Abstract
High whole-grain consumption is related to better health outcomes. The specific physiological effect of these compounds is still unrevealed, partly because the accurate estimation of the intake of whole grains from dietary assessments is difficult and prone to bias, due to the complexity of the estimation of the intake by the consumer. A biomarker of whole-grain intake and type of whole-grain intake would be useful for quantifying the exposure to whole-grain intake. In this review, we aim to review the evidence on the potential biomarkers for whole-grain intake in the literature. We conducted a systematic search in Medline, Embase, Web of Science, and the Cochrane database. In total, 39 papers met the inclusion criteria following the PRISMA guidelines and were included. The relative validity, responsiveness, and reproducibility of these markers were assessed for short-, medium-, and long-term exposure as important criteria for the potential use of these biomarkers from a clinical and research perspective. We found three major groups of biomarkers: (1) alkylresorcinol, as well as its homologs and metabolites, assessed in plasma, adipose tissue biopsies, erythrocyte membranes, and urine; (2) avenacosides, assessed in urine samples; and (3) benzoxazinoid-derived phenylacetamide sulfates, assessed in blood and urine samples. The reviewed biomarkers may be used for improved assessment of associations between whole-grain intake and health outcomes.
Keywords:
whole grains; cereal fibers; rye; wheat; oat; barley; benzoxazinoid; avenacosides; biomarker; alkylresorcinol 1. Introduction
Whole grains (WGs) are a rich origin of dietary fibers and numerous bioactive compounds. Each one of these compounds has various physiological functions [1]. Recent epidemiological studies suggest that the intake of WG components seems to be associated with a lower risk of various chronic lifestyle-associated diseases, particularly cancer, type 2 diabetes, obesity, and cardiovascular diseases [2,3,4,5,6], as well as better health and treatment outcomes in some inflammation-related chronic diseases [7,8], and they contribute to the human–microbe symbiosis [9,10]. Furthermore, a correlation between WGs and a greater nutrient intake and improved quality of diet was reported [11,12]. WGs are defined as “consisting of the intact, ground, cracked, or flaked caryopsis of the grain whose principal anatomical components, the starchy endosperm, germ, and bran, are present in the same relative proportions as they exist in the intact grain” [13]. Many countries promote WG consumption in their dietary guidelines [14]. The Danish official dietary guidelines recommend citizens to prioritize WG components in their diets [15]. Children over 10 years and adults are recommended to eat at least 75 grams of WG each day [16].
However, the precise mechanism of the positive physiological effects offered by WG remains unresolved [1]. The weak accuracy of assessing habitual diet intake is a common obstacle in nutritional data [17]. These data rely on self-reported dietary assessment methods that are often subject to recall bias and prone to random and systematic measurement errors [18,19]. Intake of WG may be captured with some accuracy by methods like diet history interviews specifically focusing on their intake, using the double portion method or food frequency questionnaire (FFQ) inventories designed to particularly capture WG in the diet [20,21]. However, many of the common FFQ inventories, as well as the diet record or diet recall methods, may not capture WGs in foods because they do not provide specific information on the particular product consumed of a given food or food group [22]. The use of a biomarker has the potential to measure the intake of a given nutrient objectively and with less variation, which may lead to a strengthening of the correlations between WG intake and the reduced risk of certain diseases [23,24]. A biomarker was defined by the International Program on Chemical Safety, led by the World Health Organization (WHO) and in collaboration with the United Nations and the International Labor Organization as “any substance, structure, or process that can be measured in the body or its products, can influence or predict the incidence of outcome or disease, and can be classified into markers of exposure, effect, and susceptibility” [25].
Different biomarkers of WG intake were assessed and reported during the last few decades. The evidence for their validity is difficult to synthesize because of the multitude of biomarkers and different study approaches, which makes it challenging to get a broad overview of this topic. We, therefore, performed a systematic review of results from the published literature on the validity of the biomarkers of WGs and cereal fibers that were reported in human studies in order to assess their potential use from a clinical and research perspective.
2. Materials and Methods
2.1. Search Methods
The research question was defined using the Population, Intervention, Comparator outcome, and study design criteria (PICO) [26], as presented in Table 1. We conducted a systematic search in Medline, Embase, Web of Science, and Cochrane databases for papers assessing the relative validity, responsiveness, and reproducibility of biomarkers of WG and cereal-fiber intake in humans. The used search terms are shown in Table S1 (Supplementary Materials). The cut-off date of 20 September 2019 was used with an initial limit date applied to 1975. The search was restricted to human studies, with no restrictions on the age range, gender, ethnicity or health status of the participants. The search terms were designed to limit the search to papers that provided information on biomarkers for WG intake. The search was, however, extended to articles assessing biomarkers for dietary-fiber intake, as these studies may include evidence on WG biomarkers. Relevant articles were also manually identified using the reference lists of the identified studies.

Table 1.
The description of the PICO 1 criteria used for this review.
2.2. Selection Criteria
We included published, peer-reviewed randomized controlled cross-over and parallel studies, case–control studies, cohorts, and cross-sectional studies that evaluated or validated biomarkers of WG consumption in humans. Case reports, conference abstracts, systematic reviews, and papers in other languages than English were excluded.
2.3. Data Collection and Analysis
Two reviewers (M.J. and S.B.S.) screened the papers following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [27]; any discrepancies were resolved by mutual consensus, and, when necessary, a third reviewer was added (V.A.). We used Cochrane’s online systematic review software “Covidence” in this process [28]. The variables of interest are listed in the included tables (Tables 2–4, and Table S2, Supplementary Materials). Briefly, we collected descriptive variables such as the study design, country and year of publication, and patient characteristics (age, number, sex, and comorbidities), exposure variables such as the type of the targeted fibers, method of report of the exposure, recall period if a questionnaire was used, outcome variables such as the examined potential biomarkers, used biological material, and the results.
2.4. Data Analysis
In order to evaluate the capability of the evaluated biomarkers, various statistical methods and related coefficients were used when assessing the association between WG intake and biomarkers in the included papers. In this review, we used the guide of Evans in the interpretation of both Pearson’s and Spearman’s correlations, as it provides a more detailed classification compared to Cohen’s guide [29,30]. Briefly, a statistically significant r less than 0.20 is considered very weak, 0.20 to 0.39 is considered weak, 0.40 to 0.59 is considered moderate, 0.60 to 0.79 is considered strong, and 0.80 or greater is considered a very strong correlation. We took into account the confidence interval and the p-value in the interpretation of the results, as these classifications referred to linear associations. In the assessment of the reported intraclass correlation coefficient (ICC), values less than 0.5 were considered poor, values between 0.5 and 0.75 were considered moderate, values between 0.75 and 0.9 were considered good, and values greater than 0.90 were considered as having excellent reliability, based on the 95% confidence interval (CI) [31]. We reported the confidence interval (CI), as well as the standard deviation (SD) or the standard error (SE), of the mean in studies when these values were reported.
Due to heterogeneity in study design, measurement, and analysis methods in different studies, the Cochrane risk of bias checklists assessment was not considered suitable for use in the present review. Instead, we used a scale based on our methodological and clinical knowledge in the field (Table S2, Supplementary Materials). The protocol of this systematic review was registered in the International prospective register of systematic reviews (PROSPERO) under CRD42019137708.
