Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
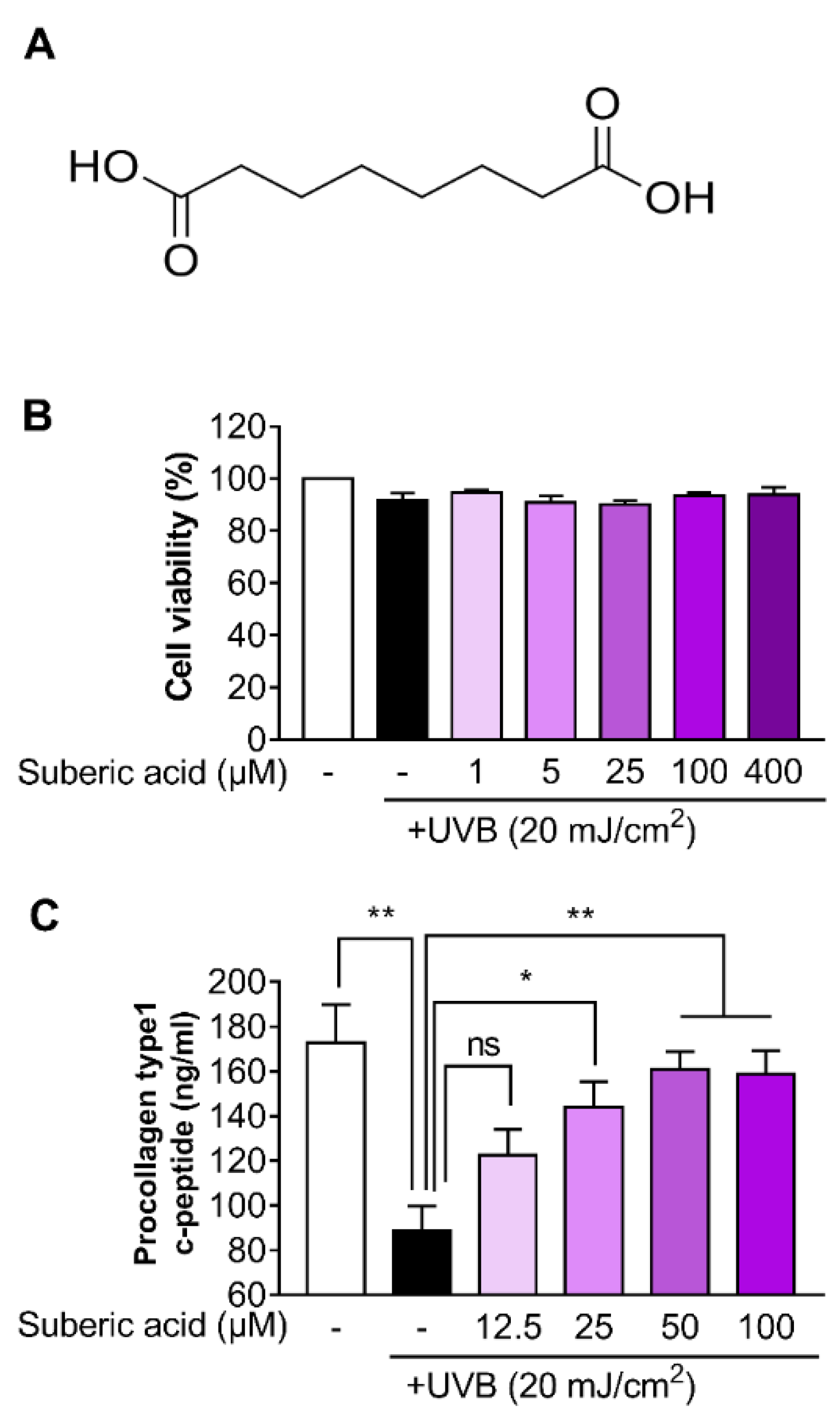
2.3. Cell Viability Assay
2.4. Procollagen I C-terminal Peptide Determination
2.5. Animal Experiments
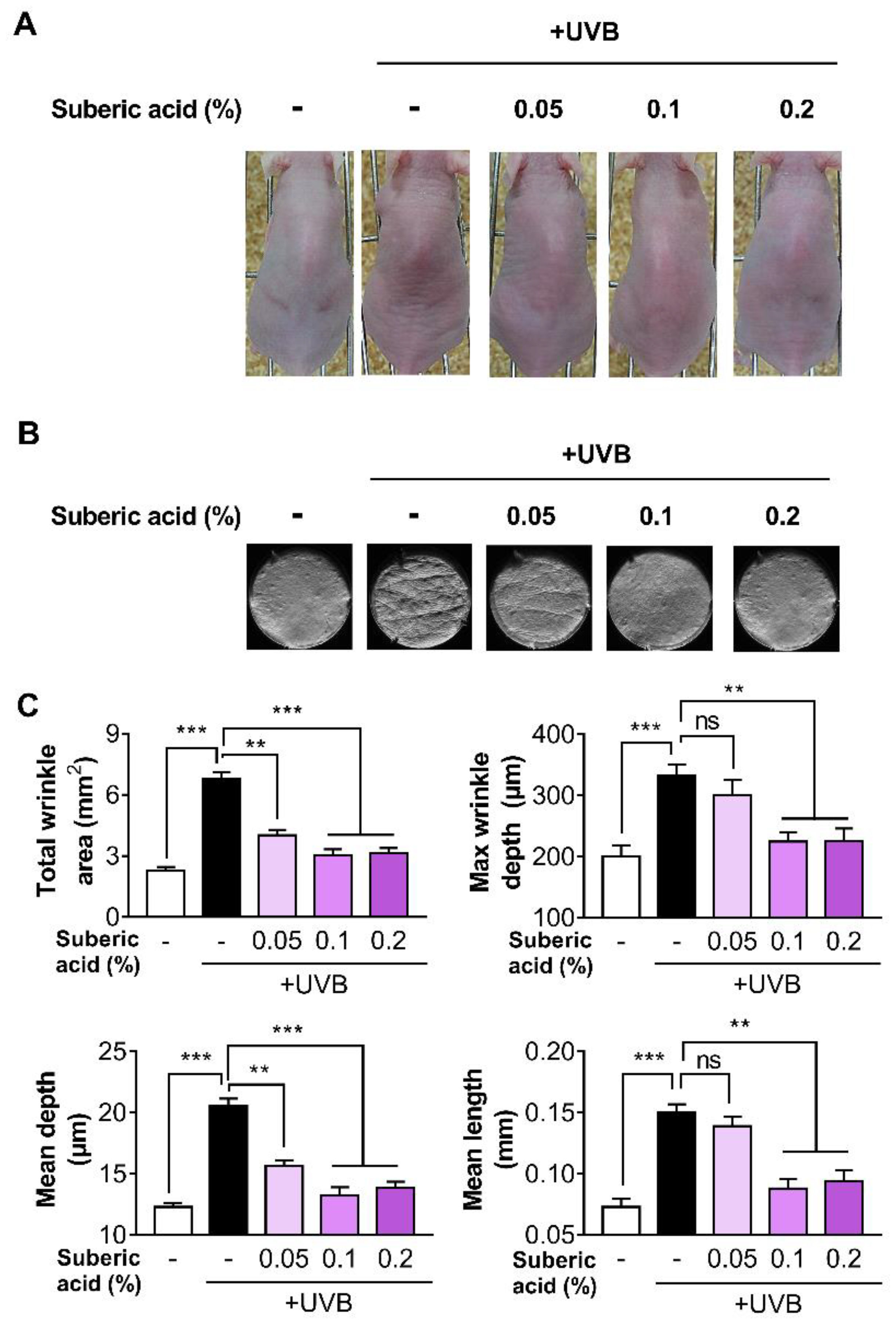
