Abstract
Whether circulating fatty acids (FAs) play a causal role in the development of cardiovascular disease (CVD) remains unclear. We conducted a Mendelian randomisation study to explore the associations between plasma phospholipid FA levels and 15 CVDs. Summary-level data from the CARDIoGRAMplusC4D, MEGASTROKE, and Atrial Fibrillation consortia and UK Biobank were used. Sixteen single-nucleotide polymorphisms (SNPs) associated with ten plasma FAs were used as instrumental variables. SNPs in or close to the FADS1 gene were associated with most FAs. We performed a secondary analysis of the association between a functional variant (rs174547) in FADS1, which encodes 𝛥5-desaturase (a key enzyme in the endogenous FA synthesis), and CVD. Genetic predisposition to higher plasma α-linolenic, linoleic, and oleic acid levels was associated with lower odds of large-artery stroke and venous thromboembolism, whereas higher arachidonic and stearic acid levels were associated with higher odds of these two CVDs. The associations were driven by SNPs in or close to FADS1. In the secondary analysis, the minor allele of rs174547 in FADS1 was associated with significantly lower odds of any ischemic stroke, large-artery stroke, and venous thromboembolism and showed suggestive evidence of inverse association with coronary artery disease, abdominal aortic aneurysm and aortic valve stenosis. Genetically higher plasma α-linolenic, linoleic, and oleic acid levels are inversely associated with large-artery stroke and venous thromboembolism, whereas arachidonic and stearic acid levels are positively associated with these CVDs. The associations were driven by FADS1, which was also associated with other CVDs.
1. Introduction
Cardiovascular disease (CVD) is one of the leading causes of mortality and disability among men and women worldwide [1,2]. It was estimated that CVD caused 16.7 deaths worldwide in 2010, and the number was projected to increase to 23.3 million in 2030 [1]. As a modifiable risk factor, suboptimal diet is an important preventable risk factor for CVD [3]. Among dietary factors, the types of fatty acids (FAs) consumed have been considered to play an important role in the prevention of CVD [4]. It has been proposed that increased intake of unsaturated FAs, particularly polyunsaturated fatty acids (PUFA), and decreased intake of saturated fatty acids (SFA) will decrease the incidence of CVD by lowering low-density lipoprotein cholesterol and triglyceride levels [4,5,6], improving insulin sensitivity [6], and reducing systemic inflammation [6]. However, available evidence on the associations of specific FAs with CVD risk is inconsistent or limited [7,8,9,10].
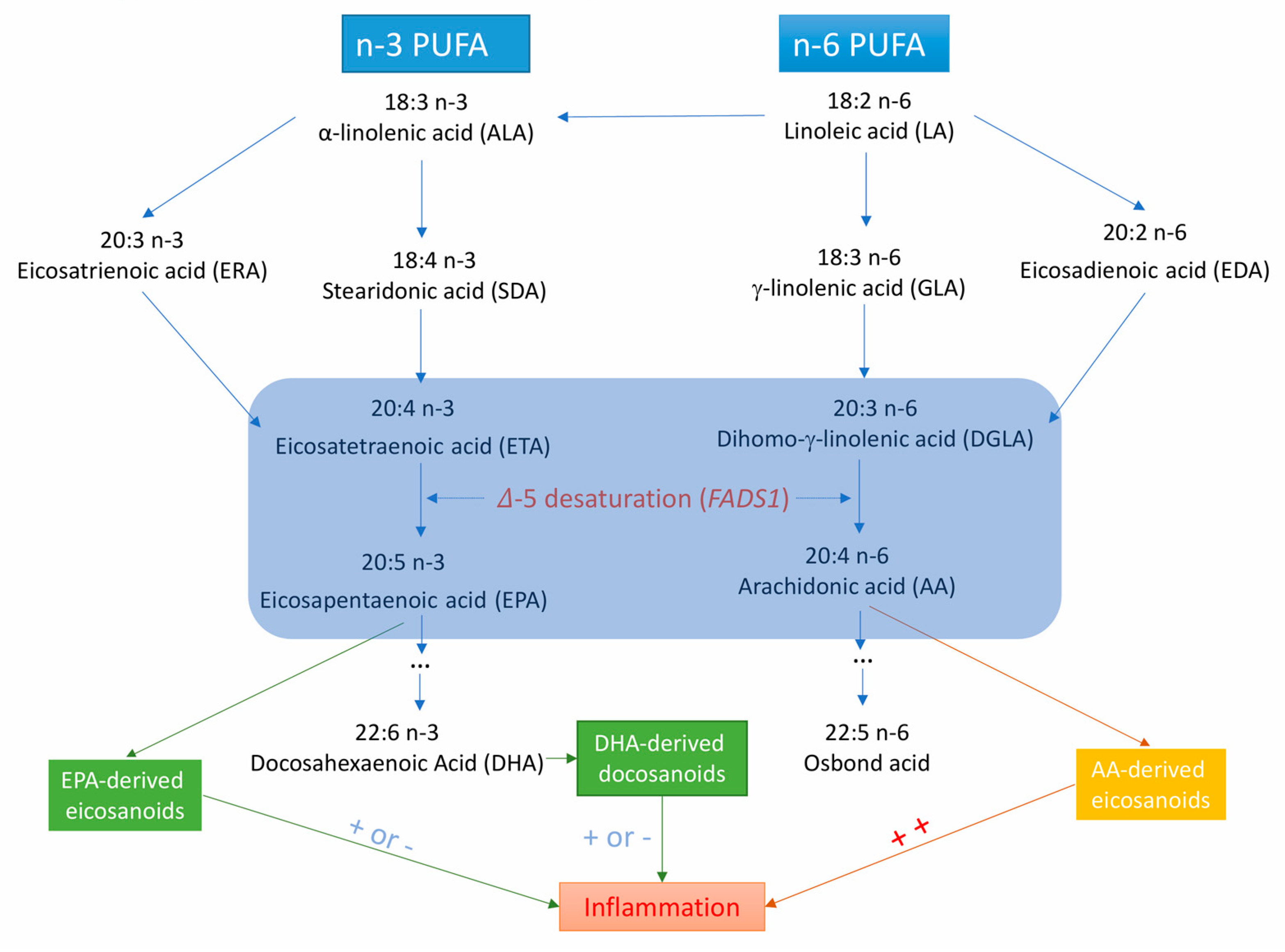
Fatty acid desaturase 1 (FADS1) gene encodes 𝛥5-desaturase, which is the key rate-limiting enzyme in the endogenous synthesis of long-chain PUFAs (Figure 1) [11]. 𝛥5-desaturase produces arachidonic acid (AA) and eicosapentaenoic acid (EPA) from dihomo-γ-linolenic acid and eicosatetraenoic acid, respectively [11]. Several observational studies have shown that 𝛥5-desaturase activity (measured by the ratio of AA to dihomo-γ-linolenic acid) is associated with the risk of metabolic syndrome [12], type 2 diabetes mellitus [13], and certain CVDs [14,15]. However, data on the associations of 𝛥5-desaturase activity or FADS1 variants with CVDs are limited.