3. Results
3.1. Overview of the Studies Included
We included 39 studies that met the inclusion criteria for this review (Figure 1). These studies involved 7002 participants; of them, 96 were included in more than one study [32,33,34,35]. The health status of these participants was reported in 32 studies; seven of these included 914 free-living subjects (Table 1). Seventeen studies evaluated alkylresorcinols (AR) as a biomarker for WG intake in plasma (P-AR) [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50], one study in erythrocyte membranes [51], and three studies in adipose tissue [43,47,50]. AR metabolites were evaluated in urine in 12 studies [32,33,34,39,42,52,53,54,55,56,57,58], and in plasma in five studies [52,56,59,60,61]; one study evaluated plasma benzoxazinoid compounds (N-(2-hydroxyphenyl) acetamide (HPAA) and hydroxy-N-(2-hydroxyphenyl) acetamide (HHPAA sulfate)) as biomarkers of whole-grain rye intake (WGR) [62]; one study evaluated urinary avenacosides as a biomarker for oat intake [63]. Seven studies applied a non-targeted metabolomic approach [64,65,66,67,68,69,70]. This review included nine cross-over [36,37,38,39,40,42,44,64,65] and five parallel randomized controlled studies [41,43,45,62,66], three case–control studies [46,56,60], 16 cohort studies [32,33,34,35,47,51,52,54,55,57,58,59,61,63,67,68], and six cross-sectional studies [48,49,50,53,69,70]. Fourteen studies were conducted in Sweden [32,33,35,38,39,44,47,50,55,57,60,65,68,70], 10 in Finland [34,40,43,51,52,59,61,62,64,66], seven in the United States of America (USA) [42,49,53,54,58,63,67], two in Denmark [37,48], two in the United Kingdom (UK) [36,45], two as multicenter European studies [41,46], one in Spain [69], and one in Latvia [56]. The main characteristics, the targeted biomarkers, and main findings of the included studies are presented in Table 2. Studies applying a non-targeted metabolomic approach are described in Table 3, and the main results are presented in Table 4.
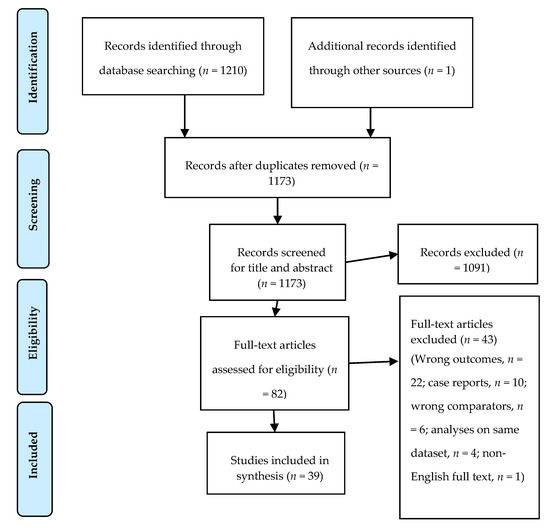
Figure 1.
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flow chart of database literature search and study selection [28].

Table 2.
The summary of the characteristics and the main results of the included studies.

Table 3.
The main characteristics of the included studies applying a non-targeted metabolomic approach.

Table 4.
The reported metabolites and main results of the included studies applying a non-targeted metabolomic approach.
3.2. Quality Assessment and Assessment of the Risk of Bias in the Included Studies
Table S2 (Supplementary Materials) shows the quality assessment and the assessment of the risk of bias of the included studies. Of 39 studies, 32 studies reported their inclusion and exclusion criteria [32,33,34,35,36,37,40,41,42,43,44,45,46,47,49,52,53,54,55,56,58,59,60,61,62,63,64,65,66,67,69,70], whereas 27 studies reported the health status of the included subjects [34,35,36,37,39,40,41,42,43,44,45,46,49,52,53,56,58,59,60,61,62,63,64,65,66,67,70]. Only one study reported the blinding of the assessors [65], and nine studies reported that the used questionnaires was validated specifically for the study [32,33,34,45,46,52,57,59,69]. Twenty-three studies reported a recall period for the used questionnaire of less than 10 days [32,33,34,35,36,37,38,39,41,42,43,44,45,49,52,55,56,59,60,64,65,66,70], and 19 studies used the questionnaire data to assess a period over one week [36,37,38,40,41,43,44,45,47,48,49,50,51,53,54,64,65,66,69]. Nineteen of 27 studies assessing biomarkers in plasma used fasting samples [34,36,37,38,39,42,43,44,45,47,50,51,52,56,59,61,62,65,66]. Of studies assessing biomarkers in urine, nine of 18 studies used 24-h urine [32,39,42,55,58,64,67,68,70], and three used spot urine [33,54,69].
3.3. Reported Biomarkers
3.3.1. Alkylresorcinols in Plasma
Seventeen studies that assessed alkylresorcinol in plasma (P-AR) were included in this review [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. Thirteen studies reported the relative validity of P-AR and reported the correlation between P-AR and the exposure to different WGs [34,35,36,37,38,40,41,43,45,46,47,48,49]. For the correlation between P-AR and whole-grain rye (WGR), four studies reported a weak correlation [35,37,46,48]. One study reported a weak correlation among the men, and a moderate correlation among the women [47]. Three studies reported a moderate correlation between P-AR and whole-grain wheat (WGW) [35,38,45], one study reported a very weak correlation [37], and one study reported a weak correlation among women; no correlation was reported among men [47]. Three studies reported a moderate correlation between P-AR and whole-grain rye and wheat (WGR + WGW) [35,37]. Wu et al. reported a weak correlation among men and a moderate correlation among women [47]. Five studies reported a weak correlation with cereal fibers [34,35,37,38,49], while one study reported a very weak correlation [48]. Two studies reported a moderate correlation to total fibers [36,41], and two other studies reported a weak correlation [38,49], while one reported a very weak correlation [48]. The correlation between P-AR and total WG intake was reported to be weak in four studies [37,45,47,49], very weak in one [46], moderate in one [36], and strong in one study [43]. The study design, sampling condition and time, the used dietary assessment method, and the reported correlations to the intake are illustrated in Figure 2. Correlation coefficients, CI intervals and p-values are presented in Table 2 when available in the included papers.
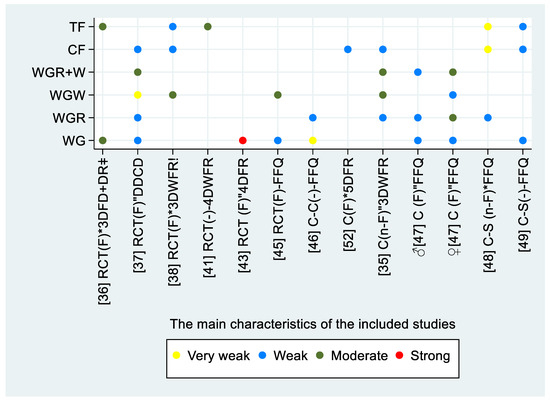
Figure 2.
The reported correlations between the intake of whole grains and alkylresorcinol concentration in plasma. The figure includes information on study design, sampling condition, sampling time, and dietary assessment method on the horizontal axis. The vertical axis represents the type of the exposure. Abbreviations: study design—randomised controlled trial, RCT; case–control, CC; cohort, C; cross-sectional, C-S; sampling condition; fasting, (F); non-fasting, (n-F); sampling time—* within 24 h since last intervention/intake; “ later than 24 h after; dietary assessment method—three-day food diaries, 3DFR; daily dietary compliance diaries, DDCD; three-day weighted food records, 3DWFR; four-day food intake records, 4DFR; food frequency questionnaire, FFQ; whole grain, WG; whole-grain rye, WGR; whole-grain wheat, WGW; cereal fiber, CF; total fiber, TF; correlation—very weak, r < 0.20; weak, 0.20 ≤ r ≤ 0.39; moderate, 0.40 ≤ r ≤ 0.59; strong, 0.60 ≤ r ≤ 0.79; ǂ analyzed separately; ! pooled data.