2.6. Assessment of Wrinkle Formation
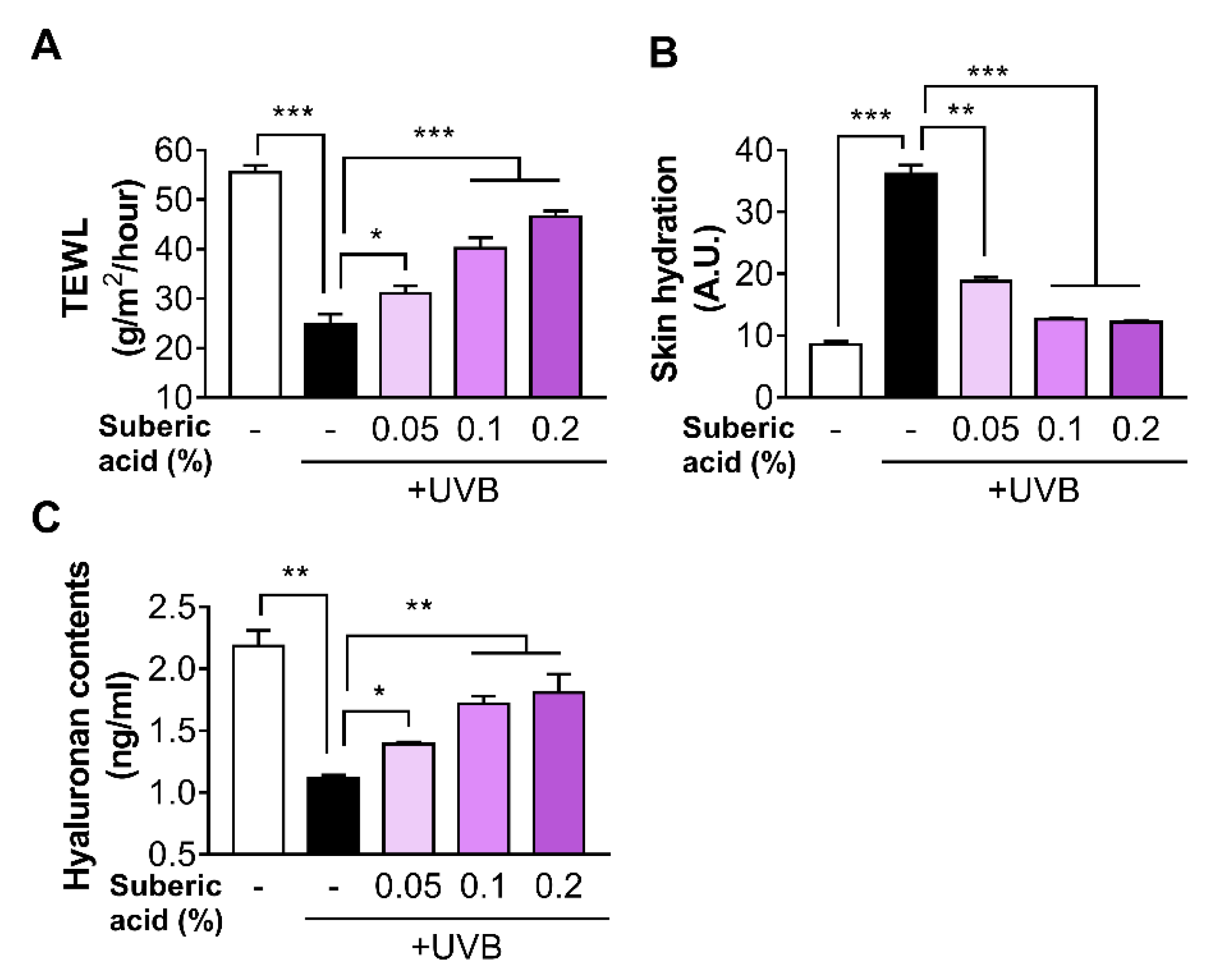
2.7. Determination of Skin Hyaluronic Acid
2.8. Transepidermal Water Loss (TEWL) and Skin Hydration Determination
