Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects, Study Design, and Treatment Protocol
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
3.1. Comparisons between Survivors and Non-Survivors
3.2. Baseline Characteristics and Clinical Outcomes between Study Groups
3.3. Physiological Characteristics between Study Groups
3.4. Septic Cardiomyopathy
3.5. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Ortega, A.; Suberviola, B.; Garcia-Astudillo, L.A.; Holanda, M.S.; Ortiz, F.; Llorca, J.; Delgado-Rodríguez, M. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: Results of a three-year follow-up quasi-experimental study. Crit. Care Med. 2010, 38, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Keh, D.; Boehnke, T.; Weber-Cartens, S.; Schulz, C.; Ahlers, O.; Bercker, S.; Volk, H.D.; Doecke, W.D.; Falke, K.J.; Gerlach, H. Immunologic and hemodynamic effects of "low-dose" hydrocortisone in septic shock: A double-blind, randomized, placebo-controlled, crossover study. Am. J. Respir. Crit. Care Med. 2003, 167, 512–520. [Google Scholar] [CrossRef]
- Marik, P.E.; Pastores, S.M.; Annane, D.; Meduri, G.U.; Sprung, C.L.; Arlt, W.; Keh, D.; Briegel, J.; Beishuizen, A.; Dimopoulou, I.; et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit. Care Med. 2008, 36, 1937–1949. [Google Scholar] [CrossRef]
- Annane, D.; Bellissant, E.; Sebille, V.; Lesieur, O.; Mathieu, B.; Raphael, J.C.; Gajdos, P. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. Br. J. Clin. Pharmacol. 1998, 46, 589–597. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Bruegger, D.; Rehm, M.; Conzen, P.; Welsch, U.; Becker, B.F. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007, 107, 776–784. [Google Scholar] [CrossRef]
- Téblick, A.; Peeters, B.; Langouche, L.; Van den Berghe, G. Adrenal function and dysfunction in critically ill patients. Nat. Rev. Endocrinol. 2019, 15, 417–427. [Google Scholar] [CrossRef]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive glucocorticoid therapy in patients with septic shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef]
- Venkatesh, B.; Cohen, J. Why the adjunctive corticosteroid treatment in critically Ill patients with septic shock (ADRENAL) trial did not show a difference in mortality. Crit. Care Med. 2019, 47, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Annane, D. Why my steroid trials in septic shock were “Positive”. Crit. Care Med. 2019, 47, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic signatures in sepsis and a differential response to steroids. from the VANISH randomized trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef]