The sensitivity of AR to the WG dose change is poor, both in short- and long-term exposure. McKeown et al. reported in their study that the short-term dose response of the mean of P-AR on the WGW was significantly higher after two one-week interventions of three and six daily servings of WGW than at the wash-out (>/3.1-fold higher). No significant dose–response difference was found between the two interventions [42]. In another study, Ross et al. assessed the long-term dose response of P-AR in three intervention groups of WG in a parallel randomized controlled trial with three intervention groups of low, medium, and high intake of WG. After 16 weeks, a significant difference in P-AR was shown between the group with the low WG intake and the other groups. No significant difference in P-AR was demonstrated between the medium and the high WG intake groups [45]. A study from Andersson et al. showed a strong correlation between P-AR homologs C17:0 and rye intake, and a moderate correlation between C21:0 and the wheat intake. The authors reported a moderate correlation between the C17:0/C21:0 ratio and the WGR and a moderate inverse correlation with the WGW intake [35]. A similar good specificity of the C17:0/C21:0 ratio with the rye/wheat intake was reported in three other controlled intervention conditions [38,39,40]. No correlation was observed between AR homologs and barley, oat, corn, or rice intake [35], while a weaker correlation with the WG intake was observed in subjects with higher consumption of non-A- containing grains [36]. Landberg et al. reported good short-term reproducibility (six weeks) of plasma AR under intervention conditions where the intake of WGR was high and kept constant [44]. The reproducibility of plasma AR was poor (ICC = 0.47; 95% CI: (0.27, 0.67)) over a 2–3-month period among free-living subjects [35].
3.3.2. Alkylresorcinol in Adipose Tissue Biopsies
Three studies evaluated the correlation between WG intake and AR in adipose tissue biopsies. In a cross-sectional study design, Jansson et al. reported a moderate correlation between WG bread and total AR in adipose tissue [50]. In a randomized cross-over parallel study of WG and refined-grain (RF) interventions over 12 weeks, Wu et al. reported a strong correlation between WG intake and both P-AR (r = 0.60–0.72, p < 0.05) and AR in adipose tissue (r = 0.60–0.84, p < 0.05), and a higher P-AR and AR in adipose tissue in the WG than the RF intervention [43]. In a retrospective cohort study design, Wu et al. evaluated AR in adipose tissue biopsies as a biomarker of long-term WG wheat and rye intake in women (over one, seven, and 17 years) and in men (over one, two, and 14 years), and they found weak correlations with WG, WGR, WGW, and WGR + WGW intake in both genders except for a moderate correlation with WGR in women over one, seven, and 17 years [47].
3.3.3. Alkylresorcinol in Erythrocyte Membrane
Only one study addressed AR in the human erythrocyte membrane (EM) as a biomarker of WGW and WGR intake. In a parallel controlled study design, Linko et al. demonstrated that AR is incorporated and can be measured in the human EM in vivo. They also demonstrated a good symmetric progression of AR in plasma and EM in response to the WGR, WGW, and WG barley intake. No AR was detected in a subject gluten-free diet, nor was it detected in EM or plasma [51]. The composition of AR homologs differed between plasma and EM samples. The average percentage of C17:0 was significantly higher in plasma (13% CI (6, 16)) compared to the average percentage in EM (5% CI (3, 9)) (p < 0.001). In contrast, the average percentage of C25:0 was higher in EM (5% CI (4, 9)) compared to plasma (12% CI (10, 13)) (p < 0.001) [51].
3.3.4. Alkylresorcinol Metabolites in Plasma
Five studies assessed the plasma levels of the two AR metabolites, 3,5-dihydroxybenozoic acid (DHBA) and the 3-(3,5-dihydroxyphenyl)-1-propanoic acid (DHPPA), as potential biomarkers for WG intake [34,56,59,60,61]. Drake et al. reported in a case–control study design a very weak correlation between DHBA, DHPPA, and DHBA + DHPPA with cereal fibers, a week correlation with total WG, high-fiber bread, and total fiber intake, and a very weak inverse correlation with low-fiber bread in non-fasting samples [60]. Another study found a moderate correlation between both metabolites and their sum and total cereal fiber in the fasting samples [59], while no significant correlations were detected between these metabolites and vegetable, berry, or fruit fiber intake in another study [59]. Soderholm et al. reported good responsiveness of both metabolites in response to the range of WGR intake. After a washout period and a single WGR dose intake, the plasma concentration of DHBA and DHPPA raised simultaneously and reached a c-max after six hours (DHBA-tmax = 6.1 ± 0.5 h and DHPPA-tmax = 6.4 ± 0.7 h). The concentration of each metabolite at 25 h was slightly but significantly higher than at baseline. The t1/2 of DHPPA was longer compared to DHBA (t1/2-DHPPA = 16.3 ± 1.8 h, t1/2-DHBA = 10.1 ± 0.8 h) [61].
3.3.5. Alkylresorcinol Metabolites in Urine
Three studies assessed the relative validity of AR metabolites in spot urine; all studies addressed validity among free-living subjects. Four studies addressed AR metabolites in 12–36-h urine. DHPPA and DHBA are the major relative components of AR metabolites [32,58]. The proportion of the individual metabolite in the total AR metabolite excretion in 24-h urine was 42% for DHPPA, 33% for DHBA, 13% for DHCA, 9% for DHBA-glycine, and 2% for DHPPTA [32]. Landberg et al. estimated the four-day intake of WG. It correlated with the mean creatinine-adjusted AR metabolite concentrations from four spot-urine samples collected during the same period (days one, two, 13, and 14): DHBA (r = 0.49, p < 0.05), DHPPA (r = 0.38, p < 0.05), DHCA (r = 0.49, p < 0.05), DHBA-glycine (r = 0.42, p < 0.05), and DHPPTA (non-significant). Generally moderate to very strong correlations between metabolites were reported (except a very weak correlation between DHPPTA and DHPPA) [33]. In another study where DHBA, DHPPA, and DHBA + DHPPA were assessed as long-term biomarkers (2–3 years), very weak to weak correlations were reported with WG, cereal fiber, and total fiber intake, as well as a poor reproducibility of these metabolites between the two time points [54]. Marklund et al. compared the relative validity of DHBA, DHPPA, and DHBA + DHPPA between spot urine and 24-h urine. In spot urine, they reported a moderate correlation between DHPPA and WG, WGR, and WGR + WGW. The association with WGW was not significant. Similar results were reported for DHBA, except that, here, the authors found a strong correlation with WGR + WGW. In 24-h urine, slightly better correlations were reported for DHBA + DHPPA, and similar correlations were reported for the two other metabolites [55]. DHBA, DHPPA, and DHBA + DPPPA did not correlate with either the oat, barley, or rice intake. The authors reported a poor reproducibility of the concentration of DHBA and DHPPA in 24-h urine (ICC = 0.46–0.51), and a poor reproducibility in spot urine between two occasions three months apart, even when no difference in WG consumption between two occasions was observed [55].
