Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Selection of Plasma Fatty Acids and Instrumental Variables
2.3. Outcome Data Sources
2.4. Statistical Analysis
2.5. Pleiotropy Assessment
3. Results
3.1. Plasma FAs and CVDs
3.2. FADS1 and CVD
3.3. Pleotropic Associations
4. Discussion
4.1. Plasma FAs and Ischemic Stroke
4.2. Plasma FAs and Venous Thromboembolism
4.3. Plasma FAs and other CVDs
4.4. FADS1 and CVD
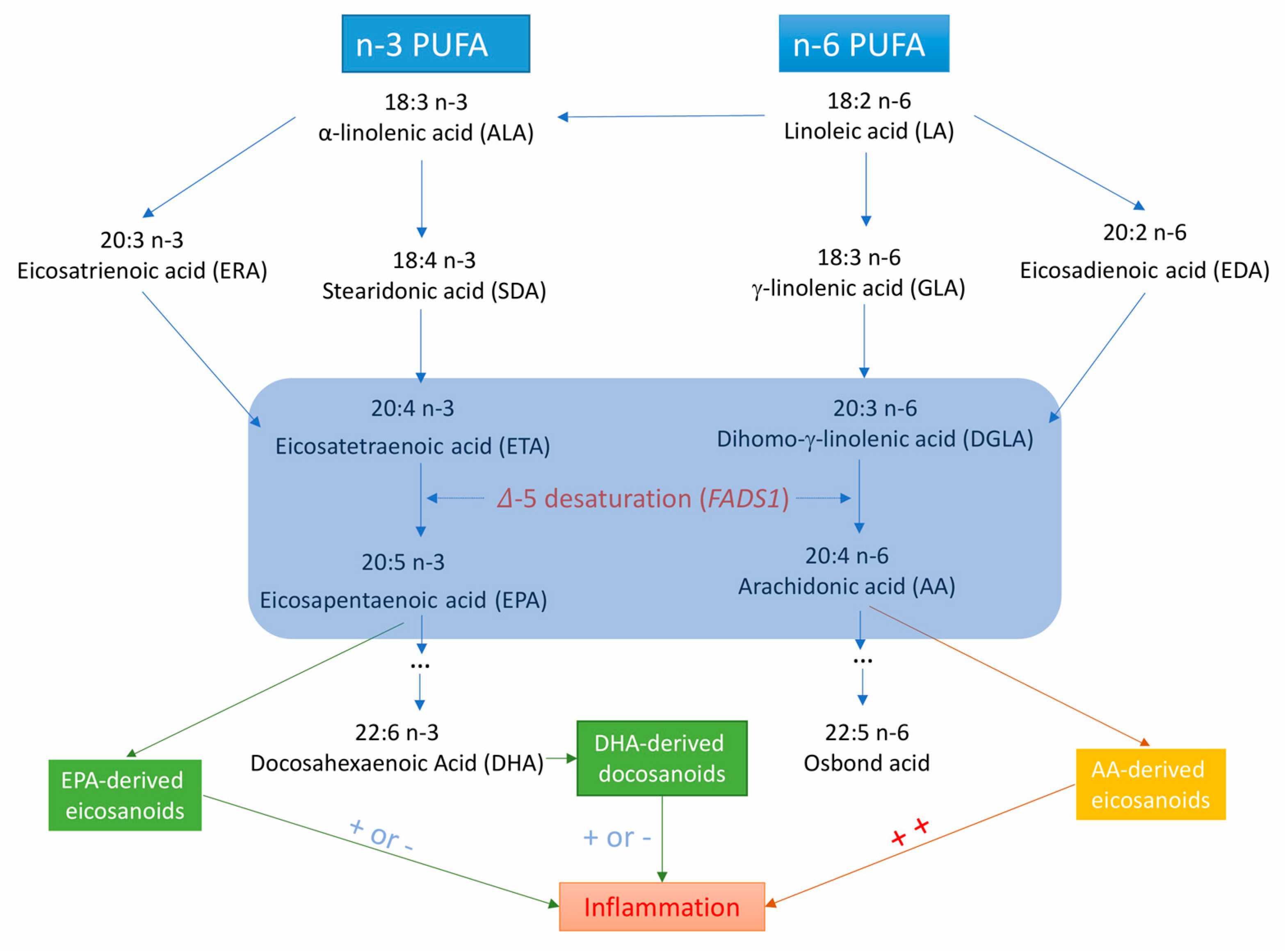
4.5. Possible Mechanisms
4.6. Strengths and Limiations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bansilal, S.; Castellano, J.M.; Fuster, V. Global burden of CVD: Focus on secondary prevention of cardiovascular disease. Int. J. Cardiol. 2015, 201 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013, The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries; 1990–2017, a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary; circulating; and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B.; et al. Association between fish consumption; long chain omega 3 fatty acids; and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 2012, 345, e6698. [Google Scholar] [CrossRef]
- Isaksen, T.; Evensen, L.H.; Johnsen, S.H.; Jacobsen, B.K.; Hindberg, K.; Brækkan, S.K.; Hansen, J.B. Dietary intake of marine n-3 polyunsaturated fatty acids and future risk of venous thromboembolism. Res. Pract. Thromb. Haemost. 2018, 13, 59–69. [Google Scholar] [CrossRef]
- Tosi, F.; Sartori, F.; Guarini, P.; Olivieri, O.; Martinelli, N. Delta-5 and delta-6 desaturases: Crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv. Exp. Med. Biol. 2014, 824, 61–81. [Google Scholar]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Aros, F.; Lamuela-Raventós, R.M.; et al. Plasma fatty acid composition; estimated desaturase activities; and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kröger, J.; Zietemann, V.; Enzenbach, C.; Weikert, C.; Jansen, E.H.; Döring, F.; Joost, H.G.; Boeing, H.; Schulze, M.B. Erythrocyte membrane phospholipid fatty acids; desaturase activity; and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 2011, 93, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Sundstrom, J.; Vessby, B.; Cederholm, T.; Riserus, U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am. J. Clin. Nutr. 2008, 88, 203–209. [Google Scholar] [CrossRef]
- Lu, Y.; Vaarhorst, A.; Merry, A.H.; Dollé, M.E.; Hovenier, R.; Imholz, S.; Schouten, L.J.; Heijmans, B.T.; Müller, M.; Slagboom, P.E.; et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS ONE 2012, 7, e41681. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.C.; Bhattacharya, S.; et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef]