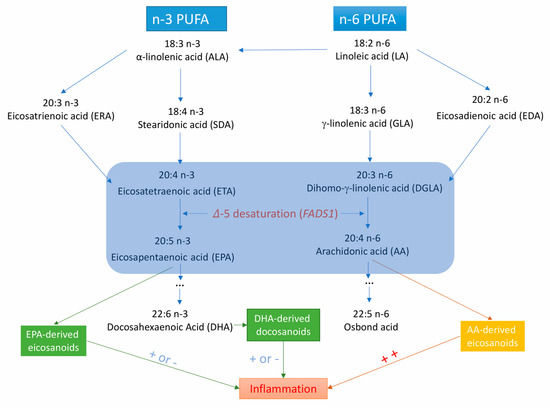
Figure 1.
Metabolic and inflammation-related pathways for long-chain polyunsaturated fatty acids from the essential ɑ-linolenic acid and linoleic acid. ++ indicates that AA-derived eicosanoids are strong pro-inflammatory factors; + or − means EPA- and DHA-derived eicosanoids or docosanoids are less inflammatory or anti-inflammatory.
Mendelian randomisation (MR) is a method that strengthens causal inference between risk factors and outcomes by exploiting genetic variants as instrumental variables of an exposure [16]. This technique minimizes confounding as genetic variants are randomly assorted at meiosis, thereby having no association with self-selected lifestyle factors and behaviors. In addition, it overcomes reverse causality since allelic randomisation antedates the disease’s onset.
We conducted a two-sample MR study to explore the associations of plasma phospholipid FA levels with risk of 15 CVDs. Since 𝛥5-desaturase plays a major role in FA synthesis, we performed a secondary analysis to investigate the associations of a functional variant (rs174547) within the FADS1 gene, as a proxy for 𝛥5-desaturase activity, with CVD.
2. Methods
2.1. Study Design
We employed a two-sample MR design in the present study. Information of data sources used in this MR study and definitions for each CVD are summarized in Table S1–Table S3. Studies included in the genome-wide association studies (GWASs) had been approved by a relevant institutional review board and participants had provided informed consent. The present MR study has been approved by the Swedish Ethical Review Authority.
2.2. Selection of Plasma Fatty Acids and Instrumental Variables
Selection of individual FAs was based on available data from meta-analyses of GWASs of 8631 to 8961 individuals of European ancestry (Table S1) [17,18,19]. Ten individual FAs potentially associated with CVD were included in this MR study: α-linolenic acid (ALA); EPA; docosapentaenoic acid (DPA); docosahexaenoic acid (DHA); linoleic acid (LA); AA; palmitoleic acid (POA); oleic acid (OA); palmitic acid (PA) and stearic acid (SA). Since the concentrations (represented as the percentage of total FAs) of individual FAs vary largely, change in standard deviation (SD) was adapted as the unit. SDs for each FA were obtained from a population-based cohort study of middle-aged and older white participants of the Atherosclerosis Risk in Communities Study [20] and are provided in Table S4.
Information of the 16 single-nucleotide polymorphisms (SNPs) selected as instrumental variables for individual FAs in this MR study is summarized in Table S4. All SNPs were associated with plasma FAs at the level of genome-wide significance (p < 5×10−8). Two SNPs (rs780093 and rs780094) located in the GCKR locus were excluded because GCKR is a highly pleiotropic locus associated with a number of phenotypes, such as body mass index, alcohol intake, and serum calcium levels, which are potential confounders in analyses of plasma FA levels and CVD. All SNPs for each individual FA were in different gene regions and in linkage equilibrium.
Rs174547 in the FADS1 gene was used as a proxy for 𝛥5-desaturase activity in the secondary analysis. The minor (C) allele of rs174547 is associated with lower FADS1 gene expression in human liver [21] and is also associated with plasma phospholipid PUFA levels in the direction anticipated from reduced 𝛥5-desaturase activity [22]. Rs174547 explained a large variation in levels of several FAs, especially AA (up to 37.6%) (Table S4).
2.3. Outcome Data Sources
Summary-level data for the CVD outcomes were acquired from the CARDIoGRAMplusC4D consortium for coronary artery disease [23], the MEGASTROKE consortium for ischemic stroke and its subtypes [24], the Atrial Fibrillation consortium for AF [25], and the UK Biobank for nine other major CVDs (heart failure, aortic valve stenosis, abdominal and thoracic aortic aneurysms, transient ischemic attack, intracerebral and subarachnoid hemorrhages, venous thromboembolism, and peripheral vascular disease) [26] (Table S1). Our analyses of data from the UK Biobank included 367,643 participants after exclusion of non-European individuals (to reduce population stratification), related individuals (third-degree relatives or closer), low call rate, and excess heterozygosity (3 or more standard deviations from the mean).