Guyman et al. reported that the excretion of DHPPA in 12-h urine was 44% higher in whole-grain wheat and rye consumers than non-consumers after adjusting for body mass index (BMI), and energy and fiber intake [53]. Aubertin-Leheundre et al. evaluated the relative validity of the concentration of DHBA and DHPPA in 72-h urine as biomarkers for rye and cereal-fiber intake, based on a five-day food record that was initiated two days before the specimen collection. They reported a weak correlation between cereal fibers and DHBA and a moderate one with DHPPA. Generally, DHBA and DHPPA correlated modestly with P-AR and all AR homologs [34]. In a similar study set-up, Aubertin-Leuheundre observed a strong correlation between WGR intake and DHBA, and a moderate correlation with DHPPA. The authors observed a slightly weaker correlations between the total fiber intake and DHBA (r = 0.443 p < 0.05) and DHPPA (r = 0.390 p < 0.05) [52]. Recently, Wierzbicka et al. reported a strong correlation between the WGR intake and DHCA in 24-h urine samples on two occasions 2–3 months apart. A moderate correlation was observed for WGR with DHBA and DHPPTA (5-(3,5-dihydroxyphenyl) pentanoic acid), and for WGW intake with DHBA, DHPPA, and DHCA (3,5dihydroxycinnamic acid) on the first occasion. No correlations were observed between WGW and these metabolites on the second occasion, which was explained by a lower and less stable WGW intake on the second occasion. The intake of WG oats, barley and maize, did not correlate with the AR metabolite concentrations. DHCA-amide (3,5-dihydroxycinnamic acid amide) did not correlate with AR intake even if it was the metabolite with the highest urinary excretion, and the authors suggested that DHCA-amide may have a precursor other than AR [32]. McKeown et al. reported a good responsiveness of 24-h urine DHBA and DHPPA to the WG intake in an RCT study design. DHBA, DHPPA, and DHBA + DHPPA excreted in urine after WGW intake were higher compared with washout and higher when the WG intake increased from three to six servings daily [42]. Similar findings were reported in other studies [39,58]. The pharmacokinetic parameters indicate that the half-life (t1/2) of DHBA is slightly longer than DHPPA in urine (15.9 h vs. 14.8 h) [58].
3.3.6. Avenacosides
Wang et al. investigated the metabolism and pharmacokinetics of avenacosides as a biomarker of oat intake in humans, and it was reported to be absent in urine after the washout period, and present two hours after the ingestion of a single dose of WG oat. Only a trace of these metabolites was present 36 h after the exposure [63].
3.3.7. Benzoxazinoid-Derived Phenylacetamide Sulfates
One study assessed the plasma profile of the double-hexose-conjugate of 2,4-dihyxdoxy 1,4-benzoxazin-3one (DIBOA) in subjects after consuming WGR, WGW, and refined grain bread [62]. Fasting plasma samples were collected at four time points during 24 h after the exposure and were analyzed by an LC–quadrupole time-of-flight (QTOF)-MS approach [62]. Hydroxy-N-(2-hydroxyphenyl) acetamide (HHPAA) and N-(2hydroxyphenyl) acetamide (HPAA) appeared in the plasma 60 min after the intake of WGR bread and reached their maximal concentration at 120 min and 60 min, respectively. Both metabolites were absent after 24 h of exposure. HHPPA and HPAA were also detected in other studies assessing plasma and urine metabolites after WG exposure by a non-targeted metabolomic approach [64,67,69,70] (Table 3).
3.3.8. Untargeted Metabolomics Studies
Seven metabolomic studies with an untargeted approach met the inclusion criteria of this review [64,65,66,67,68,69,70]. The majority of them aimed to elucidate metabolites associated with WG intake. Two of these studies explored metabolites in fasting plasma [65,66], two in 24-h urine [64,70], one in 24-h urine collected at six different time-points [67], one in two-day 24-h urine [68], and one in spot urine [69]. Table 3 shows the main characteristics of these studies. The reported databases, known metabolites, and the main results are listed in Table 4.
4. Discussion
In this study, we systematically reviewed the available potential biomarkers for WG intake in humans. We found three major groups of biomarkers: (1) AR, as well as its homologs and metabolites, assessed in plasma, adipose tissue biopsies, erythrocyte membranes, and urine; (2) avenacosides, assessed in urine samples; and (3) benzoxazinoid-derived phenylacetamide sulfates, assessed in blood and urine samples.
AR, its homologs, and metabolites were the predominant group of the assessed biomarkers for WG intake. They showed good responsiveness and generally a moderate to strong short-term relative validity for WGR and WGW intake. However, some studies reported weaker correlations when assessing the relative validity of AR and its metabolites as biomarkers of the WG, WGR, and WGW intake. Different factors may contribute to these differences, such as the design, the methods, and the set-up of the studies. Stronger correlations were generally observed in RCTs compared to studies with a cross-sectional design. Yet, one study with an RCT design reported a non-significant difference in the mean concentration of P-AR between groups with high and low intake of WG [37]. Secondly, the included subjects across the assessed studies had different health status and comorbidities. The absorption, metabolism, and excretion processes of these compounds may vary depending on the clinical status of the involved organs and tissues [23,71,72,73], and may, thus, contribute to the discrepant results. Thirdly, the concentration of AR in WGR and WGW was previously found to vary widely between 360 and 3200 μg/g and 317 and 1429 μg/g, respectively [74,75,76]. This may contribute to explaining that the concentration of AR, its homologs, and metabolites in biological samples varied with the type of consumed WG in the different studies. Lastly, AR and its homologs are present in high concentrations in WGR and WGW, and in very low concentrations in maize, peas, triticale, and barley grains; they are absent in oat and rice [77,78]. The intake of oat, spelt, maize, millet, rice, and sorghum and other non-AR-containing WG contributes to the total WG intake, but not to the concentrations of AR, its homologs, or its metabolites [36,43,47,55]. In an included WG intervention study, the 12 subjects with the relatively lowest concentrations of plasma AR consumed more WG oats and less WG wheat, rye, barley, rice, and corn, compared to the rest of the subjects [36]. Thus, the concentration of AR, its homologs, and metabolites can be misleading in populations and subjects where WGR and WGW are not the primary content of WG. This review includes studies on different ethnical and geographical populations, which may also argue for the varying results.
Avenacosides were suggested as a marker for WG oat intake, primarily because these phytochemical steroid glycosides are uniquely produced in oats [79,80]. The evidence on whether avenacosides might serve as a biomarker for WG oat intake is still limited, and further research is needed. Perhaps the determination of the concentration of Avenacosides in urine could be complementary to the concentration of AR as biomarkers for WG intake, but further research is needed. Benzoxazinoid, primarily DIBOA, is the most abundant compound in different WG bread, and HHPAA and HPAA were identified after the intake of different forms of rye bread [62,81], and they were suggested as biomarkers for WG intake. In addition to WGR and WGW, they are richly found in maize and, thus, have the potential to be supplemental for AR and avenacosides in the assessment of the total WG intake. Studies with a targeted approach assessing the relative validity and the responsiveness of these compounds to the WG exposure are needed to reveal their validity.
Different multivariate statistical models combining different metabolites were proposed and showed good potential to predict the WG intake in humans [69,70]. These models are, however, mainly based on markers described before, which are limited with their short lifetime, in combination with other metabolites like enterolactone, glucuronide, and pyrraline [69]. These metabolites are not specific for WG, as they are also present in the endosperm, food additives, and many plant foods [82]. These limitations make these statistical models less promising to serve as good indicators for WG intake in a clinical or epidemiological context.