- Wilson, J.X. Mechanism of action of vitamin C in sepsis: Ascorbate modulates redox signaling in endothelium. Biofactors 2009, 35, 5–13. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Okamoto, K.; Tanaka, H.; Makino, Y.; Makino, I. Restoration of the glucocorticoid receptor function by the phosphodiester compound of vitamins C and E, EPC-K1 (L-ascorbic acid 2-[3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6 -yl hydrogen phosphate] potassium salt), via a redox-dependent mechanism. Biochem. Pharmacol. 1998, 56, 79–86. [Google Scholar]
- Fujita, I.; Hirano, J.; Itoh, N.; Nakanishi, T.; Tanaka, K. Dexamethasone induces sodium-dependant vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. Br. J. Nutr. 2001, 86, 145–149. [Google Scholar] [CrossRef]
- De Andrade, J.A.A.; Gayer, C.R.M.; Nogueira, N.P.A.; Paes, M.C.; Bastos, V.; Neto, J.; Alves, S.C., Jr.; Coelho, R.M.; da Cunha, M.G.A.T.; Gomes, R.N. The effect of thiamine deficiency on inflammation, oxidative stress and cellular migration in an experimental model of sepsis. J. Inflamm. 2014, 11, 11. [Google Scholar] [CrossRef]
- Woolum, J.A.; Abner, E.L.; Kelly, A.; Thompson Bastin, M.L.; Morris, P.E.; Flannery, A.H. Effect of Thiamine administration on lactate clearance and mortality in patients with septic shock. Crit. Care Med. 2018, 46, 1747–1752. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, vitamin c, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jo, E.J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.K.; Lee, M.K.; Lee, K. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: Propensity score-based analysis of a before-after cohort study. J. Crit. Care 2018, 47, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Patel, B.M.; Chittock, D.R.; Russell, J.A.; Walley, K.R. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology 2002, 96, 576–582. [Google Scholar] [CrossRef]