- Guan, W.; Steffen, B.T.; Lemaitre, R.N.; Wu, J.H.Y.; Tanaka, T.; Manichaikul, A.; Foy, M.; Rich, S.S.; Wang, L.; Nettleton, J.A.; et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 2014, 7, 321–331. [Google Scholar] [CrossRef]
- Wu, J.H.; Lemaitre, R.N.; Manichaikul, A.; Guan, W.; Tanaka, T.; Foy, M.; Kabagambe, E.K.; Djousse, L.; Siscovick, D.; Fretts, A.M.; et al. Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: Results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ. Cardiovasc. Genet. 2013, 6, 171–183. [Google Scholar] [CrossRef]
- Yamagishi, K.; Nettleton, J.A.; Folsom, A.R. Plasma fatty acid composition and incident heart failure in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 965–974. [Google Scholar] [CrossRef]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bokor, S.; Dumont, J.; Spinneker, A.; Gonzalez-Gross, M.; Nova, E.; Widhalm, K.; Moschonis, G.; Stehle, P.; Amouyel, P.; De Henauw, S.; et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J. Lipid Res. 2010, 51, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Saleheen, D.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A comprehensive 1;000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar] [PubMed]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Chaffin, M.D.; Weng, L.C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018, 50, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2, an expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4983. [Google Scholar] [CrossRef]
- Bork, C.S.; Veno, S.K.; Lundbye-Christensen, S.; Jakobsen, M.U.; Tjonneland, A.; Calder, P.C.; Overvad, K.; Schmidt, E.B. Adipose tissue content of alpha-linolenic acid and the risk of ischemic stroke and ischemic stroke subtypes: A Danish case-cohort study. PLoS ONE 2018, 13, e0198927. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; de Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef]
- Veno, S.K.; Bork, C.S.; Jakobsen, M.U.; Lundbye-Christensen, S.; Bach, F.W.; Overvad, K.; Schmidt, E.B. Linoleic Acid in Adipose Tissue and Development of Ischemic Stroke: A Danish Case-Cohort Study. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H.; Yatsuya, H.; Tanabe, N.; Date, C.; Kikuchi, S.; Yamamoto, A.; Inaba, Y.; Tamakoshi, A.; JACC Study Group. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am. J. Clin. Nutr. 2010, 92, 759–765. [Google Scholar] [PubMed]