2.4. Statistical Analysis
The conventional inverse-variance weighted method with fixed-effects was used to analyze the associations between plasma FA levels and 15 CVDs. The results reported represent the odds ratios (ORs) for an SD increase in genetically predicted plasma FA levels. In sensitivity analyses, we employed SNPs, except variants in or close to the FADS1 gene, as instrumental variables for FAs to exclude the driving effect of FADS1. In the secondary analysis of 𝛥5-desaturase activity, ORs with 95% confidence intervals (CI) for each CVD per additional C allele of rs174547 in FADS1 were derived from the log ORs (beta-coefficients for the SNP-CVD associations) and standard errors. To account for multiple testing, we considered associations with p values below 3.33×10−4 (where p = 0.05/150 (10 FAs and 15 CVDs)) to represent strong evidence of causal associations in the main analysis, and p values below 0.003 (p = 0.05/15 CVDs) to represent statistical significance in the secondary analysis of rs174547. Associations with p values below 0.05 but above 3.33×10−4 and 0.003 in the main and secondary analyses, respectively, were regarded as suggestive evidence of associations. All statistical analyses were performed in Stata/SE 15.0.
2.5. Pleiotropy Assessment
We used the PhenoScanner V2 database [27] to explore pleiotropic associations of the FA-associated SNPs with other traits at the genome-wide significance level. Similar or related traits were reported and documented.
3. Results
3.1. Plasma FAs and CVDs
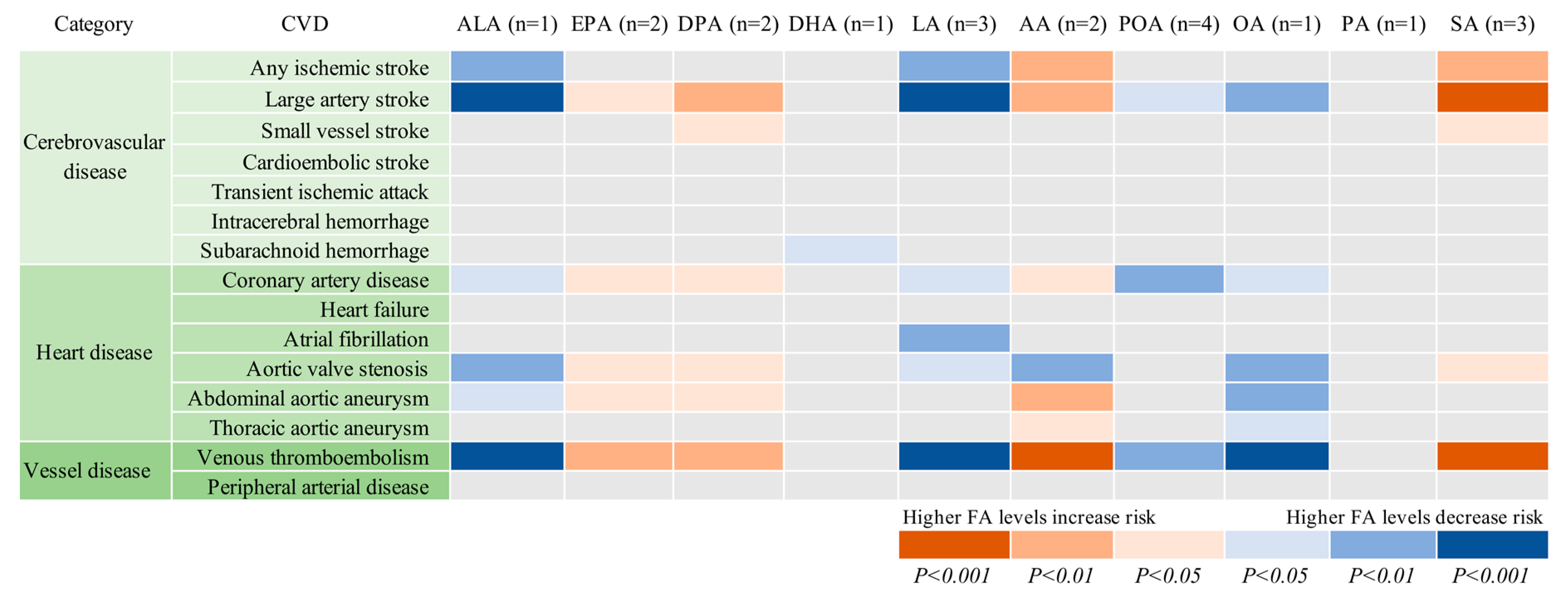
The associations between genetically predicted plasma FAs and the 15 CVDs are displayed in Table 1 and Figure 2. Genetic predisposition to higher plasma levels of ALA, LA, and OA was significantly or borderline significantly (p = 5.34×10−4) associated with lower odds of large-artery stroke and venous thromboembolism, whereas higher plasma AA and SA (only for venous thromboembolism) levels were significantly or borderline significantly (p = 3.34×10−4) associated with higher odds of these two CVDs. There was suggestive evidence of inverse associations of genetically predicted ALA, LA, OA, or POA levels with one or more of the other 10 CVDs, as well as positive associations of genetically predicted EPA, DPA, DHA, AA, or SA levels with one or more CVD. However, only a significant association between DHA and subarachnoid hemorrhage was still obtained when SNPs in or close to the FADS1 gene (rs174547, rs174538, and rs102275) were not included in the analysis.

Table 1.