- Gaies, M.G.; Gurney, J.G.; Yen, A.H.; Napoli, M.L.; Gajarski, R.J.; Ohye, R.G.; Charpie, J.R.; Hirsch, J.C. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 2010, 11, 234–238. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Griffee, M.J.; Merkel, M.J.; Wei, K.S. The role of echocardiography in hemodynamic assessment of septic shock. Crit. Care Clin. 2010, 26, 365–382. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Dhar-Mascareno, M.; Carcamo, J.M.; Golde, D.W. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic. Biol. Med. 2005, 38, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Manzetti, S.; Zhang, J.; van der Spoel, D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014, 53, 821–835. [Google Scholar] [CrossRef]
- Gibson, G.E.; Zhang, H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem. Int. 2002, 40, 493–504. [Google Scholar] [CrossRef]
- Krishnagopalan, S.; Kumar, A.; Parrillo, J.E.; Kumar, A. Myocardial dysfunction in the patient with sepsis. Curr. Opin. Crit. Care 2002, 8, 376–388. [Google Scholar] [CrossRef]
- Hao, J.; Li, W.W.; Du, H.; Zhao, Z.F.; Liu, F.; Lu, J.C.; Yang, X.C.; Cui, W. Role of vitamin C in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial KATP channel. Chem. Pharm. Bull. 2016, 64, 548–557. [Google Scholar] [CrossRef][Green Version]
- Rumbus, Z.; Matics, R.; Hegyi, P.; Zsiboras, C.; Szabo, I.; Illes, A.; Petervari, E.; Balasko, M.; Marta, K.; Miko, A.; et al. Fever Is Associated with Reduced, Hypothermia with increased mortality in septic patients: A meta-analysis of clinical trials. PLoS ONE 2017, 12, e0170152. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Zaloga, G.P. Hypothermia and cytokines in septic shock. Norasept II Study Investigators. North American study of the safety and efficacy of murine monoclonal antibody to tumor necrosis factor for the treatment of septic shock. Intensive Care Med. 2000, 26, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Wiewel, M.A.; Harmon, M.B.; van Vught, L.A.; Scicluna, B.P.; Hoogendijk, A.J.; Horn, J.; Zwinderman, A.H.; Cremer, O.L.; Bonten, M.J.; Schultz, M.J.; et al. Risk factors, host response and outcome of hypothermic sepsis. Crit. Care 2016, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Bhavani, S.V.; Carey, K.A.; Gilbert, E.R.; Afshar, M.; Verhoef, P.A.; Churpek, M.M. Identifying novel sepsis subphenotypes using temperature trajectories. Am. J. Respir. Crit. Care Med. 2019, 200, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Azad, T.D.; Donato, M.; Haynes, W.A.; Perumal, T.M.; Henao, R.; Bermejo-Martin, J.F.; Almansa, R.; Tamayo, E.; Howrylak, J.A.; et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit. Care Med. 2018, 46, 915–925. [Google Scholar] [CrossRef]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis pathophysiology, chronic critical illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Huijskens, M.