- Yamagishi, K.; Iso, H.; Kokubo, Y.; Saito, I.; Yatsuya, H.; Ishihara, J.; Inoue, M.; Tsugane, S.; JPHC Study Group. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC Study. Eur. Heart J. 2013, 34, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, B.; Sundstrom, J.; Arnlov, J.; Terent, A.; Vessby, B.; Zethelius, B.; Lind, L. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: A community-based study with long-term follow-up. Stroke 2006, 37, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Folsom, A.R.; Steffen, L.M. Plasma fatty acid composition and incident ischemic stroke in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc. Dis. 2013, 36, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yaemsiri, S.; Sen, S.; Tinker, L.F.; Robinson, W.R.; Evans, R.W.; Rosamond, W.; Wasserthiel-Smoller, S.; He, K. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke 2013, 44, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Shao, W. Monounsaturated Fatty Acid Intake and Stroke Risk: A Meta-analysis of Prospective Cohort Studies. J. Stroke Cerebrovasc. Dis. 2016, 25, 1326–1334. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Lee, K.W.; Blann, A.D.; Lip, G.Y. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb. Res. 2006, 118, 305–312. [Google Scholar] [CrossRef]
- Din, J.N.; Newby, D.E.; Flapan, A.D. Omega 3 fatty acids and cardiovascular disease—Fishing for a natural treatment. BMJ 2004, 328, 30. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.D.; Iversen, A.M.; Schmidt, E.B. n-3 polyunsaturated fatty acids and coronary thrombosis. Lipids 2001, 36, S79–S82. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Isaksen, T.; Evensen, L.H.; Brækkan, S.K.; Hansen, J.B. Dietary Intake of Marine Polyunsaturated n-3 Fatty Acids and Risk of Recurrent Venous Thromboembolism. Thromb. Haemost. 2019. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kudo, N.; Koyama, M.; Kawashima, Y. Effects of dehydroepiandrosterone on oleic acid accumulation in rat liver. Biochem. Pharmacol. 2003, 65, 1583–1591. [Google Scholar] [CrossRef]
- Yu, S.; Derr, J.; Etherton, T.D.; Kris-Etherton, P.M. Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am. J. Clin. Nutr. 1995, 61, 1129–1139. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Mariani, J.; Doval, H.C.; Nul, D.; Varini, S.; Grancelli, H.; Ferrante, D.; Tognoni, G.; Macchia, A. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: Updated systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2013, 2, e005033. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Marchioli, R.; Macchia, A.; Silletta, M.G.; Ferrazzi, P.; Gardner, T.J.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; et al. Fish oil and postoperative atrial fibrillation: The Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation (OPERA) randomized trial. JAMA 2012, 308, 2001–2011. [Google Scholar] [CrossRef]