Associations of genetically predicted plasma fatty acid levels with 15 cardiovascular diseases.
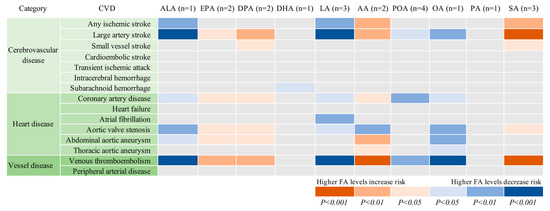
Figure 2.
Associations of plasma phospholipid fatty acids levels with 15 cardiovascular diseases. AA indicates arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; OA, oleic acid; PA, palmitic acid; POA, palmitoleic acid; SA, stearic acid. The number in parenthesis after each fatty acid represents the number of single-nucleotide polymorphisms used as instrumental variables for each fatty acid.
3.2. FADS1 and CVD
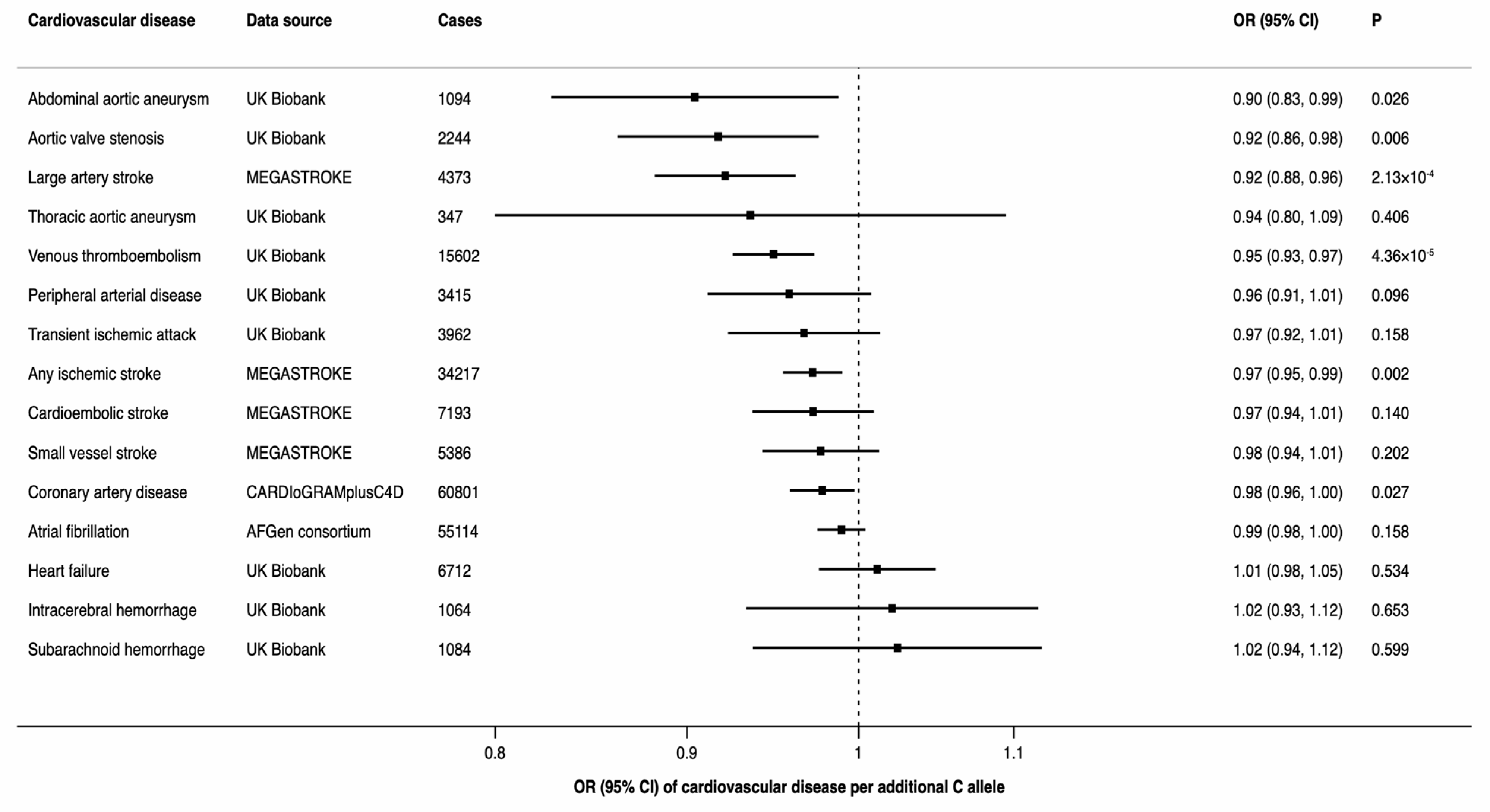
There was a statistically significant inverse association between the minor allele of rs174547 (minor allele frequency of 0.34 in UK Biobank) in FADS1 and three of the 15 CVDs, including any ischemic stroke, large-artery stroke, and venous thromboembolism (Figure 3). Moreover, there was a suggestive inverse association of rs174547 with coronary artery disease, abdominal aortic aneurysm and aortic valve stenosis (Figure 3).
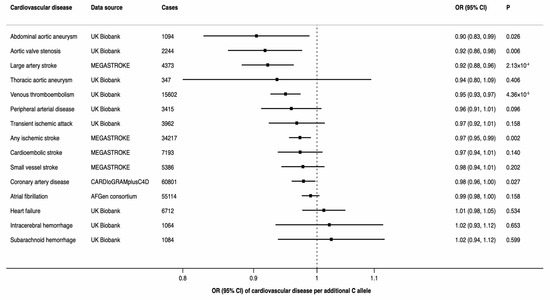
Figure 3.