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.; Tilanus, M.G.; Bos, G.M.; Wieten, L.; Germeraad, W.T. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013, 19, 2054–2067. [Google Scholar] [CrossRef]
- Wilson, J.X. Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid. Redox Signal. 2013, 19, 2129–2140. [Google Scholar] [CrossRef]
- Keh, D.; Trips, E.; Marx, G.; Wirtz, S.P.; Abduljawwad, E.; Bercker, S.; Bogatsch, H.; Briegel, J.; Engel, C.; Gerlach, H.; et al. Effect of hydrocortisone on development of shock among patients with severe sepsis: The hypress randomized clinical trial. JAMA 2016, 316, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.G.; Kim, Y.J.; Ryoo, S.M.; Hwang, S.Y.; Jo, I.J.; Chung, S.P.; Choi, S.H.; Suh, G.J.; Kim, W.Y. Early vitamin C and thiamine administration to patients with septic shock in emergency departments: Propensity score-based analysis of a before-and-after cohort study. J. Clin. Med. 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 127) | Survivors (n = 84) | Non-Survivors (n = 43) | p |
|---|---|---|---|---|
| Age, years | 77 (68–83) | 77 (68–82) | 79 (68–84) | 0.61 |
| Male sex | 75 (59) | 47 (56) | 28 (65) | 0.32 |
| Body mass index, kg/m2 | 21.3 (18.1–24.2) | 21.3 (17.9–24.2) | 21.1 (18.6–23.3) | 0.98 |
| Comorbidities | ||||
| Diabetes | 44 (35) | 28 (33) | 16 (37) | 0.66 |
| Chronic heart failure | 15 (12) | 8 (10) | 7 (16) | 0.26 |
| Chronic neurologic disease | 38 (30) | 26 (31) | 12 (28) | 0.72 |
| Chronic lung disease | 20 (16) | 11 (13) | 9 (21) | 0.25 |
| Liver cirrhosis | 10 (8) | 5 (6) | 5 (12) | 0.31 |
| Chronic kidney disease | 27 (21) | 15 (18) | 12 (28) | 0.19 |
| Malignancy | 29 (23) | 18 (21) | 11 (26) | 0.60 |
| Immunocompromised | 29 (23) | 17 (20) | 12 (28) | 0.33 |
| Nosocomial infection | 49 (39) | 29 (35) | 20 (47) | 0.19 |
| Cause of sepsis | ||||
| Pneumonia | 56 (44) | 32 (38) | 24 (56) | 0.06 |
| Urosepsis | 35 (28) | 30 (36) | 5 (12) | 0.004 |
| Gastrointestinal/biliary | 24 (19) | 18 (21) | 6 (14) | 0.31 |