- Plunde, O.; Larsson, S.C.; Artiach, G.; Carracedo, M.; Franco-Cereceda, A.; Eriksson, P. FADS1 genotype predicts aortic valve FADS mRNA expression; alters valvular omega-3 fatty acid content; and associates with aortic valve calcification. 2019; submitted for publication. [Google Scholar]
- Kwak, J.H.; Paik, J.K.; Kim, O.Y.; Jang, Y.; Lee, S.H.; Ordovas, J.M.; Lee, J.H. FADS gene polymorphisms in Koreans: Association with omega6 polyunsaturated fatty acids in serum phospholipids; lipid peroxides; and coronary artery disease. Atherosclerosis 2011, 214, 94–100. [Google Scholar] [CrossRef]
- Li, S.W.; Lin, K.; Ma, P.; Zhang, Z.L.; Zhou, Y.D.; Lu, S.Y.; Zhou, X.; Liu, S.M. FADS gene polymorphisms confer the risk of coronary artery disease in a Chinese Han population through the altered desaturase activities: Based on high-resolution melting analysis. PLoS ONE 2013, 8, e55869. [Google Scholar] [CrossRef]
- Matthan, N.R.; Ooi, E.M.; Van Horn, L.; Neuhouser, M.L.; Woodman, R.; Lichtenstein, A.H. Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: A nested case-control study within the Women’s Health Initiative observational study. J. Am. Heart Assoc. 2014, 3, e000764. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Sun, L.; Ye, L.; Shi, J.; Zhou, L.; Yang, J.; Du, B.; Song, Z.; Yu, Y.; Xie, L. A case-control study between the gene polymorphisms of polyunsaturated fatty acids metabolic rate-limiting enzymes and coronary artery disease in a Chinese Han population. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, R.; Kurl, S.; Tuomainen, T.P.; Virtanen, J.K. Associations of estimated Delta-5-desaturase and Delta-6-desaturase activities with stroke risk factors and risk of stroke: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2017, 117, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, S.; Ericson, U.; Gullberg, B.; Hedblad, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in FADS1 has little effect on the association between dietary PUFA intake and cardiovascular disease. J. Nutr. 2014, 144, 1356–1363. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, R.X.; Cao, X.L.; Wu, D.F.; Chen, W.X.; Zhou, Y.J. Association of two polymorphisms in the FADS1/FADS2 gene cluster and the risk of coronary artery disease and ischemic stroke. Int. J. Clin. Exp. Pathol. 2015, 8, 7318–7331. [Google Scholar]