Associations between rs174547 in the FADS1 gene and odds ratio of 15 cardiovascular diseases. CI indicates confidence interval; OR, odds ratio.
3.3. Pleotropic Associations
Pleiotropic associations of FA-associated SNPs with other traits are shown in Table S5. SNPs in or close to the FADS1 gene were significantly associated with several traits, including triglyceride, cholesterol and fasting glucose levels, blood cells, height, pulse rate, and heart rate. Rs16966952 in PDXDC1 was associated with fat-free mass, height, and weight. Rs10740118 in JMJD1C was associated with triglyceride levels, blood cells, diastolic blood pressure, body mass index, and education. The SNPs rs603424 nearby SCD and rs11190604 in HIF1AN were associated with blood cells and fat-free mass, respectively.
4. Discussion
The main finding of this MR study is that genetically higher plasma levels of ALA, LA, and OA were associated with lower odds of large-artery stroke and venous thromboembolism, whereas higher plasma AA and SA levels were associated with higher odds of these two CVDs. However, these and other suggestive associations of plasma levels of several FAs with CVD were driven by FADS1, encoding the 𝛥5-desaturase enzyme, which showed strong or suggestive associations with six out of 15 CVDs.
4.1. Plasma FAs and Ischemic Stroke
Observational studies of the association between individual FAs and ischemic stroke, including large-artery stroke, are relatively limited and the findings are conflicting, possibly because of small sample sizes of some studies and residual confounding. Most but not all studies support that moderate or high ALA [28] and LA [29,30] and low SA [31,32] exposure is associated with lower risk of ischemic stroke, which is in line with our results, although our findings for SA were not significant. A large Danish cohort study reported a significant inverse association between the content of LA in adipose tissue and risk of large-artery stroke [30]. However, circulating ALA levels were not significantly associated with ischemic stroke in smaller cohorts of Swedish [33] and U.S. [34] adults. With regard to MUFAs, the findings vary considerably between available studies. In two cohort studies conducted in U.S. populations, serum POA and OA levels were positively associated with risk of ischemic stroke [34,35]. However, a null association of those FAs with ischemic stroke was reported in two systematic reviews [36,37]. In our study, we found a suggestive inverse association of higher plasma OA levels with any ischemic stroke and large-artery stroke in particular. Support for a possible role of MUFAs and PUFAs for prevention of stroke comes from a large-scale randomized trial in Spain, which showed that a Mediterranean diet supplemented with either olive oil (high in OA and LA) or nuts (high in MUFAs and PUFAs) significantly reduced the incidence of total stroke [38]. However, another finding from that trial revealed that extra virgin olive oil but not normal olive oil exerted a protective effect on cardiovascular disease [39], suggesting that other minor components of extra virgin olive oil, rather than OA, explained the protective effect.
4.2. Plasma FAs and Venous Thromboembolism
Data on the associations between plasma levels of FAs and venous thromboembolism are scarce and inconsistent. In two reviews, although higher n-3 PUFAs appear to influence collagen-induced platelet aggregation in a favorable manner, there is no clear evidence of beneficial effects on fibrinolysis and blood coagulability [40,41]. Another systematic review on coronary thrombosis pointed out that the beneficial effect of n-3 PUFAs on coronary heart disease may be antiarrhythmic rather than antithrombotic [42]. In animal studies, EPA showed a potential effect against thrombosis in rats [43], and results of a cohort study of Norwegian adults showed that dietary intake of marine n-3 PUFAs was inversely associated with risk of venous thromboembolism [10] and risk of recurrent venous thromboembolism after unprovoked index events [44]. However, if anything, we observed a detrimental effect of genetically higher EPA levels on venous thromboembolism in the present study. More studies are needed to determine the association between FAs and venous thromboembolism. In addition, we observed a positive association between SA and venous thromboembolism. In vivo, SA has been found to be easily converted into OA [45], which showed an inverse association with venous thromboembolism in the present study. Thus, whether the observed positive association between SA and venous thromboembolism is direct or mediated by OA remains unclear. Previous studies on SA showed a neutral or unclear effect on plasma lipids [46]. Elucidation of the detailed mechanism behind the association between SA and venous thromboembolism warrants further study.
4.3. Plasma FAs and other CVDs
The present study found several suggestive associations between genetically predicted levels of certain FAs and CVDs, such as inverse associations of higher levels of ALA, LA, OA, and POA as well as lower levels of EPA, DPA, DHA, AA, or SA levels with coronary artery disease, aortic valve stenosis and abdominal aneurysm. The observed effects of different FAs on coronary artery disease were, overall, in line with observational studies [47,48]. Nonetheless, epidemiological studies focusing on serum FAs and aortic valve stenosis and abdominal aneurysm are limited.
With regard to atrial fibrillation, observational studies consistently concluded a null association between n-3 PUFAs and incidence of atrial fibrillation [49], which is supported by our findings. In addition, among patients undergoing cardiac surgery, supplementation of n-3 PUFAs did not decrease the risk of post-operative atrial fibrillation [50].
4.4. FADS1 and CVD
To the best of our knowledge, this is the first study to examine and show that a functional variant in FADS1 is inversely associated with abdominal aortic aneurysm, aortic valve stenosis, large-artery stroke and venous thromboembolism. In an experimental study, we found that the minor allele of rs174547 is associated with increased desaturase activity in the n-3 PUFA pathway in human aortic valves, leading to increased DHA content [51]. In the present MR study, we could not assess the role of tissue levels of DHA, but plasma DHA levels were inversely associated with subarachnoid hemorrhage only.