| Skin/soft tissue | 8 (6) | 4 (5) | 4 (9) | 0.44 |
| Concurrent bacteremia | 36 (28) | 25 (30) | 11 (26) | 0.62 |
| ARDS at ICU admission | 10 (8) | 5 (6) | 5 (12) | 0.31 |
| APACHE II score | 28 (20–34) | 25 (18–30) | 31 (28–39) | <0.001 |
| SOFA score | 12 (10–14) | 11 (10–12) | 13 (12–15) | <0.001 |
| Mechanical ventilation | 87 (69) | 48 (57) | 39 (91) | <0.001 |
| Neuromuscular blocker | 35 (28) | 15 (18) | 20 (47) | 0.001 |
| Renal replacement therapy | 41 (32) | 11 (13) | 30 (70) | <0.001 |
| Vital signs and laboratory data | ||||
| Body temperature, °C | 37.0 (36.7–38.0) | 37.2 (36.8–38.0) | 36.9 (36.4–37.8) | 0.01 |
| Mean arterial pressure, mmHg | 60 (55–65) | 60 (55–66) | 58 (54–64) | 0.20 |
| Respiratory rate, breaths/min | 28 (24–32) | 27 (24–31) | 30 (26–34) | 0.03 |
| PaO2/FiO2 | 214 (130–314) | 240 (157–350) | 147 (103–272) | 0.007 |
| Bicarbonate, mEq/L | 19.2 (16.3–22.0) | 19.4 (16.3–22.8) | 19.0 (16.1–21.2) | 0.59 |
| Creatinine, mg/dL | 1.4 (0.9–2.2) | 1.3 (0.7–2.0) | 1.7 (1.1–2.5) | 0.11 |
| White cell count, 1000/mm3 | 14.4 (8.0–21.8) | 15.5 (9.3–21.9) | 10.9 (4.1–20.9) | 0.08 |
| Total bilirubin, mg/dL | 0.9 (0.5–1.6) | 0.8 (0.5–1.6) | 0.9 (0.5–1.9) | 0.36 |
| C-reactive protein, mg/L | 135 (82–223) | 137 (85–247) | 133 (82–197) | 0.74 |
| Lactate, mmol/L | 4.0 (2.5–7.0) | 3.3 (2.3–6.4) | 6.1 (3.9–8.5) | 0.001 |
| Norepi eq dose, ug/min | 15.0 (9.4–21.3) | 13.0 (5.6–18.7) | 21.1 (12.9–32.4) | <0.001 |
| Vasoactive-inotropic score | 30.0 (18.6–48.7) | 23.5 (14.1–44.1) | 46.4 (26.7–74.7) | <0.001 |
| Echocardiography (n = 47/21) 1 | ||||
| Ejection fraction, % | 56 (42–63) | 57 (44–63) | 55 (42–61) | 0.55 |
| Septic cardiomyopathy | 22 (32) | 13 (28) | 9 (43) | 0.22 |
| Time from shock onset to vitamin C protocol, h | 6 (2–12) | 5 (1–12) | 7 (3–12) | 0.27 |
| Variable | Group A (n = 27) | Group B (n = 30) | Group C (n = 35) | Group D (n = 35) | p |
|---|---|---|---|---|---|
| Age, years | 77 (70–82) | 77 (66–81) | 78 (68–84) | 79 (64–85) | 0.88 |
| Male sex | 17 (63) | 18 (60) | 20 (57) | 20 (57) | 0.96 |
| Body mass index, kg/m2 | 21.3 (17.9–23.3) | 22.8 (19.6–25.2) | 21.6 (19.6–25.1) | 19.7 (17.5–22.3) | 0.04 |
| Comorbidities | |||||
| Diabetes | 9 (33) | 12 (40) | 11 (31) | 12 (34) | 0.91 |
| Chronic heart failure | 2 (7) | 4 (13) | 5 (14) | 4 (11) | 0.87 |