- Christensen, J.H. Omega-3 polyunsaturated Fatty acids and heart rate variability. Front. Physiol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Fernandez, ML.; West, KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dessi, M.; Noce, A.; Bertucci, P.; Manca di Villahermosa, S.; Zenobi, R.; Castagnola, V.; Addessi, E.; Di Daniele, N. Atherosclerosis; dyslipidemia; and inflammation: The significant role of polyunsaturated Fatty acids. ISRN Inflamm. 2013, 2013, 191823. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Tysseling, K.A.; Rice, J.; Pham, M.; Haji, L.; Gallis, B.M.; Baas, A.S.; Paramsothy, P.; Giachelli, C.M.; Corson, M.A.; et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
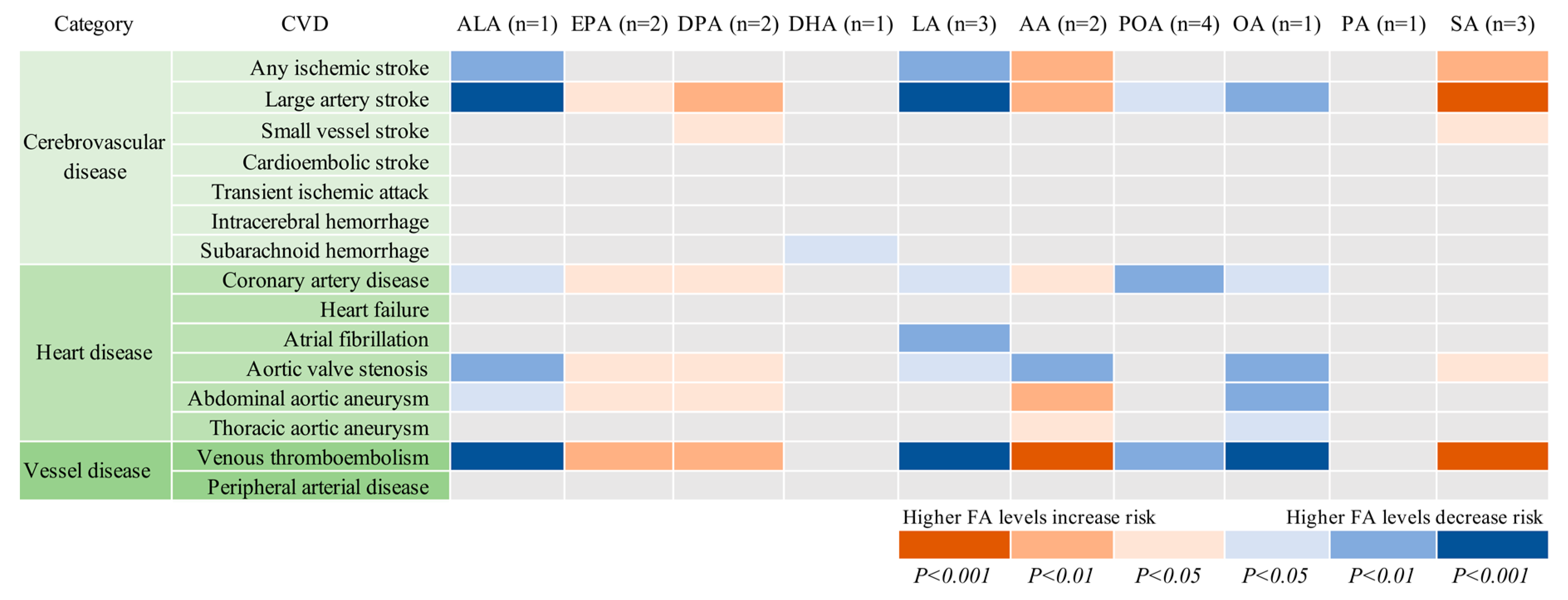

| CVD | Cases | Dataset | ALA (n = 1 SNP) | EPA (n = 2 SNPs) | DPA (n = 2 SNPs) | DHA (n = 1 SNP) | LA (n = 3 SNPs) | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Cerebrovascular disease | ||||||||||||
| Any ischemic stroke | 60 341 | MEGASTROKE | 0.93 (0.89, 0.98) | 0.002 | 1.06 (0.99, 1.13) | 0.081 | 1.04 (1.00, 1.07) | 0.060 | 1.11 (0.96, 1.27) | 0.153 | 0.96 (0.93, 0.99) | 0.006 |
| Large artery stroke | 6688 | MEGASTROKE | 0.82 (0.73, 0.91) | 2.11×10−4 | 1.17 (1.01, 1.36) | 0.035 | 1.13 (1.03, 1.23) | 0.007 | 1.28 (0.92, 1.78) | 0.142 | 0.86 (0.80, 0.93) | 8.61×10−5 |
| Small vessel stroke | 11 710 | MEGASTROKE | 0.94 (0.86, 1.03) | 0.203 | 1.13 (1.00, 1.27) | 0.060 | 1.07 (1.00, 1.15) | 0.084 | 0.85 (0.64, 1.12) | 0.240 | 0.99 (0.93, 1.05) | 0.746 |
| Cardioembolic stroke | 9006 | MEGASTROKE | 0.93 (0.85, 1.02) | 0.141 | 1.04 (0.91, 1.18) | 0.567 | 1.04 (0.97, 1.12) | 0.284 | 1.05 (0.80, 1.40) | 0.715 | 0.95 (0.89, 1.02) | 0.149 |
| Transient ischemic attack | 3962 | UK Biobank | 0.92 (0.82, 1.03) | 0.158 | 1.12 (0.95, 1.32) | 0.174 | 1.05 (0.96, 1.15) | 0.317 | 1.09 (0,76, 1.57) | 0.631 | 0.96 (0.88, 1.04) | 0.297 |
| Intracerebral hemorrhage | 1064 | UK Biobank | 1.05 (0.84, 1.32) | 0.652 | 0.90 (0.66, 1.23) | 0.506 | 1.00 (0.84, 1.19) | 0.993 | 0.74 (0.37, 1.48) | 0.387 | 1.03 (0.88, 1.20) | 0.737 |
| Subarachnoid hemorrhage | 1084 | UK Biobank | 1.06 (0.85, 1.33) | 0.599 | 1.07 (0.79, 1.46) | 0.668 | 1.06 (0.89, 1.26) | 0.549 | 0.47 (0.23, 0.93) | 0.030 * | 1.08 (0.93, 1.27) | 0.317 |
| Heart disease | ||||||||||||
| Coronary artery disease | 60 801 | CARDIoGRAMplusC4D | 0.95 (0.90, 0.99) | 0.027 | 1.08 (1.01, 1.15) | 0.038 | 1.04 (1.00, 1.08) | 0.045 | 1.00 (0.86, 1.16) | 1.000 | 0.96 (0.92, 0.99) | 0.011 |
| Heart failure | 6712 | UK Biobank | 1.03 (0.94, 1.13) | 0.533 | 1.01 (0.89, 1.15) | 0.866 | 0.99 (0.92, 1.06) | 0.762 | 0.90 (0.68, 1.19) | 0.463 | 1.02 (0.96, 1.08) | 0.604 |