Our results for FADS1 based on data from the CARDIoGRAMplusC4D consortium are in line with those of some prior smaller studies of the association of genetic variations in FADS1 or 𝛥5-desaturase activity with coronary artery disease. A case-control study, comprising 1646 adults (756 coronary artery disease cases), found that the T allele of rs174556 (strongly correlated with the C allele of rs174547) was associated with a lower risk of coronary artery disease [52]. This finding was verified by another study in an Asian population, including 505 cases and 510 controls, which found that low 𝛥5-desaturase activity was associated with lower odds of coronary artery disease [53]. In contrast, in a case-control study of 2448 postmenopausal women, 𝛥5-desaturase activity was inversely associated with coronary artery disease [54], and a small case-control study found that the frequency of the T allele of rs174556 (correlated with the C allele of rs174547) was higher among the cases than among the controls [55]. Other studies, including two prospective studies [56,57] (of which one relatively large cohort of 24,032 Swedish adults [57]) and a case-control study [58] found no significant association of SNPs in FADS1 or 𝛥5-desaturase activity (defined by the ratio of AA to dihomo-γ-linolenic acid) with risk of coronary artery disease or ischemic stroke. The inconsistency may be explained by the small sample sizes in most previous studies, by differences in proportion of n-3 and n-6 PUFAs in the diet in different populations, or by synchronization of low 𝛥5-desaturase activity and low 𝛥6-desaturase activity.
4.5. Possible Mechanisms
Variants in or near the FADS1 gene are associated with several potential intermediates, including cholesterol and triglyceride levels and heart rate. Meta-analyses of randomized controlled trials have shown that n-3 PUFA supplementation has a beneficial effect on cholesterol and triglyceride levels as well as heart rate variability [48,59]. In addition, evidence from short-term feeding trials indicates that replacement of SFAs with PUFAs or MUFAs lowers low-density lipoprotein cholesterol and triglyceride levels [5,6]. Animal and cell studies have demonstrated that FAs exert effects on lipid levels by influencing low-density lipoprotein receptor and turnover, very low-density lipoprotein secretion, lipogenesis, cholesterol 7α-hydroxylase activity, lipoprotein lipase activity and reverse cholesterol transport [60]. Therefore, plasma FAs levels play an important role in the development of CVDs via the regulation of these intermediates, in particular lipid levels.
There may be additional mechanisms whereby FAs may affect the risk of CVD, such as effects on inflammation [61,62,63] and oxidative stress [64]. 𝛥5-desaturase produces AA and EPA from dihomo-𝛾-linolenic acid and eicosatetraenoic acid, respectively [10]. AA-derived eicosanoids initiate and augment pro-inflammatory responses, whereas EPA-derived eicosanoids are less inflammatory or anti-inflammatory [65,66] and participate in the resolution of inflammation. FA-related eicosanoids may increase atherosclerotic plaque deposition, thereby facilitating atherosclerosis formation. Atherosclerosis and thrombosis development are vital pathological processes in the development of coronary artery disease and ischemic stroke [63]. In addition, in the metabolism of certain FAs (such as AA) through the cyclooxygenase pathways, excessive reactive oxygen species are generated [64]. These free radicals may damage vascular function, increase endothelial permeability, alter reactivity to vasodilators, and promote formation of focal lesions in endothelial cell membranes at very low levels by increasing vasodilation and platelet aggregability [63,64].
4.6. Strengths and Limiations
A major strength of this study is the MR approach, which minimizes confounding and reverse causality, potentially distorting the results of observational studies. In addition, we tested the association between plasma FA levels and CVD using summary-level data from large-scale genetic consortia or studies with large sample size, thereby guaranteeing high statistical power to detect weak associations. However, for certain CVDs, we had a limited number of cases, which might preclude inference about the absence of association in cases where no association was detected, such as for thoracic aortic aneurysm, intracerebral hemorrhage, and subarachnoid hemorrhage. The study populations mainly included individuals of European ancestry. Thus, bias due to population stratification was reduced. Nonetheless, population confinement limited the generalizability of our findings to other populations. Another limitation in the present study is that we only had one to four SNPs as instrumental variables for individual FAs. This confined sensitivity analyses to explore pleiotropy, which might potentially weaken the reliability of the results. However, we manually searched pleiotropic traits related to FAs. Some of the SNPs had pleiotropic associations with other traits but the SNPs may affect those traits through vertical (mediated) pleiotropy rather than through horizontal pleiotropy. A further major shortcoming is that SNPs in or close to the FADS1 gene were associated with most FAs, which explained the major proportion of variance in most plasma FA levels, and were driving the associations.
5. Conclusions
This study showed that genetic predisposition to higher plasma ALA, LA, and OA levels was associated with lower odds of large-vessel stroke and venous thromboembolism, whereas plasma AA and SA levels were positively associated with these CVDs. However, the associations were driven by FADS1, which was also significantly or suggestively associated with coronary artery disease, abdominal aortic aneurysm and aortic valve stenosis. Thus, it is recommended to reconsider the role of individual FAs in the prevention of different CVDs.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/12/3001/s1, Table S1: Information of included studies and consortia, Table S2: Definitions and sources of information for CAD, AF and ischemic stroke and its subtypes from consortia, Table S3: Definitions and sources of information for cardiovascular disease outcomes in UK Biobank, Table S4: Single-nucleotide polymorphisms selected as instrumental variables for individual FAs, Table S5: Related traits of the single-nucleotide polymorphisms associated with plasma fatty acid levels from PhenoScanner search.
Author Contributions
S.Y. designed the research, analyzed and interpreted data and wrote the manuscript. M.B. (Magnus Bäck) and M.B. (Maria Bruzelius) reviewed the manuscript. A.M.M., S.B. prepared the data and reviewed the manuscript. S.L. designed the research, analyzed and interpreted the data and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this study came from the Swedish Research Council for Health, Working Life and Welfare (Forte; Grant Number 2018-00123) and the Swedish Research Council (Vetenskapsrådet; Grant Number 2019-00977). SB is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 204623/Z/16/Z).
