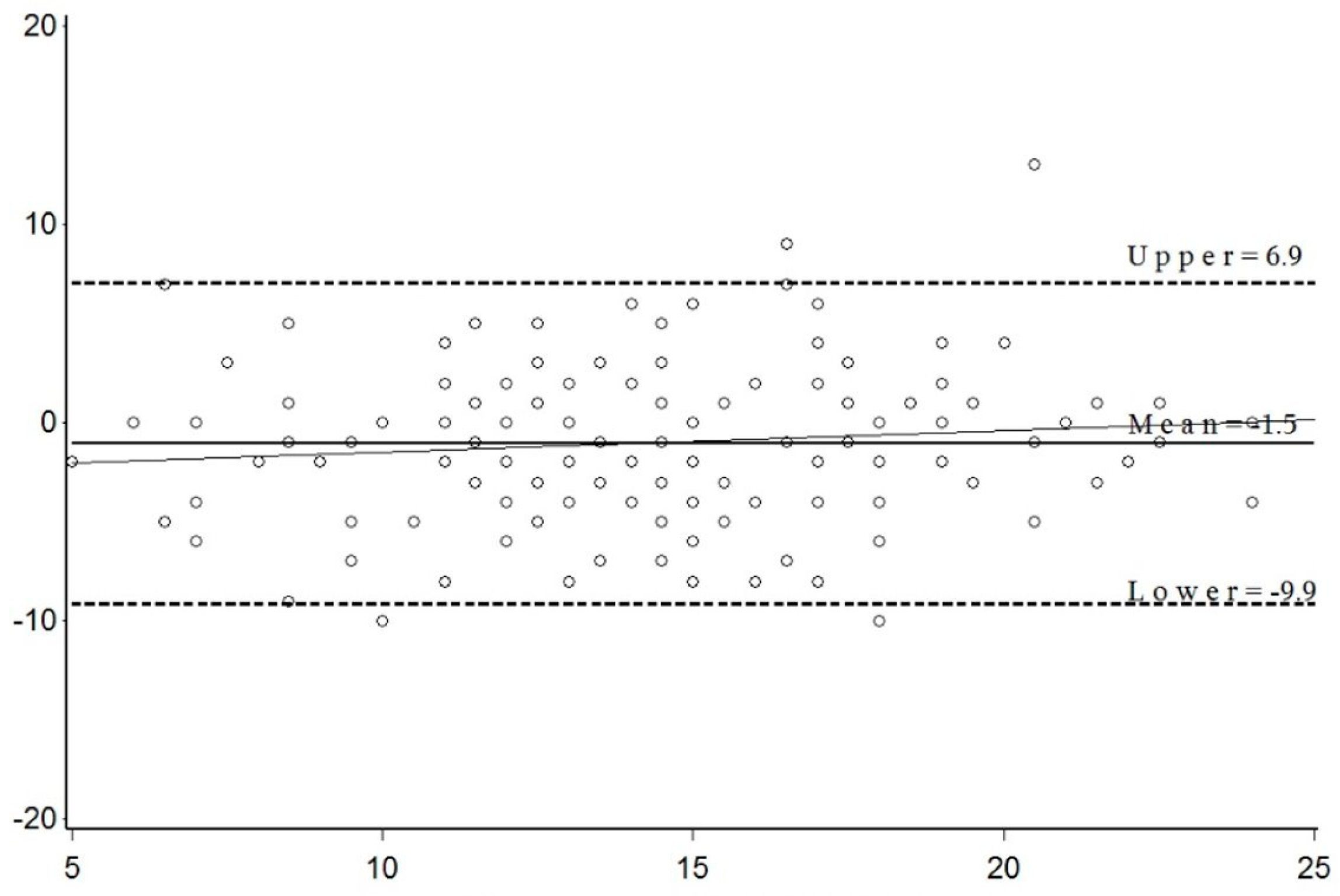
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, e2014009. [Google Scholar] [CrossRef]
- Barclay, W.; Flood, V.M.; Brand-Miller, J.C.; Mitchell, P. Validity of carbohydrate, glycaemic index and glycaemic load data obtained using a semi-quantitative food-frequency questionnaire. Public Health Nutr. 2008, 11. [Google Scholar] [CrossRef]
- Smith, W.; Mitchell, P.; Reay, E.M.; Webb, K.; Harvey, P.W.J. Validity and reproducibility of a self-administered food frequency questionnaire in older people. Aust. N. Z. J. Public Health 1998, 22, 456–463. [Google Scholar] [CrossRef]
- Flood, V.; Smith, W.T.; Webb, K.L.; Mitchell, P. Issues in assessing the validity of nutrient data obtained from a food-frequency questionnaire: Folate and vitamin B12 examples. 2004, 7, 751–756. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. National Health Survey: First Results “Consumption of Fruit, Vegetables, and Sugar Sweetened and Diet Drinks—Australia. Table 12.3 Proportion of Persons”. 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/mf/4364.0.55.001 (accessed on 29 October 2019).