Studies on AR, its homologs, and metabolites generally showed a moderate short-term reproducibility, but a relatively poor reproducibility in the assessment of the medium- to long-term exposure to WG in the blood and urine [35,55,83,84]. To our knowledge, no study assessed the reproducibility of avenacosides and benzoxazinoid compounds. However, it is known that AR, avenacosides, and benzoxazinoid compounds share a short lifetime (<24 h) in the blood and urine. A poorly reproducible biomarker requires an extensive number of samples, at different time points, and it can still lead to biased judgments of the biomarker–disease correlation [85]. Thus, these compounds may serve as a good supplement to food frequency questionnaires and as qualitative markers of compliance in research. Their value as quantitative markers in the assessment of a habitual longer-term WG intake is, however, limited [62]. In this context of finding trustful markers of long-term intake, which is essential in both research and clinical work, other biological sample sites were investigated. The concentration of AR and its homologs in biopsies from adipose tissues generally showed a similar relative validity and reproducibility compared to those measured in plasma [43,47,50]. Equally important to the need for a validated and standardized analysis method, factors like the low price, high sensitivity, specificity, reliability, and especially less invasion determine whether a biomarker is good [86,87]. Compared to a simple peripheral blood drawing procedure, the tissue extraction procedure may be more invasive, expensive, time-consuming, and related to several clinical complications. These considerations make adipose tissues as a biomaterial less optimal in this context. Other attempts were made to assess AR in other, less invasive biological samples. It was demonstrated that AR is present in the erythrocyte membrane in subjects consuming WG. The evidence whether the concentration of AR in EM could serve as a biomarker for WG intake is still limited, and further research is needed [51]. To date, trustful biomarkers measuring the medium- and long-term WG intake are still missing.
There are several limitations to the results of this review that we should acknowledge. Firstly, this review included studies with different design and methods, which made a meta-analysis of the results not suitable. Nonetheless, the integration of the results of all these studies helped to cover the topic broadly. Secondly, our review did not interpret the difference in results across the countries, where the different studied populations were included. Different communities may have a different intake of the different types of WG, which could be important to take into consideration when comparing the results of these studies. Thirdly, we decided to use a generic checklist in the assessment of the quality and risk of bias of the included articles, notwithstanding the design, measurement, and analysis methods in these studies. We acknowledge that this decision might cause some design- and analysis-specific bias to be missed. Fourthly, the health status of the populations assessed in the majority of the included studies was different. Populations with one reported diagnosis may also suffer from other unreported diseases, which made these groups non-comparable.
Recently, Landberg et al. published a scoping review of biomarkers of different cereal types including whole and refined grains, pasta, rice, and pseudo-cereals [88]. To our knowledge, this work is the first systematic review that collected the results on the validity of the available biomarkers of WG intake in humans. The methods used in this review followed the PRISMA guidelines for systematic reviews, a study protocol was registered before the study start, and the contribution of different experts strengthened the quality of the reported data. Findings from this review have important implications for epidemiological and clinical research. In the future, more research might approach and reveal biomarkers with a greater reproducibility and validity in the assessment of the medium- to long-term consumption of WG. RCT and observational study designs have different advantages in nutritional research [18]. Future studies may benefit from the Consort and the Strobe guidelines that were established to improve the quality of these studies [89,90].
5. Conclusions
This review evaluated potential biomarkers for whole-grain intake in humans. Because biomarkers can accurately assess intake, they can be used for improved assessment of associations between whole-grain intake and health outcomes. Alkylresorcinol and their metabolites showed good responsiveness and short-term relative validity for whole-grain rye and wheat intake. They may potentially be used in research when the assessment of the short-term intake of whole-grain rye and wheat is needed. Their poor medium- to long-term reproducibility is a substantial limitation to their use in clinical settings. Furthermore, other whole grains like oats and maize would contribute to whole-grain intake and cannot be captured by these markers. Avenacosides are present in oat and were suggested as a biomarker for whole-grain oat intake. Potentially, avenacosides could serve as a supplementary marker to alkylresorcinol in the assessment of whole-grain intake, but the evidence is still limited. Benzoxazinoid derivates were proposed as potential markers for whole-grain rye and wheat intake, but, like alkylresorcinol, they are limited by their short half-lives. More research is needed to compare the relative validity and responsiveness of these derivates to alkylresorcinol. Metabolomic studies showed a potential validity when various compounds were combined in a model to assess whole grain intake. To date, biomarkers for the assessment of the medium- and long-term whole-grain intake are missing. The concentration of the revealed biomarkers may rather serve as a supplement to food frequency questionnaires and qualitative markers of compliance rather than as trustful markers of intake measure.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/12/2994/s1: Table S1: The search strategy used in different databases; Table S2. Quality assessment and the assessment of the risk of bias in the included studies
Author Contributions
Conceptualization, M.J.; formal analysis, M.J., S.B.S., and V.A.; data curation, M.J., S.B.S., and V.A.; writing—original draft preparation, M.J.; writing—review and editing, M.J., S.B.S., V.A., and B.L.H.; visualization, M.J. and S.B.S.; supervision, V.A.
Funding
This study was funded by the “European Union’s Horizon 2020 research and innovation program, grant number 733100”, The Region of Southern Denmark”, “The Hospital of Southern Jutand”, “The University of Southern Denmark”, and “Knud og Edith Eriksens mindefond”.
Acknowledgments
The authors want to thank Jitka Stilund Hansen and the Library at the University of Southern Denmark for help with the database search. We also want to thank Charlotte Leboeuf-Yde, for helping with the methodology of this review.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| WG | whole grain |
| FFQ | food frequency questionnaire |
| CI | 95% confidence interval |
| SD | standard deviation |
| SE | standard error |
| ICC | intraclass correlation coefficient |
| AR | alkylresorcinol |
| P-AR | plasma alkylresorcinol |
| WGR | whole-grain rye |
| WGW | whole-grain wheat |
| 3DFDs | 3-day food diaries |
| DRs | daily records |
| h | hours |
| RF | refined grain |
| EM | erythrocyte membrane |
| DHBA | 3,5-dihydroxybenozoic acid |
| DHPPA | 3-(3,5-dihydroxyphenyl)-1-propanoic acid |
| DHCA | 3,5-dihydroxycinnamic acid |
| DHBA-glycine | 2-(3,5-dihydroxybenzamido)acetic acid |
| DHPPTA | 5-(3,5-dihydroxyphenyl)pentanoic acid |
| DHCA-amide | 3,5dihydroxycinnamic acid amide |
| DIBOA | 2,4-dihyxdoxy 1,4-benzoxazin-3one |
| HHPAA | hydroxy-N-(2-hydroxyphenyl) acetamide |
| HPAA | N-(2hydroxyphenyl) acetamide |
References
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Brownlee, I.A. Whole-grain foods and chronic disease: Evidence from epidemiological and intervention studies. Proc. Nutr. Soc. 2015, 74, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrugger, S.; Maerkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frokiaer, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylan hydrolyzates as immunomodulators in Caco-2 and HT-29 colon cancer cell lines. Food Funct. 2017, 8, 220–231. [Google Scholar] [CrossRef]
- Andersen, V.; Holmskov, U.; Sorensen, S.B.; Jawhara, M.; Andersen, K.W.; Bygum, A.; Hvid, L.; Grauslund, J.; Wied, J.; Glerup, H.; et al. A Proposal for a Study on Treatment Selection and Lifestyle Recommendations in Chronic Inflammatory Diseases: A Danish Multidisciplinary Collaboration on Prognostic Factors and Personalised Medicine. Nutrients 2017, 9, 499. [Google Scholar] [CrossRef]
- Awika, J.M.; Rose, D.J.; Simsek, S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018, 9, 1389–1409. [Google Scholar] [CrossRef]
- Derrien, M.; Veiga, P. Rethinking Diet to Aid Human-Microbe Symbiosis. Trends Microbial. 2017, 25, 100–112. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Nicklas, T.A.; Zanovec, M.; Cho, S. Whole-grain consumption is associated with diet quality and nutrient intake in adults: The National Health and Nutrition Examination Survey, 1999–2004. J. Am. Diet. Assoc. 2010, 110, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Hur, I.Y.; Reicks, M. Relationship between whole-grain intake, chronic disease risk indicators, and weight status among adolescents in the National Health and Nutrition Examination Survey, 1999–2004. J. Acad. Nutr. Diet. 2012, 112, 46–55. [Google Scholar] [CrossRef] [PubMed]
- The American Association of Cereal Chemists. The American Association of Cereal Chemists—AACCI Definitions of Whole Grain/Sprouted Grain/Whole Grain Product. Available online: https://www.aaccnet.org/initiatives/definitions/Pages/WholeGrain.aspx (accessed on 3 December 2019).