Acknowledgments
Summary-level data for genetic associations with the cardiovascular disease outcomes have been contributed by the CARDIoGRAMplusC4D, MEGASTROKE and Atrial Fibrillation consortia and the UK Biobank study. The authors thank all investigators for sharing these data. The research based on the UK Biobank was conducted under Application Number 29202.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AA: arachidonic acid; ALA: α-linolenic acid; CI: confidence interval; CVDs: cardiovascular diseases; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; FAs: fatty acids; GWAS: genome-wide association study; LA: Linoleic acid; MUFA: monounsaturated fatty acid; OA: oleic acid; OR: odds ratio; PA: palmitic acid; POA: palmitoleic acid; PUFA, polyunsaturated fatty acid; SA: stearic acid; SD: standard deviation; SFA: saturated fatty acid; SNP: single-nucleotide polymorphism.
References
- Bansilal, S.; Castellano, J.M.; Fuster, V. Global burden of CVD: Focus on secondary prevention of cardiovascular disease. Int. J. Cardiol. 2015, 201 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T.; et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013, The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries; 1990–2017, a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary; circulating; and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B.; et al. Association between fish consumption; long chain omega 3 fatty acids; and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 2012, 345, e6698. [Google Scholar] [CrossRef]
- Isaksen, T.; Evensen, L.H.; Johnsen, S.H.; Jacobsen, B.K.; Hindberg, K.; Brækkan, S.K.; Hansen, J.B. Dietary intake of marine n-3 polyunsaturated fatty acids and future risk of venous thromboembolism. Res. Pract. Thromb. Haemost. 2018, 13, 59–69. [Google Scholar] [CrossRef]
- Tosi, F.; Sartori, F.; Guarini, P.; Olivieri, O.; Martinelli, N. Delta-5 and delta-6 desaturases: Crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv. Exp. Med. Biol. 2014, 824, 61–81. [Google Scholar]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Aros, F.; Lamuela-Raventós, R.M.; et al. Plasma fatty acid composition; estimated desaturase activities; and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kröger, J.; Zietemann, V.; Enzenbach, C.; Weikert, C.; Jansen, E.H.; Döring, F.; Joost, H.G.; Boeing, H.; Schulze, M.B. Erythrocyte membrane phospholipid fatty acids; desaturase activity; and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 2011, 93, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Sundstrom, J.; Vessby, B.; Cederholm, T.; Riserus, U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am. J. Clin. Nutr. 2008, 88, 203–209. [Google Scholar] [CrossRef]
- Lu, Y.; Vaarhorst, A.; Merry, A.H.; Dollé, M.E.; Hovenier, R.; Imholz, S.; Schouten, L.J.; Heijmans, B.T.; Müller, M.; Slagboom, P.E.; et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS ONE 2012, 7, e41681. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Tanaka, T.; Tang, W.; Manichaikul, A.; Foy, M.; Kabagambe, E.K.; Nettleton, J.A.; King, I.B.; Weng, L.C.; Bhattacharya, S.; et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011, 7, e1002193. [Google Scholar] [CrossRef]
- Guan, W.; Steffen, B.T.; Lemaitre, R.N.; Wu, J.H.Y.; Tanaka, T.; Manichaikul, A.; Foy, M.; Rich, S.S.; Wang, L.; Nettleton, J.A.; et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 2014, 7, 321–331. [Google Scholar] [CrossRef]
- Wu, J.H.; Lemaitre, R.N.; Manichaikul, A.; Guan, W.; Tanaka, T.; Foy, M.; Kabagambe, E.K.; Djousse, L.; Siscovick, D.; Fretts, A.M.; et al. Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: Results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ. Cardiovasc. Genet. 2013, 6, 171–183. [Google Scholar] [CrossRef]
- Yamagishi, K.; Nettleton, J.A.; Folsom, A.R. Plasma fatty acid composition and incident heart failure in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 965–974. [Google Scholar] [CrossRef]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bokor, S.; Dumont, J.; Spinneker, A.; Gonzalez-Gross, M.; Nova, E.; Widhalm, K.; Moschonis, G.; Stehle, P.; Amouyel, P.; De Henauw, S.; et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J. Lipid Res. 2010, 51, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Nikpay, M.; Goel, A.; Won, H.H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Saleheen, D.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A comprehensive 1;000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar] [PubMed]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Chaffin, M.D.; Weng, L.C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018, 50, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2, an expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4983. [Google Scholar] [CrossRef]
- Bork, C.S.; Veno, S.K.; Lundbye-Christensen, S.; Jakobsen, M.U.; Tjonneland, A.; Calder, P.C.; Overvad, K.; Schmidt, E.B. Adipose tissue content of alpha-linolenic acid and the risk of ischemic stroke and ischemic stroke subtypes: A Danish case-cohort study. PLoS ONE 2018, 13, e0198927. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; de Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef]
- Veno, S.K.; Bork, C.S.; Jakobsen, M.U.; Lundbye-Christensen, S.; Bach, F.W.; Overvad, K.; Schmidt, E.B. Linoleic Acid in Adipose Tissue and Development of Ischemic Stroke: A Danish Case-Cohort Study. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H.; Yatsuya, H.; Tanabe, N.; Date, C.; Kikuchi, S.; Yamamoto, A.; Inaba, Y.; Tamakoshi, A.; JACC Study Group. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am. J. Clin. Nutr. 2010, 92, 759–765. [Google Scholar] [PubMed]