- Radd-Vagenas, S.; Fiatarone Singh, M.A.; Daniel, K.; Noble, Y.; Jain, N.; O’Leary, F.; Mavros, Y.; Heffernan, M.; Meiklejohn, J.; Guerrero, Y.; et al. Validity of the Mediterranean Diet and Culinary Index (MediCul) for Online Assessment of Adherence to the “Traditional” Diet and Aspects of Cuisine in Older Adults. Nutrients 2018, 10, 1913. [Google Scholar] [CrossRef]
- Tanaka, M. Multidisciplinary team approach for elderly patients. Geriatr. Gerontol. Int. 2003, 3, 69–72. [Google Scholar] [CrossRef]
- Tang, D.; Mitchell, P.; Flood, V.; Kifley, A.; Hayes, A.; Liew, G.; Gopinath, B. Dietary intervention in patients with age-related macular degeneration: Protocol for a randomised controlled trial. BMJ Open 2019, 9, e024774. [Google Scholar] [CrossRef]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119. [Google Scholar] [CrossRef]
- Flood, V.M.; Smith, W.; Rochtchina, E.; Wang, J.J.; Mitchell, P. Assembling a nutrient database for a large cohort study: Blue Mountains Eye Study. Food Aust. 2008, 60, 37–40. [Google Scholar]
- Willett, W.C.; Sampson, L.; Browne, M.L.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Speizer, F.E. The use of a self-administered questionnaire to assess diet four years in the past. Am. J. Epidemiol. 1988, 127, 188–199. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Eat for Health Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Food Standards Australia New Zealand. Australian Food Composition Database. 2019. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/afcd/Pages/foodsearch.aspx (accessed on 29 October 2019).
- Marks, K.; Rutishauser, I.H.E.; Riley, M. Monitoring Food Habits in the Australian Population Using Short Questions; Australian Food and Nutrition Monitoring Unit: Canberra, Australia, 2001. [Google Scholar]
- Nunes, S.; Alves, D.; Barreto, P.; Raimundo, M.; Da Luz Cachulo, M.; Farinha, C.; Lains, I.; Rodrigues, J.; Almeida, C.; Ribeiro, L.; et al. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study-Report 4. Nutrition 2018, 51, 6–12. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, H.; Kwon, O.; Chang, N. Associations between fruits, vegetables, vitamin, A.; beta-carotene and flavonol dietary intake, and age-related macular degeneration in elderly women in Korea: The Fifth Korea National Health and Nutrition Examination Survey. Eur. J. Clin. Nutr. 2018, 72, 161–167. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Bringmann, A.; Hollborn, M.; Kohen, L.; Wiedemann, P. Intake of dietary salt and drinking water: Implications for the development of age-related macular degeneration. Mol. Vis. 2016, 22, 1437–1454. [Google Scholar]
- Chan, J.; Knutsen, S.F.; Blix, G.G.; Lee, J.W.; Fraser, G.E. Water, other fluids, and fatal coronary heart disease: The Adventist health study. Am. J. Epidemiol. 2002, 155, 827–833. [Google Scholar] [CrossRef]
- Lieberman, H.R. Hydration and cognition: A critical review and recommendations for future research. J. Am. Coll. Nutr. 2007, 26, 555S–561S. [Google Scholar] [CrossRef]
- Wilson, M.-M.G.; Morley, J.E. Impaired cognitive function and mental performance in mild dehydration. Eur. J. Clin. Nutr. 2003, 2, S24. [Google Scholar] [CrossRef]
- Ministry of Health. Nutrient Reference Values: Water. 2014. Available online: https://www.nrv.gov.au/nutrients/water (accessed on 29 October 2019).