| Atrial fibrillation | 65 446 | AFGen | 0.97 (0.94, 1.01) | 0.158 | 1.05 (0.99, 1.10) | 0.085 | 1.02 (0.99, 1.05) | 0.265 | 0.99 (0.88, 1.11) | 0.813 | 0.97 (0.94, 0.99) | 0.009 |
| Aortic valve stenosis | 2244 | UK Biobank | 0.81 (0.69, 0.94) | 0.006 | 1.32 (1.07, 1.64) | 0.011 | 1.17 (1.04, 1.33) | 0.011 | 0.93 (0.58, 1.51) | 0.779 | 0.88 (0.79, 0.98) | 0.024 |
| Abdominal aortic aneurysm | 1094 | UK Biobank | 0.78 (0.62, 0.97) | 0.025 | 1.41 (1.04, 1.92) | 0.027 | 1.26 (1.06, 1.50) | 0.010 | 0.60 (0.30, 1.19) | 0.140 | 0.87 (0.74, 1.02) | 0.077 |
| Thoracic aortic aneurysm | 347 | UK Biobank | 0.85 (0.57, 1.25) | 0.406 | 1.04 (0.60, 1.79) | 0.895 | 1.05 (0.77, 1.43) | 0.761 | 1.52 (0.45, 5.14) | 0.504 | 0.95 (0.72, 1.25) | 0.708 |
| Vessel disease | ||||||||||||
| Peripheral arterial disease | 3415 | UK Biobank | 0.90 (0.79, 1.02) | 0.096 | 1.18 (1.00, 1.41) | 0.058 | 1.09 (0.99, 1.21) | 0.085 | 0.93 (0.63, 1.37) | 0.696 | 0.93 (0.85, 1.01) | 0.087 |
| Venous thromboembolism | 15 602 | UK Biobank | 0.88 (0.82, 0.93) | 4.39×10−5 | 1.13 (1.03, 1.23) | 0.007 | 1.08 (1.03, 1.13) | 0.003 | 1.14 (0.93, 1.38) | 0.202 | 0.92 (0.88, 0.96) | 1.95×10−4 |
| CVD | Cases | Dataset | AA (n = 2 SNPs) | POA (n = 4 SNPs) | OA (n = 1 SNP) | PA (n = 1 SNP) | SA (n = 3 SNPs) | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |||
| Cerebrovascular disease | ||||||||||||
| Any ischemic stroke | 60 341 | MEGASTROKE | 1.03 (1.01, 1.06) | 0.002 | 0.97 (0.90, 1.05) | 0.422 | 0.87 (0.80, 0.95) | 0.002 | 0.90 (0.77, 1.05) | 0.164 | 1.12 (1.04, 1.21) | 0.003 |
| Large artery stroke | 6688 | MEGASTROKE | 1.10 (1.04, 1.15) | 3.34×10−4 | 0.82 (0.68, 0.98) | 0.031 | 0.68 (0.55, 0.85) | 5.34×10−4 | 0.90 (0.62, 1.32) | 0.591 | 1.35 (1.12, 1.61) | 0.001 |
| Small vessel stroke | 11 710 | MEGASTROKE | 1.03 (0.99, 1.08) | 0.136 | 0.95 (0.84, 1.09) | 0.464 | 0.89 (0.75, 1.06) | 0.196 | 0.86 (0.62, 1.18) | 0.345 | 1.19 (1.03, 1.39) | 0.021 |
| Cardioembolic stroke | 9006 | MEGASTROKE | 1.04 (0.99, 1.08) | 0.099 | 0.90 (0.76, 1.06) | 0.210 | 0.84 (0.69, 1.01) | 0.057 | 0.98 (0.71, 1.34) | 0.874 | 1.08 (0.92, 1.26) | 0.344 |
| Transient ischemic attack | 3962 | UK Biobank | 1.04 (0.99, 1.10) | 0.144 | 0.96 (0.77, 1.19) | 0.687 | 0.84 (0.66, 1.06) | 0.139 | 1.05 (0.70, 1.58) | 0.805 | 1.11 (0.91, 1.36) | 0.285 |
| Intracerebral hemorrhage | 1064 | UK Biobank | 0.97 (0.88, 1.08) | 0.605 | 0.88 (0.59, 1.32) | 0.526 | 1.15 (0.73, 1.82) | 0.535 | 1.32 (0.61, 2.89) | 0.482 | 0.81 (0.56, 1.19) | 0.281 |