- Yamagishi, K.; Iso, H.; Kokubo, Y.; Saito, I.; Yatsuya, H.; Ishihara, J.; Inoue, M.; Tsugane, S.; JPHC Study Group. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC Study. Eur. Heart J. 2013, 34, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, B.; Sundstrom, J.; Arnlov, J.; Terent, A.; Vessby, B.; Zethelius, B.; Lind, L. Metabolic risk factors for stroke and transient ischemic attacks in middle-aged men: A community-based study with long-term follow-up. Stroke 2006, 37, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Folsom, A.R.; Steffen, L.M. Plasma fatty acid composition and incident ischemic stroke in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc. Dis. 2013, 36, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yaemsiri, S.; Sen, S.; Tinker, L.F.; Robinson, W.R.; Evans, R.W.; Rosamond, W.; Wasserthiel-Smoller, S.; He, K. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke 2013, 44, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Shao, W. Monounsaturated Fatty Acid Intake and Stroke Risk: A Meta-analysis of Prospective Cohort Studies. J. Stroke Cerebrovasc. Dis. 2016, 25, 1326–1334. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Lee, K.W.; Blann, A.D.; Lip, G.Y. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb. Res. 2006, 118, 305–312. [Google Scholar] [CrossRef]
- Din, J.N.; Newby, D.E.; Flapan, A.D. Omega 3 fatty acids and cardiovascular disease—Fishing for a natural treatment. BMJ 2004, 328, 30. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.D.; Iversen, A.M.; Schmidt, E.B. n-3 polyunsaturated fatty acids and coronary thrombosis. Lipids 2001, 36, S79–S82. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Isaksen, T.; Evensen, L.H.; Brækkan, S.K.; Hansen, J.B. Dietary Intake of Marine Polyunsaturated n-3 Fatty Acids and Risk of Recurrent Venous Thromboembolism. Thromb. Haemost. 2019. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kudo, N.; Koyama, M.; Kawashima, Y. Effects of dehydroepiandrosterone on oleic acid accumulation in rat liver. Biochem. Pharmacol. 2003, 65, 1583–1591. [Google Scholar] [CrossRef]
- Yu, S.; Derr, J.; Etherton, T.D.; Kris-Etherton, P.M. Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am. J. Clin. Nutr. 1995, 61, 1129–1139. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Mariani, J.; Doval, H.C.; Nul, D.; Varini, S.; Grancelli, H.; Ferrante, D.; Tognoni, G.; Macchia, A. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: Updated systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2013, 2, e005033. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Marchioli, R.; Macchia, A.; Silletta, M.G.; Ferrazzi, P.; Gardner, T.J.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; et al. Fish oil and postoperative atrial fibrillation: The Omega-3 Fatty Acids for Prevention of Post-Operative Atrial Fibrillation (OPERA) randomized trial. JAMA 2012, 308, 2001–2011. [Google Scholar] [CrossRef]
- Plunde, O.; Larsson, S.C.; Artiach, G.; Carracedo, M.; Franco-Cereceda, A.; Eriksson, P. FADS1 genotype predicts aortic valve FADS mRNA expression; alters valvular omega-3 fatty acid content; and associates with aortic valve calcification. 2019; submitted for publication. [Google Scholar]
- Kwak, J.H.; Paik, J.K.; Kim, O.Y.; Jang, Y.; Lee, S.H.; Ordovas, J.M.; Lee, J.H. FADS gene polymorphisms in Koreans: Association with omega6 polyunsaturated fatty acids in serum phospholipids; lipid peroxides; and coronary artery disease. Atherosclerosis 2011, 214, 94–100. [Google Scholar] [CrossRef]
- Li, S.W.; Lin, K.; Ma, P.; Zhang, Z.L.; Zhou, Y.D.; Lu, S.Y.; Zhou, X.; Liu, S.M. FADS gene polymorphisms confer the risk of coronary artery disease in a Chinese Han population through the altered desaturase activities: Based on high-resolution melting analysis. PLoS ONE 2013, 8, e55869. [Google Scholar] [CrossRef]
- Matthan, N.R.; Ooi, E.M.; Van Horn, L.; Neuhouser, M.L.; Woodman, R.; Lichtenstein, A.H. Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: A nested case-control study within the Women’s Health Initiative observational study. J. Am. Heart Assoc. 2014, 3, e000764. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Sun, L.; Ye, L.; Shi, J.; Zhou, L.; Yang, J.; Du, B.; Song, Z.; Yu, Y.; Xie, L. A case-control study between the gene polymorphisms of polyunsaturated fatty acids metabolic rate-limiting enzymes and coronary artery disease in a Chinese Han population. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, R.; Kurl, S.; Tuomainen, T.P.; Virtanen, J.K. Associations of estimated Delta-5-desaturase and Delta-6-desaturase activities with stroke risk factors and risk of stroke: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2017, 117, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, S.; Ericson, U.; Gullberg, B.; Hedblad, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in FADS1 has little effect on the association between dietary PUFA intake and cardiovascular disease. J. Nutr. 2014, 144, 1356–1363. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, R.X.; Cao, X.L.; Wu, D.F.; Chen, W.X.; Zhou, Y.J. Association of two polymorphisms in the FADS1/FADS2 gene cluster and the risk of coronary artery disease and ischemic stroke. Int. J. Clin. Exp. Pathol. 2015, 8, 7318–7331. [Google Scholar]
- Christensen, J.H. Omega-3 polyunsaturated Fatty acids and heart rate variability. Front. Physiol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Fernandez, ML.; West, KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dessi, M.; Noce, A.; Bertucci, P.; Manca di Villahermosa, S.; Zenobi, R.; Castagnola, V.; Addessi, E.; Di Daniele, N. Atherosclerosis; dyslipidemia; and inflammation: The significant role of polyunsaturated Fatty acids. ISRN Inflamm. 2013, 2013, 191823. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Tysseling, K.A.; Rice, J.; Pham, M.; Haji, L.; Gallis, B.M.; Baas, A.S.; Paramsothy, P.; Giachelli, C.M.; Corson, M.A.; et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).