- Chong, E.W.-T.; Simpson, J.A.; Robman, L.D.; Hodge, A.M.; Aung, K.Z.; English, D.R.; Giles, G.G.; Guymer, R.H. Red Meat and Chicken Consumption and Its Association with Age-related Macular Degeneration. Am. J. Epidemiol. 2009, 169, 867–876. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C. and, E.; beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Heart Foundation. Meat, Poultry and Seafood. 2019. Available online: https://www.heartfoundation.org.au/healthy-eating/food-and-nutrition/protein-foods/meat-poultry-and-seafood (accessed on 29 October 2019).
- Panagiotakos, D.; Kalogeropoulos, N.; Pitsavos, C.; Roussinou, G.; Palliou, K.; Chrysohoou, C.; Stefanadis, C. Validation of the MedDietScore via the determination of plasma fatty acids. Int. J. Food Sci. Nutr. 2009, 60, 168–180. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Moreno-Franco, B.; Ordovás, J.M.; Leon, M.; Casasnovas, J.A.; Penalvo, J.L. Design and development of an instrument to measure overall lifestyle habits for epidemiological research: The Mediterranean Lifestyle (MEDLIFE) index. Public Health Nutr. 2015, 18, 959–967. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Ciccarone, E.; Di Castelnuovo, A.; Salcuni, M.; Siani, A.; Giacco, A.; Donati, M.B.; De Gaetano, G.; Capani, F.; Iacoviello, L.; Gendiabe, I. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with Type 2 diabetes. J. Thromb. Haemost. 2003, 1, 1744–1752. [Google Scholar] [CrossRef]
- Goulet, J.; Lamarche, B.; Nadeau, G.; Lemieux, S.; Lemieux, S. Effect of a nutritional intervention promoting the Mediterranean food pattern on plasma lipids, lipoproteins and body weight in healthy French-Canadian women. Atherosclerosis 2003, 170, 115–124. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007, 125, 671–679. [Google Scholar] [CrossRef]
- Chiu, C.J.; Klein, R.; Milton, R.C.; Gensler, G.; Taylor, A. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br. J. Ophthalmol. 2009, 93, 1241–1246. [Google Scholar] [CrossRef]
- Kaushik, S.; Wang, J.J.; Flood, V.; Tan, J.S.L.; Barclay, A.W.; Wong, T.Y.; Brand-Miller, J.; Mitchell, P. Dietary glycemic index and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 1104–1110. [Google Scholar] [CrossRef]
- Chiu, C.-J.; Milton, R.C.; Klein, R.; Gensler, G.; Taylor, A. Dietary carbohydrate and the progression of age-related macular degeneration: A prospective study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2007, 86, 1210–1218. [Google Scholar] [CrossRef]
- Grains and Legumes Nutrition Council. Lifting the Lid on Legumes: The Benefits of Choosing Legumes. 2013. Available online: https://www.glnc.org.au/wp-content/uploads/2011/04/GLN_LiftingtheLid_WEB.pdf (accessed on 29 October 2019).
- Mohn, E.S.; Johnson, E.J. Lutein and Cognition Across the Lifespan. Nutr. Today 2017, 52, 183–189. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and Zeaxanthin-Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Tang, D.; Burlutsky, G.; Flood, V.M.; Mitchell, P. Consumption of eggs and the 15-year incidence of age-related macular degeneration. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Heart Foundation. Eggs. Available online: https://www.heartfoundation.org.au/healthy-eating/food-and-nutrition/protein-foods/eggs (accessed on 29 October 2019).