- Slavin, J.; Tucker, M.; Harriman, C.; Jonnalagadda, S.S. Whole Grains: Definition, Dietary Recommendations, and Health Benefits. Cereal Foods World 2013, 58, 191–198. [Google Scholar] [CrossRef]
- Ministry of Environment and Food of Denmark. The Official Dietary Guidelines. 2018. Available online: https://www.foedevarestyrelsen.dk/english/Food/Nutrition/The_dietary_recommendations/Pages/default.aspx (accessed on 10 December 2018).
- Foedevarestyrelsen. Rugbrød til aftensmad er også sund fornuft. 2017. Available online: https://www.foedevarestyrelsen.dk/Nyheder/Aktuelt/Sider/Nyheder_2017/Rugbr%C3%B8d_til_aftensmad_er_ogs%C3%A5_sund_fornuft.aspx# (accessed on 3 December 2019).
- Kaaks, R.J. Biochemical markers as additional measurements in studies of the accuracy of dietary questionnaire measurements: Conceptual issues. Am. J. Clin. Nutr. 1997, 65, 1232S–1239S. [Google Scholar] [CrossRef]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding Nutritional Epidemiology and Its Role in Policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef]
- Tasevska, N.; Midthune, D.; Tinker, L.F.; Potischman, N.; Lampe, J.W.; Neuhouser, M.L.; Beasley, J.M.; Van Horn, L.; Prentice, R.L.; Kipnis, V. Use of a urinary sugars biomarker to assess measurement error in self-reported sugars intake in the nutrition and physical activity assessment study (NPAAS). Cancer Epidemiol. Prev. Biomark. 2014, 23, 2874–2883. [Google Scholar] [CrossRef]
- Ross, A.B.; Pineau, N.; Kochhar, S.; Bourgeois, A.; Beaumont, M.; Decarli, B. Validation of a FFQ for estimating whole-grain cereal food intake. Br. J. Nutr. 2009, 102, 1547–1551. [Google Scholar] [CrossRef]
- Ross, A.B.; Kristensen, M.; Seal, C.J.; Jacques, P.; McKeown, N.M. Recommendations for reporting whole-grain intake in observational and intervention studies. Am. J. Clin. Nutr. 2015, 101, 903–907. [Google Scholar] [CrossRef]
- McKeown, N.M.; Jacques, P.F.; Seal, C.J.; de Vries, J.; Jonnalagadda, S.S.; Clemens, R.; Webb, D.; Murphy, L.A.; van Klinken, J.W.; Topping, D.; et al. Whole grains and health: From theory to practice—Highlights of The Grains for Health Foundation’s Whole Grains Summit 2012. J. Nutr. 2013, 143, 744S–758S. [Google Scholar] [CrossRef]
- Ross, A.B.; Kamal-Eldin, A.; Aman, P. Dietary alkylresorcinols: Absorption, bioactivities, and possible use as biomarkers of whole-grain wheat- and rye-rich foods. Nutr. Rev. 2004, 62, 81–95. [Google Scholar] [CrossRef]
- Kaaks, R.; Ferrari, P.; Ciampi, A.; Plummer, M.; Riboli, E. Uses and limitations of statistical accounting for random error correlations, in the validation of dietary questionnaire assessments. Public Health Nutr. 2002, 5, 969–976. [Google Scholar] [CrossRef] [PubMed]
- The United Nations Environment Programme; The World Health Organization. Biomarkers in Risk Assessment: Validity and Validation; The International Programme on Chemical Safety (IPCS): Geneva, Switzerland, 2001; Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 3 December 2019).
- Van Loveren, C.; Aartman, I.H. The PICO (Patient-Intervention-Comparison-Outcome) question. Ned. Tijdschr. Tandheelkd. 2007, 114, 172–178. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA StatementThe PRISMA Statement. Ann. Int. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 3 December 2019).
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co: Belmont, CA, USA, 1996. [Google Scholar]
- Cohen, L. Measurement of Life Events; Cohen, L.H., Ed.; Life Events and Psychological Functioning; Sage: Newbury Park, CA, USA, 1988; pp. 11–30. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, R.; Zamaratskaia, G.; Kamal-Eldin, A.; Landberg, R. Novel urinary alkylresorcinol metabolites as biomarkers of whole grain intake in free-living Swedish adults. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Landberg, R.; Wierzbicka, R.; Shi, L.; Nybacka, S.; Kamal-Eldin, A.; Hedblad, B.; Lindroos, A.K.; Winkvist, A.; Forslund, H.B. New alkylresorcinol metabolites in spot urine as biomarkers of whole grain wheat and rye intake in a Swedish middle-aged population. Eur. J. Clin. Nutr. 2018, 72, 1439. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Koskela, A.; Marjamaa, A.; Adlercreutz, H. Plasma alkylresorcinols and urinary alkylresorcinol metabolites as biomarkers of cereal fiber intake in Finnish women. Cancer Epidemiol. Prev. Biomark. 2008, 17, 2244–2248. [Google Scholar] [CrossRef]
- Andersson, A.; Marklund, M.; Diana, M.; Landberg, R. Plasma alkylresorcinol concentrations correlate with whole grain wheat and rye intake and show moderate reproducibility over a 2- to 3-month period in free-living Swedish adults. J. Nutr. 2011, 141, 1712–1718. [Google Scholar] [CrossRef]
- Ampatzoglou, A.; Atwal, K.; Maidens, C.; Williams, C.; Ross, A.; Thielecke, F.; Jonnalagadda, S.; Kennedy, O.; Yaqoob, P. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J. Nutr. 2014, 145, 215–221. [Google Scholar] [CrossRef]
- Biltoft-Jensen, A.; Damsgaard, C.; Andersen, E.; Ygil, K.; Andersen, R.; Ege, M.; Christensen, T.; Thorsen, A.; Tetens, I.; Wu, H.; et al. Validation of Reported Whole-Grain Intake from a Web-Based Dietary Record against Plasma Alkylresorcinol Concentrations in 8- to 11-Year-Olds Participating in a Randomized Controlled Trial. J. Nutr. 2016, 146, 377–383. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Andersson, A.; Vessby, B.; Aman, P. Alkylresorcinols as biomarkers of whole-grain wheat and rye intake: Plasma concentration and intake estimated from dietary records. Am. J. Clin. Nutr. 2008, 87, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Aman, P.; Friberg, L.; Vessby, B.; Adlercreutz, H.; Kamal-Eldin, A. Dose response of whole-grain biomarkers: Alkylresorcinols in human plasma and their metabolites in urine in relation to intake. Am. J. Clin. Nutr. 2009, 89, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Linko, A.M.; Juntunen, K.S.; Mykkanen, H.M.; Adlercreutz, H. Whole-grain rye bread consumption by women correlates with plasma alkylresorcinols and increases their concentration compared with low-fiber wheat bread. J. Nutr. 2005, 135, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Magnusdottir, O.; Landberg, R.; Gunnarsdottir, I.; Cloetens, L.; Åkesson, B.; Önning, G.; Jonsdottir, S.; Rosqvist, F.; Schwab, U.; Herzig, K.; et al. Plasma alkylresorcinols reflect important whole-grain components of a healthy Nordic diet. J. Nutr. 2013, 143, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.; Marklund, M.; Ma, J.; Ross, A.; Lichtenstein, A.; Livingston, K.; Jacques, P.; Rasmussen, H.; Blumberg, J.; Chen, C. Comparison of plasma alkylresorcinols (AR) and urinary AR metabolites as biomarkers of compliance in a short-term, whole-grain intervention study. Eur. J. Nutr. 2016, 55, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K.; Uusitupa, M.; Schwab, U.; Wolk, A.; Landberg, R. Alkylresorcinols in adipose tissue biopsies as biomarkers of whole-grain intake: An exploratory study of responsiveness to advised intake over 12 weeks. Eur. J. Clin. Nutr. 2015, 69, 1244–1248. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Andersson, S.O.; Johansson, J.E.; Zhang, J.X.; Hallmans, G.; Aman, P. Reproducibility of plasma alkylresorcinols during a 6-week rye intervention study in men with prostate cancer. J. Nutr. 2009, 139, 975–980. [Google Scholar] [CrossRef]
- Ross, A.B.; Bourgeois, A.; Macharia, H.N.U.; Kochhar, S.; Jebb, S.A.; Brownlee, I.A.; Seal, C.J. Plasma alkylresorcinols as a biomarker of whole-grain food consumption in a large population: Results from the WHOLEheart Intervention Study. Am. J. Clin. Nutr. 2012, 95, 204–211. [Google Scholar] [CrossRef]
- Knudsen, M.D.; Kyro, C.; Olsen, A.; Dragsted, L.O.; Skeie, G.; Lund, E.; Aman, P.; Nilsson, L.M.; Bueno-de-Mesquita, H.B.; Tjonneland, A.; et al. Self-Reported Whole-Grain Intake and Plasma Alkylresorcinol Concentrations in Combination in Relation to the Incidence of Colorectal Cancer. Am. J. Epidemiol. 2014, 179, 1188–1196. [Google Scholar] [CrossRef]
- Wu, H.; Mhd Omar, N.A.; Hakansson, N.; Wolk, A.; Michaelsson, K.; Landberg, R. Evaluation of alkylresorcinols in adipose tissue biopsies as a long-term biomarker of whole-grain wheat and rye intake in free-living Swedish men and women. Public Health Nutr. 2018, 21, 1933–1942. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Aman, P.; Christensen, J.; Overvad, K.; Tjonneland, A.; Olsen, A. Determinants of plasma alkylresorcinol concentration in Danish post-menopausal women. Eur. J. Clin. Nutr. 2011, 65, 94–101. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Hruby, A.; Landberg, R.; Herrington, D.M.; Lichtenstein, A.H. Plasma alkylresorcinols, biomarkers of whole-grain intake, are not associated with progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Public Health Nutr. 2016, 19, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.; Landberg, R.; Kamal-Eldin, A.; Wolk, A.; Vessby, B.; Aman, P. Presence of alkylresorcinols, potential whole grain biomarkers, in human adipose tissue. Br. J. Nutr. 2010, 104, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Linko, A.M.; Adlercreutz, H. Whole-grain rye and wheat alkylresorcinols are incorporated into human erythrocyte membranes. Br. J. Nutr. 2005, 93, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Koskela, A.; Samaletdin, A.; Adlercreutz, H. Responsiveness of urinary and plasma alkylresorcinol metabolites to rye intake in finnish women. Cancers 2010, 2, 513–522. [Google Scholar] [CrossRef]
- Guyman, L.A.; Adlercreutz, H.; Koskela, A.; Li, L.; Beresford, S.A.A.; Lampe, J.W. Urinary 3-(3,5-dihydroxyphenyl)-1-propanoic acid, an alkylresorcinol metabolite, is a potential biomarker of whole-grain intake in a US population. J. Nutr. 2008, 138, 1957–1962. [Google Scholar] [CrossRef]
- Landberg, R.; Townsend, M.K.; Neelakantan, N.; Sun, Q.; Sampson, L.; Spiegelman, D.; van Dam, R.M. Alkylresorcinol metabolite concentrations in spot urine samples correlated with whole grain and cereal fiber intake but showed low to modest reproducibility over one to three years in U.S. women. J. Nutr. 2012, 142, 872–877. [Google Scholar] [CrossRef]
- Marklund, M.; Landberg, R.; Andersson, A.; Aman, P.; Kamal-Eldin, A. Alkylresorcinol metabolites in urine correlate with the intake of whole grains and cereal fibre in free-living Swedish adults. Br. J. Nutr. 2013, 109, 129–136. [Google Scholar] [CrossRef]
- Meija, L.; Krams, I.; Cauce, V.; Samaletdin, A.; Soderholm, P.; Meija, R.; Larmane, L.; Lejnieks, A.; Lietuvietis, V.; Adlercreutz, H. Alkylresorcinol Metabolites in Urine and Plasma as Potential Biomarkers of Rye and Wheat Fiber Consumption in Prostate Cancer Patients and Controls. Nutr. Cancer Int. J. 2015, 67, 258–265. [Google Scholar] [CrossRef]
- Ross, A.B.; Aman, P.; Kamal-Eldin, A. Identification of cereal alkylresorcinol metabolites in human urine - potential biomarkers of wholegrain wheat and rye intake. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 809, 125–130. [Google Scholar] [CrossRef]
- Zhu, Y.; Shurlknight, K.L.; Chen, X.; Sang, S. Identification and pharmacokinetics of novel alkylresorcinol metabolites in human urine, new candidate biomarkers for whole-grain wheat and rye intake. J. Nutr. 2014, 144, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Koskela, A.; Samaletdin, A.; Adlercreutz, H. Plasma alkylresorcinol metabolites as potential biomarkers of whole-grain wheat and rye cereal fibre intakes in women. Br. J. Nutr. 2010, 103, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Sonestedt, E.; Gullberg, B.; Bjartell, A.; Olsson, H.; Adlercreutz, H.; Tikkanen, M.J.; Wirfalt, E.; Wallstrom, P. Plasma alkylresorcinol metabolites as biomarkers for whole-grain intake and their association with prostate cancer: A Swedish nested case-control study. Cancer Epidemiol. Prev. Biomark. 2014, 23, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, P.P.; Koskela, A.H.; Lundin, J.E.; Tikkanen, M.J.; Adlercreutz, H.C. Plasma pharmacokinetics of alkylresorcinol metabolites: New candidate biomarkers for whole-grain rye and wheat intake. Am. J. Clin. Nutr. 2009, 90, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Keski-Rahkonen, P.; Lappi, J.; Katina, K.; Pekkinen, J.; Savolainen, O.; Timonen, O.; Paananen, J.; Mykkanen, H.; Poutanen, K. The postprandial plasma rye fingerprint includes benzoxazinoid-derived phenylacetamide sulfates. J. Nutr. 2014, 144, 1016–1022. [Google Scholar] [CrossRef]