| Subarachnoid hemorrhage | 1084 | UK Biobank | 0.98 (0.88, 1.08) | 0.673 | 1.18 (0.78, 1.77) | 0.441 | 1.20 (0.76, 1.87) | 0.437 | 0.78 (0.36, 1.69) | 0.528 | 1.19 (0.82, 1.73) | 0.372 |
| Heart disease | ||||||||||||
| Coronary artery disease | 60 801 | CARDIoGRAMplusC4D | 1.02 (1.00, 1.05) | 0.042 | 0.89 (0.82, 0.97) | 0.008 | 0.90 (0.81, 0.99) | 0.036 | 1.03 (0.88, 1.22) | 0.691 | 1.02 (0.94, 1.11) | 0.620 |
| Heart failure | 6712 | UK Biobank | 0.99 (0.95, 1.03) | 0.535 | 0.89 (0.76, 1.05) | 0.180 | 1.06 (0.89, 1.27) | 0.524 | 0.93 (0.68, 1.27) | 0.631 | 1.02 (0.87, 1.18) | 0.825 |
| Atrial fibrillation | 65 446 | AFGen | 1.01 (1.00, 1.03) | 0.180 | 1.04 (0.97, 1.11) | 0.262 | 0.95 (0.88, 1.02) | 0.137 | 1.12 (0.98, 1.28) | 0.087 | 1.01 (0.95, 1.08) | 0.748 |
| Aortic valve stenosis | 2244 | UK Biobank | 1.11 (1.03, 1.19) | 0.004 | 0.80 (0.60, 1.07) | 0.128 | 0.66 (0.48, 0.90) | 0.008 | 0.91 (0.53, 1.55) | 0.724 | 1.33 (1.03, 1.73) | 0.031 |
| Abdominal aortic aneurysm | 1094 | UK Biobank | 1.13 (1.02, 1.25) | 0.022 | 0.88 (0.59, 1.32) | 0.537 | 0.61 (0.39, 0.96) | 0.032 | 1.39 (0.64, 2.99) | 0.405 | 1.23 (0.85, 1.78) | 0.282 |
| Thoracic aortic aneurysm | 347 | UK Biobank | 1.08 (0.90, 1.30) | 0.388 | 1.07 (0.52, 2.21) | 0.852 | 0.70 (0.32, 1.55) | 0.376 | 1.03 (0.26, 4.04) | 0.965 | 1.26 (0.65, 2.44) | 0.501 |
| Vessel disease | ||||||||||||
| Peripheral arterial disease | 3415 | UK Biobank | 1.05 (0.99, 1.11) | 0.104 | 0.92 (073, 1.16) | 0.493 | 0.82 (0.64, 1.06) | 0.135 | 1.06 (0.68, 1.64) | 0.803 | 1.14 (0.92, 1.40) | 0.242 |
| Venous thromboembolism | 15 602 | UK Biobank | 1.07 (1.04, 1.10) | 1.90×10−5 | 0.85 (0.76, 0.95) | 0.006 | 0.77 (0.68, 0.87) | 4.09×10−5 | 0.92 (0.74, 1.14) | 0.446 | 1.22 (1.10, 1.36) | 2.32×10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Bäck, M.; Bruzelius, M.; Mason, A.M.; Burgess, S.; Larsson, S. Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients 2019, 11, 3001. https://doi.org/10.3390/nu11123001
Yuan S, Bäck M, Bruzelius M, Mason AM, Burgess S, Larsson S. Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients. 2019; 11(12):3001. https://doi.org/10.3390/nu11123001
Chicago/Turabian StyleYuan, Shuai, Magnus Bäck, Maria Bruzelius, Amy M. Mason, Stephen Burgess, and Susanna Larsson. 2019. "Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study" Nutrients 11, no. 12: 3001. https://doi.org/10.3390/nu11123001
APA StyleYuan, S., Bäck, M., Bruzelius, M., Mason, A. M., Burgess, S., & Larsson, S. (2019). Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients, 11(12), 3001. https://doi.org/10.3390/nu11123001