| Chronic neurologic disease | 9 (33) | 8 (27) | 10 (29) | 11 (31) | 0.95 |
| Chronic lung disease | 7 (26) | 2 (7) | 8 (23) | 3 (9) | 0.09 |
| Liver cirrhosis | 1 (4) | 1 (3) | 5 (14) | 3 (9) | 0.38 |
| Chronic kidney disease | 3 (11) | 6 (20) | 8 (23) | 10 (29) | 0.41 |
| Malignancy | 6 (22) | 6 (20) | 8 (23) | 9 (26) | 0.96 |
| Immunocompromised | 5 (19) | 6 (20) | 7 (20) | 11 (31) | 0.56 |
| Nosocomial infection | 12 (44) | 14 (47) | 11 (31) | 12 (34) | 0.52 |
| Cause of sepsis | |||||
| Pneumonia | 11 (41) | 15 (50) | 13 (37) | 17 (49) | 0.68 |
| Urosepsis | 7 (26) | 8 (27) | 13 (37) | 7 (20) | 0.45 |
| Gastrointestinal/biliary | 7 (26) | 6 (20) | 5 (14) | 6 (17) | 0.69 |
| Skin/soft tissue | 0 | 4 (13) | 1 (3) | 3 (9) | 0.17 |
| Concurrent Bacteremia | 5 (19) | 8 (27) | 14 (40) | 9 (26) | 0.29 |
| ARDS at ICU admission | 2 (7) | 3 (10) | 3 (9) | 2 (6) | 0.97 |
| APACHE II score | 30 (26–35) | 28 (21–34) | 28 (19–33) | 26 (19–32) | 0.45 |
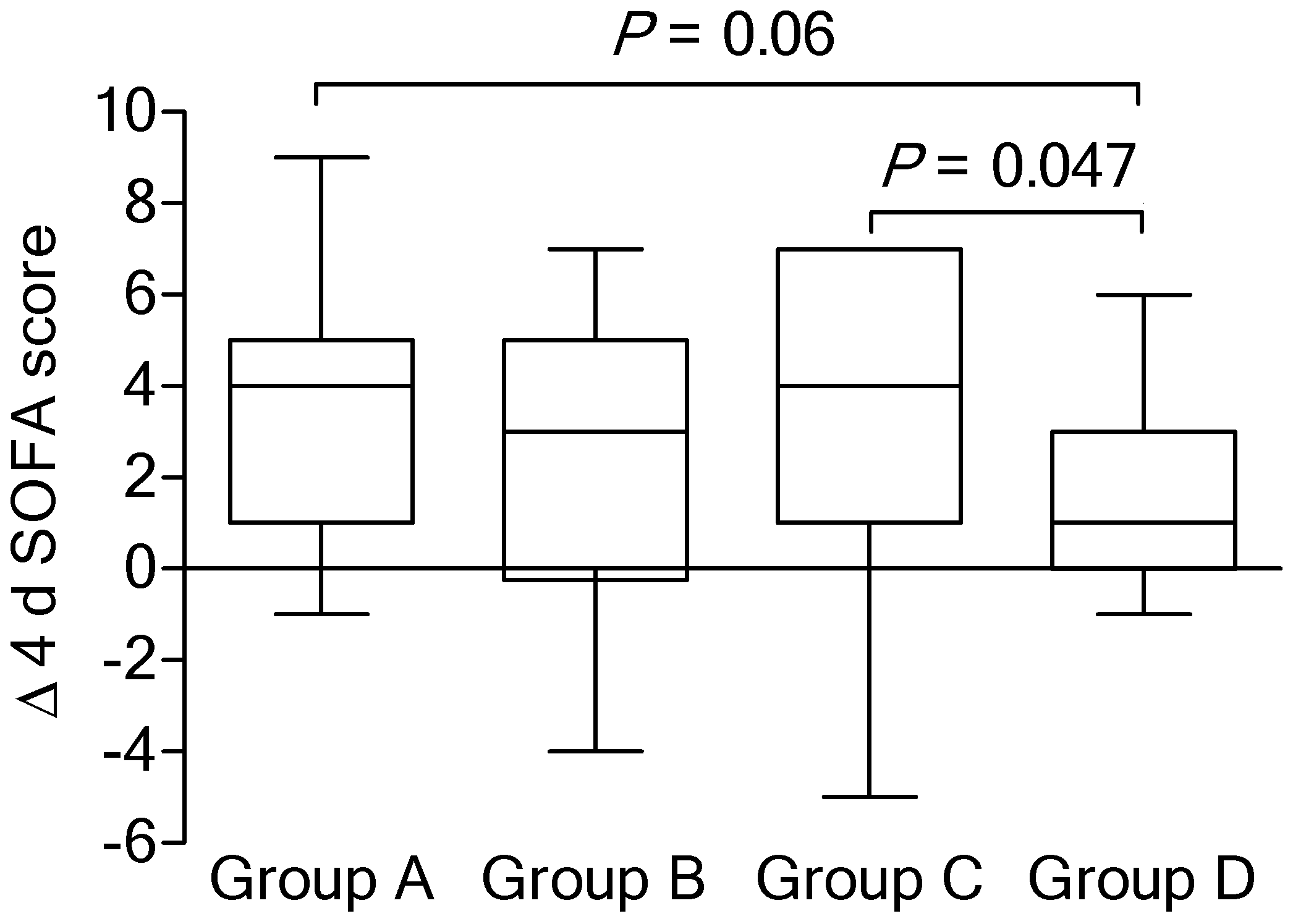
| SOFA score | 11 (10–13) | 13 (11–14) | 12 (11–13) | 12 (10–14) | 0.15 |
| Mechanical ventilation | 21 (78) | 23 (77) | 22 (63) | 21 (60) | 0.30 |
| Neuromuscular blocker | 8 (30) | 11 (37) | 10 (29) | 6 (17) | 0.36 |
| Renal replacement therapy | 7 (26) | 8 (27) | 14 (40) | 12 (34) | 0.58 |
| Vital signs and laboratory data | |||||
| Body temperature, °C | 37.8 (37.4–38.2) | 38.2 (37.8–38.5) | 36.8 (36.4–36.9) | 36.7 (36.5–37.0) | <0.001 |
| Mean arterial pressure, mmHg | 59 (57–66) | 60 (52–64) | 62 (56–68) | 58 (53–64) | 0.35 |
| Respiratory rate, breaths/min | 28 (26–33) | 29 (27–34) | 27 (24–32) | 26 (24–31) | 0.14 |
| PaO2/FiO2 | 232 (152–314) | 158 (99–208) | 260 (162–340) | 250 (120–351) | 0.048 |
| Bicarbonate, mEq/L | 19.5 (17.1–22.4) | 19.2 (16.3–20.7) | 19.0 (14.4–20.9) | 19.5 (17.4–22.8) | 0.75 |
| Creatinine, mg/dL | 1.5 (0.7–1.9) | 1.4 (1.0–1.9) | 1.4 (1.0–2.6) | 1.3 (0.7–2.2) | 0.84 |
| White cell count, 1000/mm3 | 21.9 (19.1–29.8) | 8.1 (3.7–10.9) | 21.9 (16.5–27.7) | 8.1 (3.2–11.6) | <0.001 |
| Total bilirubin, mg/dL | 0.7 (0.4–1.3) | 1.0 (0.6–1.9) | 0.7 (0.5–2.6) | 1.0 (0.6–1.5) | 0.26 |
| C-reactive protein, mg/L | 135 (57–239) | 150 (95–302) | 140 (92–221) | 115 (75–186) | 0.20 |
| Lactate, mmol/L | 3.9 (2.6–7.0) | 4.0 (3.1–6.1) | 4.2 (2.3–6.9) | 3.2 (2.1–7.2) | 0.72 |
| Norepi eq dose, ug/min | 16.0 (10.2–19.1) | 14.9 (10.4–21.1) | 16.0 (9.6–28.3) | 14.8 (6.5–20.7) | 0.70 |