- Australian Bureau of Statistics. Australian Health Survey: Nutrition First Results—Foods and Nutrients, 2011–12: Discretionary Foods. 2014. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.007main+features12011-12 (accessed on 29 October 2019).
- Adams, M.K.; Chong, E.W.; Williamson, E.; Aung, K.Z.; Makeyeva, G.A.; Giles, G.G.; English, D.R.; Hopper, J.; Guymer, R.H.; Baird, P.N.; et al. 20/20—Alcohol and age-related macular degeneration: The Melbourne Collaborative Cohort Study. Am. J. Epidemiol. 2012, 176, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.; Kreis, A.J.; Wong, T.Y.; Simpson, J.A.; Guymer, R.H. Alcohol consumption and the risk of age-related macular degeneration: A systematic review and meta-analysis. Am. J. Ophthalmol. 2008, 145, 707–715. [Google Scholar] [CrossRef]
- Fraser-Bell, S.; Wu, J.; Klein, R.; Azen, S.P.; Varma, R. Smoking, alcohol intake, estrogen use, and age-related macular degeneration in Latinos: The Los Angeles Latino Eye Study. Am. J. Ophthalmol. 2006, 141, 79–87. [Google Scholar] [CrossRef]
- Australian Government Department of Health. How Much Alcohol Is Safe to Drink? 2019. Available online: https://www.health.gov.au/health-topics/alcohol/about-alcohol/how-much-alcohol-is-safe-to-drink. (accessed on 29 October 2019).
- Cougnard-Grégoire, A.; Merle, B.M.J.; Korobelnik, J.-F.; Rougier, M.-B.; Delyfer, M.-N.; Le Goff, M.; Samieri, C.; Dartigues, J.-F.; Delcourt, C. Olive Oil Consumption and Age-Related Macular Degeneration: The Alienor Study. PLoS ONE 2016, 11, e0160240. [Google Scholar] [CrossRef]
- Nidhi, B.; Mamatha, B.S.; Baskaran, V. Olive oil improves the intestinal absorption and bioavailability of lutein in lutein-deficient mice. Eur. J. Nutr. 2014, 53, 117–126. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Nicklett, E.J.; Kadell, A.R. Fruit and vegetable intake among older adults: A scoping review. Maturitas 2013, 75, 305–312. [Google Scholar] [CrossRef]
- U.S. Department for Agriculture and Center for Nutrition Policy and Promotion. Fruit and vegetable consumption by older Americans. Nutrition Insight 2007, 34, 143–156. [Google Scholar]
- Drewnowski, A.; Rehm, C.D. Vegetable Cost Metrics Show That Potatoes and Beans Provide Most Nutrients Per Penny. PLoS ONE 2013, 8, e63277. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.U.; Pilli, S.; Telander, D.G.; Morse, L.S.; Park, S.S. Survey of patients with age-related macular degeneration: Knowledge and adherence to recommendations. Can. J. Ophthalmol. 2013, 48, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.T.; Goggin, M. Awareness of and compliance with recommended dietary supplement among age-related macular degeneration patients. Clin. Exp. Ophthalmol. 2006, 34, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hochstetler, B.S.; Scott, I.U.; Kunselman, A.R.; Thompson, K.; Zerfoss, E. Adherence to recommendations of the age-related eye disease study in patients with age-related macular degeneration. Retina 2010, 30, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Martin Bland, J.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Radd-Vagenas, S.; Fiatarone Singh, M.A.; Inskip, M.; Mavros, Y.; Gates, N.; Wilson, G.C.; Jain, N.; Meiklejohn, J.; Brodaty, H.; Wen, W.; et al. Reliability and validity of a Mediterranean diet and culinary index (MediCul) tool in an older population with mild cognitive impairment. Br. J. Nutr. 2018, 120, 1189–1200. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Review of Ophthalmology. Calcium Intake and Age-Related Macular Degeneration. 2019. Available online: https://www.reviewofophthalmology.com/article/calcium-intake-and-agerelated-macular-degeneration (accessed on 29 October 2019).