- Wang, P.; Yang, J.; Yerke, A.; Sang, S. Avenacosides: Metabolism, and potential use as exposure biomarkers of oat intake. Mol. Nutr. Food Res. 2017, 61, 1700196. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Barri, T.; Hanhineva, K.; Juntunen, K.; Dragsted, L.O.; Mykkanen, H.; Poutanen, K. UPLC-QTOF/MS metabolic profiling unveils urinary changes in humans after a whole grain rye versus refined wheat bread intervention. Mol. Nutr. Food Res. 2013, 57, 412–422. [Google Scholar] [CrossRef]
- Johansson-Persson, A.; Barri, T.; Ulmius, M.; Onning, G.; Dragsted, L. LC-QTOF/MS metabolomic profiles in human plasma after a 5-week high dietary fiber intake. Anal. Bioanal. Chem. 2013, 405, 4799–4809. [Google Scholar] [CrossRef]
- Hanhineva, K.; Lankinen, M.A.; Pedret, A.; Schwab, U.; Kolehmainen, M.; Paananen, J.; de Mello, V.; Sola, R.; Lehtonen, M.; Poutanen, K.; et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J. Nutr. 2015, 145, 7–17. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Wang, P.; Sha, W.; Sang, S.M. Urinary Biomarkers of Whole Grain Wheat Intake Identified by Non-targeted and Targeted Metabolomics Approaches. Sci. Rep. 2016, 6, 36278. [Google Scholar] [CrossRef]
- Coulomb, M.; Gombert, A.; Moazzami, A.A. Metabolomics study of cereal grains reveals the discriminative metabolic markers associated with anatomical compartments. Ital. J. Food Sci. 2015, 27, 142–150. [Google Scholar]
- Garcia-Aloy, M.; Llorach, R.; Urpi-Sarda, M.; Tulipani, S.; Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Corella, D.; Fito, M.; Estruch, R.; Serra-Majem, L.; et al. Nutrimetabolomics fingerprinting to identify biomarkers of bread exposure in a free-living population from the PREDIMED study cohort. Metabolomics 2015, 11, 155–165. [Google Scholar] [CrossRef]
- Hanhineva, K.; Brunius, C.; Andersson, A.; Marklund, M.; Juvonen, R.; Keski-Rahkonen, P.; Auriola, S.; Landberg, R. Discovery of urinary biomarkers of whole grain rye intake in free-living subjects using nontargeted LC-MS metabolite profiling. Mol. Nutr. Food Res. 2015, 59, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Kamal-Eldin, A.; Lundin, E.A.; Zhang, J.X.; Hallmans, G.; Aman, P. Cereal alkylresorcinols are absorbed by humans. J. Nutr. 2003, 133, 2222–2224. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M.; Stromberg, E.A.; Laerke, H.N.; Bach Knudsen, K.E.; Kamal-Eldin, A.; Hooker, A.C.; Landberg, R. Simultaneous pharmacokinetic modeling of alkylresorcinols and their main metabolites indicates dual absorption mechanisms and enterohepatic elimination in humans. J. Nutr. 2014, 144, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Shepherd, M.J.; Knudsen, K.E.B.; Glitso, L.V.; Bowey, E.; Phillips, J.; Rowland, I.; Guo, Z.X.; Massy, D.J.R.; Aman, P.; et al. Absorption of dietary alkylresorcinols in ileal-cannulated pigs and rats. Br. J. Nutr. 2003, 90, 787–794. [Google Scholar] [CrossRef]
- Tłuścik, F. Localization of the alkylresorcinols in rye and wheat caryopses. Acta Soc. Bot. Polon. 1978, 47, 211–218. [Google Scholar] [CrossRef]
- Suresh, G.; Dan, P.; Ann-Christine, S.; Per, Å. Analysis of alkyl- and alkenylresorcinols in triticale, wheat and rye. J. Sci. Food Agric. 1988, 45, 43–52. [Google Scholar] [CrossRef]
- Wieringa, G.W. On the Occurrence of Growth Inhibiting Substances in Rye; Institute for Storage and Processing of Agricultural Produce: Wageningen, The Netherlands, 1967. [Google Scholar]
- Ross, A.B.; Shepherd, M.J.; Schupphaus, M.; Sinclair, V.; Alfaro, B.; Kamal-Eldin, A.; Aman, P. Alkylresorcinols in cereals and cereal products. J. Agric. Food Chem. 2003, 51, 4111–4118. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Salmenkallio-Marttila, M.; Rouau, X.; Åman, P. Localization of alkylresorcinols in wheat, rye and barley kernels. J. Cereal Sci. 2008, 48, 401–406. [Google Scholar] [CrossRef]
- Sang, S.; Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, P.; Wu, W.; Zhao, Y.; Idehen, E.; Sang, S. Steroidal Saponins in Oat Bran. J. Agric. Food Chem. 2016, 64, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Rogachev, I.; Aura, A.M.; Aharoni, A.; Poutanen, K.; Mykkanen, H. Qualitative characterization of benzoxazinoid derivatives in whole grain rye and wheat by LC-MS metabolite profiling. J. Agric. Food Chem. 2011, 59, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ross, A.B.; Aman, P.; Kamal-Eldin, A. Alkylresorcinols as markers of whole grain wheat and rye in cereal products. J. Agric. Food Chem. 2004, 52, 8242–8246. [Google Scholar] [CrossRef]
- Landberg, R.; Aman, P.; Hallmans, G.; Johansson, I. Long-term reproducibility of plasma alkylresorcinols as biomarkers of whole-grain wheat and rye intake within Northern Sweden Health and Disease Study Cohort. Eur. J. Clin. Nutr. 2013, 67, 259–263. [Google Scholar] [CrossRef]
- Montonen, J.; Landberg, R.; Kamal-Eldin, A.; Aman, P.; Boeing, H.; Steffen, A.; Pischon, T. Reliability of fasting plasma alkylresorcinol metabolites concentrations measured 4 months apart. Eur. J. Clin. Nutr. 2012, 66, 968–970. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Natarajan, L.; Sun, X.; Rock, C.L.; Pierce, J.P.; Women’s Healthy, E.; Living Study, G. Reliability of plasma carotenoid biomarkers and its relation to study power. Epidemiology 2008, 19, 338–344. [Google Scholar] [CrossRef]
- Crews, H.; Alink, G.; Andersen, R.; Braesco, V.; Holst, B.; Maiani, G.; Ovesen, L.; Scotter, M.; Solfrizzo, M.; van den Berg, R.; et al. A critical assessment of some biomarker approaches linked with dietary intake. Br. J. Nutr. 2001, 86 (Suppl. 1), S5–S35. [Google Scholar] [CrossRef]
- Weber, P. Role of biomarkers in nutritional science and industry—A comment. Br. J. Nutr. 2001, 86 (Suppl. 1), S93–S95. [Google Scholar] [CrossRef][Green Version]
- Landberg, R.; Hanhineva, K.; Tuohy, K.; Garcia-Aloy, M.; Biskup, I.; Llorach, R.; Yin, X.; Brennan, L.; Kolehmainen, M. Biomarkers of cereal food intake. Genes Nutr. 2019, 14, 28. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).