| Vasoactive-inotropic score | 32.0 (21.6–48.1) | 25.9 (17.0–45.6) | 38.0 (18.9–50.6) | 27.0 (13.5–49.3) | 0.71 |
| Echocardiography (n = 17/15/21/15) 1 | |||||
| Ejection fraction, % | 56 (36–64) | 54 (37–63) | 56 (42–60) | 59 (54–64) | 0.40 |
| Septic cardiomyopathy | 7 (41) | 6 (60) | 6 (29) | 3 (20) | 0.52 |
| Time from shock onset to vitamin C protocol, h | 7 (3–12) | 11 (4–20) | 4 (1–8) | 4 (1–8) | 0.03 |
| Variable | Group A (n = 27) | Group B (n = 30) | Group C (n = 35) | Group D (n = 35) | p |
|---|---|---|---|---|---|
| Net fluid retention 1, mL | |||||
| Day 1 | 1363 (183–2145) | 2355 (1584–3169) | 2215 (296–2954) | 1839 (977–3058) | 0.12 |
| Day 2 | 674 (−13–1281) | 723 (−252–1624) | 650 (−12–1434) | 1335 (476–2030) | 0.34 |
| Day 3 | 610 (−279–932) | 230 (−388–750) | 390 (−94–947) | 560 (−138–1713) | 0.43 |
| Day 4 | 234 (−238–696) | 280 (−255–850) | 392 (−379–1178) | 453 (−210–1139) | 0.79 |
| Vasopressor weaning | 24 (89) | 20 (69) | 23 (66) | 22 (63) | 0.12 |
| Vasopressor-free days at day 28 | 21.4 ± 9.0 | 17.2 ± 12.1 | 16.7 ± 12.3 | 15.3 ± 12.5 | 0.30 |
| Ventilator weaning (n = 21/22/22/21) 2,3 | 15 (71) | 14 (64) | 8 (36) | 6 (29) | 0.01 |
| Ventilator-free days at day 28 | 13.1 ± 11.1 | 13.4 ± 10.8 | 8.2 ± 11.2 | 5.6 ± 9.5 | 0.07 |
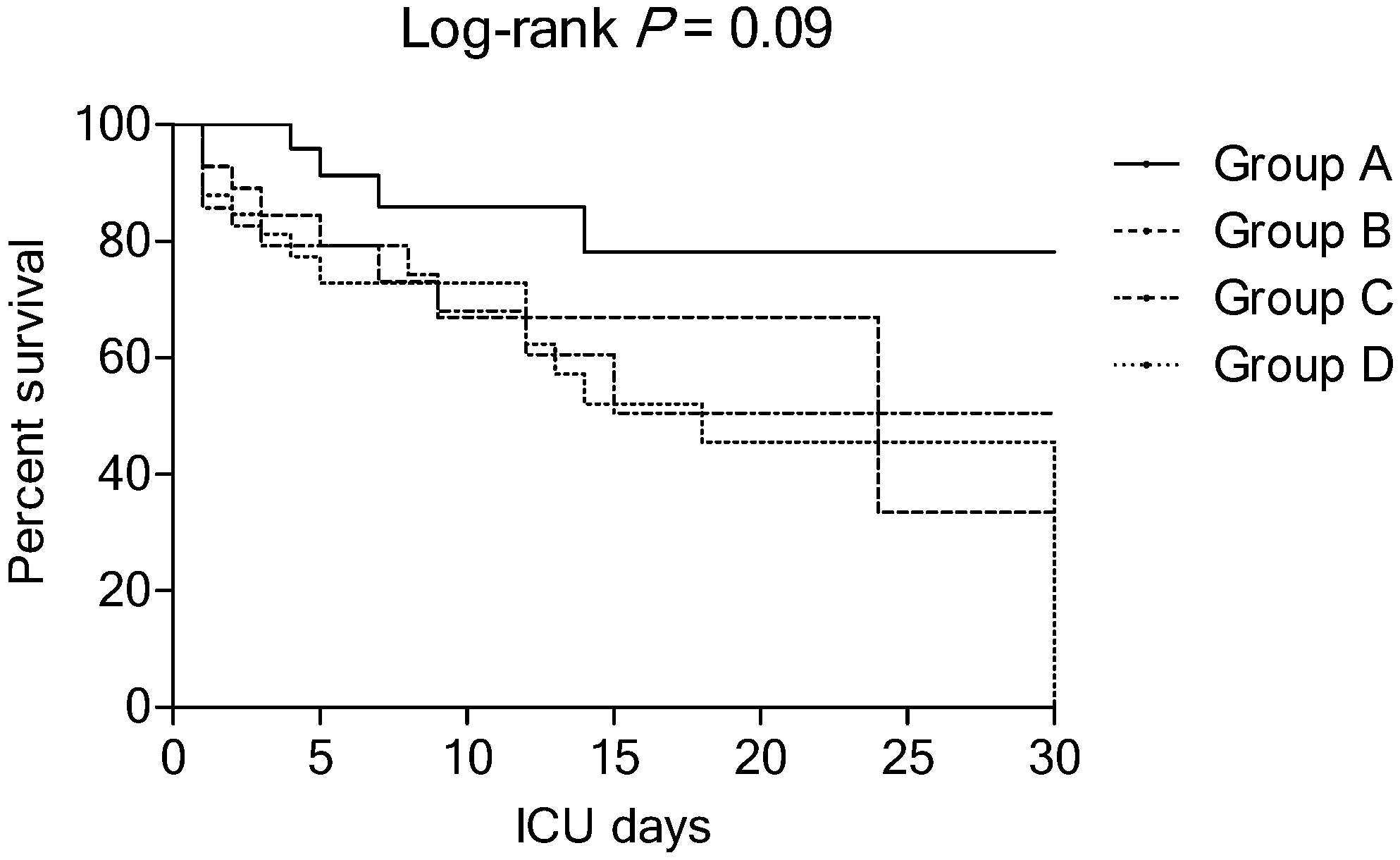
| ICU mortality | 4 (15) | 10 (33) | 12 (34) | 17 (49) | 0.051 |
| Hospital mortality | 6 (22) | 13 (43) | 15 (43) | 19 (54) | 0.09 |
| Superinfection | 4 (15) | 6 (20) | 3 (9) | 6 (17) | 0.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-Y.; Jung, J.-W.; Choi, J.C.; Shin, J.W.; Kim, J.Y. Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis. Nutrients 2019, 11, 2976. https://doi.org/10.3390/nu11122976
Kim W-Y, Jung J-W, Choi JC, Shin JW, Kim JY. Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis. Nutrients. 2019; 11(12):2976. https://doi.org/10.3390/nu11122976
Chicago/Turabian StyleKim, Won-Young, Jae-Woo Jung, Jae Chol Choi, Jong Wook Shin, and Jae Yeol Kim. 2019. "Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis" Nutrients 11, no. 12: 2976. https://doi.org/10.3390/nu11122976
APA StyleKim, W.-Y., Jung, J.-W., Choi, J. C., Shin, J. W., & Kim, J. Y. (2019). Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis. Nutrients, 11(12), 2976. https://doi.org/10.3390/nu11122976