- Gopinath, B.; Flood, V.M.; Louie, J.C.Y.; Wang, J.J.; Burlutsky, G.; Rochtchina, E.; Mitchell, P. Consumption of dairy products and the 15-year incidence of age-related macular degeneration. Br. J. Nutr. 2014, 111, 1673–1679. [Google Scholar] [CrossRef]
- Ali, Z.C.; Silvioli, R.; Rajai, A.; Aslam, T.M. Feasibility of Use of a Mobile Application for Nutrition Assessment Pertinent to Age-Related Macular Degeneration (MANAGER2). Transl. Vis. Sci. Technol. 2017, 6. [Google Scholar] [CrossRef]
- Downie, L.E.; Keller, P.R. The Self-Reported Clinical Practice Behaviors of Australian Optometrists as Related to Smoking, Diet and Nutritional Supplementation. PLoS ONE 2015, 10, e0124533. [Google Scholar] [CrossRef] [PubMed]
- Martin, L. Targeting modifiable risk factors in age-related macular degeneration in optometric practice in Sweden. Clin. Optom. 2017, 9, 77–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
| <80 Years | ≥80 Years | |
|---|---|---|
| Age (years) | 78 (5.7) | 86 (3.5) |
| Females | 53 (60.9) | 36 (52.9) |
| Caucasian ethnicity | 66 (79.5) | 61 (93.8) |
| Lives alone (%) | 13 (14.9) | 23 (33.8) |
| History of other medical conditions (%): | ||
| Heart attack (%) | 7 (8.0) | 10 (14.7) |
| Angina (%) | 4 (4.6) | 2 (2.9) |
| Other cardiac (%) | 21 (24) | 21 (30.9) |
| Stroke/Transient Ischemic Attack (%) | 3 (3.4) | 12 (17.6) |
| High blood pressure (%) | 56 (64.4) | 42 (61.8) |
| High cholesterol (%) | 42 (48.3) | 37 (54.4) |
| Diabetes/pre-diabetes (%) | 21 (24.1) | 15 (22.1) |
| Kidney disease (%) | 6 (6.9) | 4 (5.9) |
| Arthritis (%) | 40 (46.0) | 41 (60.3) |
| Other illness or major operation (%) | 54 (62.1) | 45 (66.2) |
| History of cataracts (%) | 52 (59.8) | 63 (92.6) |
| History of glaucoma (%) | 9 (10.3) | 14 (20.6) |
| Type of AMD by eyes | N = 140 | N = 114 |
| No AMD (%) | 28 (20) | 22 (19) |
| Early AMD (%) | 6 (4.3) | 0 (0.0) |
| Dry AMD (%) | 24 (17) | 8 (7.0) |
| Wet AMD (%) | 70 (50) | 69 (61) |
| Dry and Wet (%) | 12 (8.6) | 15 (13) |
| Eyes with wet AMD, receiving treatment with | N = 79 | N = 80 |
| Eylea (%) | 54 (68) | 52 (65) |
| Lucentis (%) | 24 (30) | 28 (35) |
| Avastin (%) | 1 (1.3) | 0 (0.0) |
| Food Group | Age (years) | FFQ Daily Mean Intake (SD) | SDQ-AMD Daily Mean Intake (SD) | Correlation Coefficient * | p-Value ** |
|---|---|---|---|---|---|
| Fruits | <80 | 1.79 (1.72) | 1.84 (1.06) | 0.19 | 0.08 |
| ≥80 | 1.87 (2.80) | 1.79 (1.07) | 0.33 | 0.01 | |
| Vegetables | <80 | 3.20 (1.92) | 2.25 (1.32) | 0.15 | 0.18 |
| ≥80 | 3.09 (2.07) | 1.91 (1.10) | 0.09 | 0.45 | |
| Dark green leafy | <80 | 0.11 (0.15) | 0.16 (0.24) | 0.18 | 0.09 |
| vegetables | ≥80 | 0.12 (0.17) | 0.16 (0.29) | 0.31 | <0.01 |
| Red meat | <80 | 0.37 (0.35) | 0.28 (0.22) | 0.16 | 0.13 |
| ≥80 | 0.37 (0.27) | 0.36 (0.23) | 0.31 | <0.01 | |
| Processed meat | <80 | 0.32 (0.35) | 0.17 (0.23) | 0.28 | <0.01 |
| ≥80 | 0.37 (0.40) | 0.19 (0.23) | 0.01 | 0.93 | |
| White Meat | <80 | 0.29 (0.35) | 0.26 (0.18) | 0.29 | <0.01 |
| ≥80 | 0.25 (0.22) | 0.23 (0.19) | 0.14 | 0.24 | |
| Fish/Seafood | <80 | 0.33 (0.28) | 0.28 (0.29) | 0.35 | <0.001 |
| ≥80 | 0.38 (0.35) | 0.24 (0.17) | 0.58 | <0.0001 | |
| Eggs | <80 | 0.35 (0.25) | 0.46 (0.33) | 0.32 | <0.01 |
| ≥80 | 0.29 (0.28) | 0.37 (0.27) | 0.50 | <0.0001 | |
| Legumes | <80 | 0.14 (0.24) | 0.11 (0.17) | 0.27 | 0.01 |
| ≥80 | 0.11 (0.18) | 0.10 (0.14) | 0.07 | 0.57 | |
| Nuts | <80 | 0.65 (1.00) | 0.55 (0.57) | 0.48 | <0.0001 |
| ≥80 | 0.62 (1.02) | 0.37 (0.49) | 0.54 | <0.0001 | |
| Low GI | <80 | 1.51 (1.50) | 1.01 (0.86) | 0.27 | 0.01 |
| ≥80 | 1.34 (1.02) | 1.16 (0.74) | 0.50 | <0.0001 | |
| High GI | <80 | 0.42 (0.54) | 0.30 (0.49) | 0.24 | 0.02 |
| ≥80 | 0.56 (0.72) | 0.36 (0.56) | 0.36 | <0.01 | |
| Biscuits and cakes, ice cream, sugary drinks, takeaway, processed potato | <80 | 2.01 (1.47) | 1.10 (0.82) | 0.43 | <0.0001 |
| ≥80 | 2.47 (1.63) | 1.34 (1.18) | 0.26 | 0.03 | |
| Water | <80 | 5.58 (2.19) | 4.86 (2.58) | 0.11 | 0.32 |
| ≥80 | 5.71 (1.83) | 4.31 (2.06) | 0.46 | <0.0001 | |
| Alcohol | <80 | 0.89 (1.69) | 0.65 (1.40) | 0.84 | <0.0001 |
| ≥80 | 0.69 (1.01) | 0.47 (0.82) | 0.81 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, D.; Mitchell, P.; Liew, G.; Burlutsky, G.; Flood, V.; Gopinath, B. Evaluation of a Novel Tool for Screening Inadequate Food Intake in Age-Related Macular Degeneration Patients. Nutrients 2019, 11, 3031. https://doi.org/10.3390/nu11123031
Tang D, Mitchell P, Liew G, Burlutsky G, Flood V, Gopinath B. Evaluation of a Novel Tool for Screening Inadequate Food Intake in Age-Related Macular Degeneration Patients. Nutrients. 2019; 11(12):3031. https://doi.org/10.3390/nu11123031
Chicago/Turabian StyleTang, Diana, Paul Mitchell, Gerald Liew, George Burlutsky, Victoria Flood, and Bamini Gopinath. 2019. "Evaluation of a Novel Tool for Screening Inadequate Food Intake in Age-Related Macular Degeneration Patients" Nutrients 11, no. 12: 3031. https://doi.org/10.3390/nu11123031
APA StyleTang, D., Mitchell, P., Liew, G., Burlutsky, G., Flood, V., & Gopinath, B. (2019). Evaluation of a Novel Tool for Screening Inadequate Food Intake in Age-Related Macular Degeneration Patients. Nutrients, 11(12), 3031. https://doi.org/10.3390/nu11123031