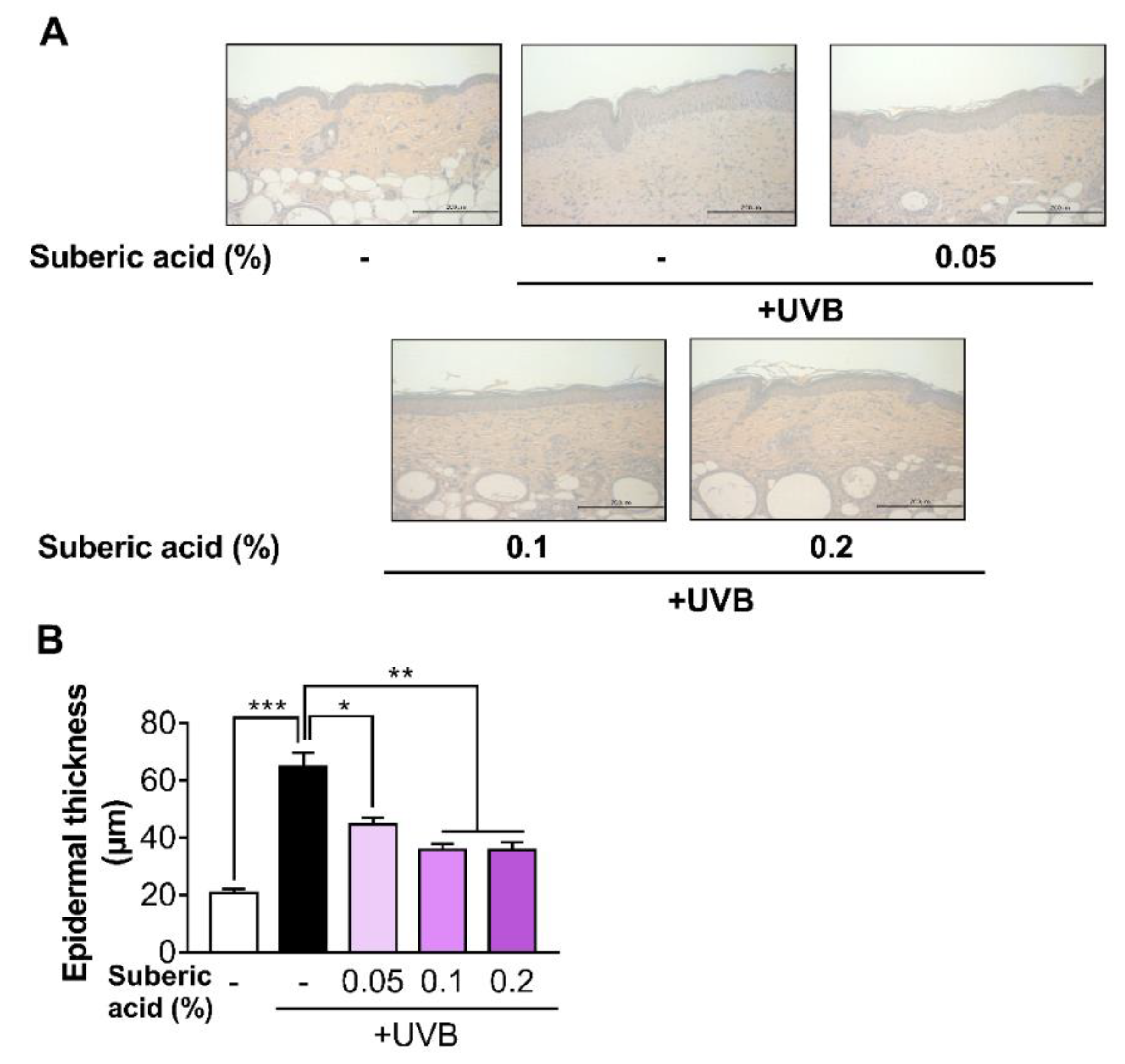
2.9. Histological Analysis
2.10. Cyclic Adenosine Monophosphate (cAMP) Assay
2.11. Western Blotting
2.12. Reverse Transcription and Quantitative Real-Time PCR
2.13. Statistical Analysis
3. Results
3.1. Suberic Acid Alleviates Collagen Loss in UVB-Exposed Hs68 Dermal Fibroblasts
3.2. Suberic Acid Reduces UVB-Induced Wrinkle Formation in Hairless Mice
3.3. Suberic Acid Prevents UVB-Induced Skin Dryness in Hairless Mice
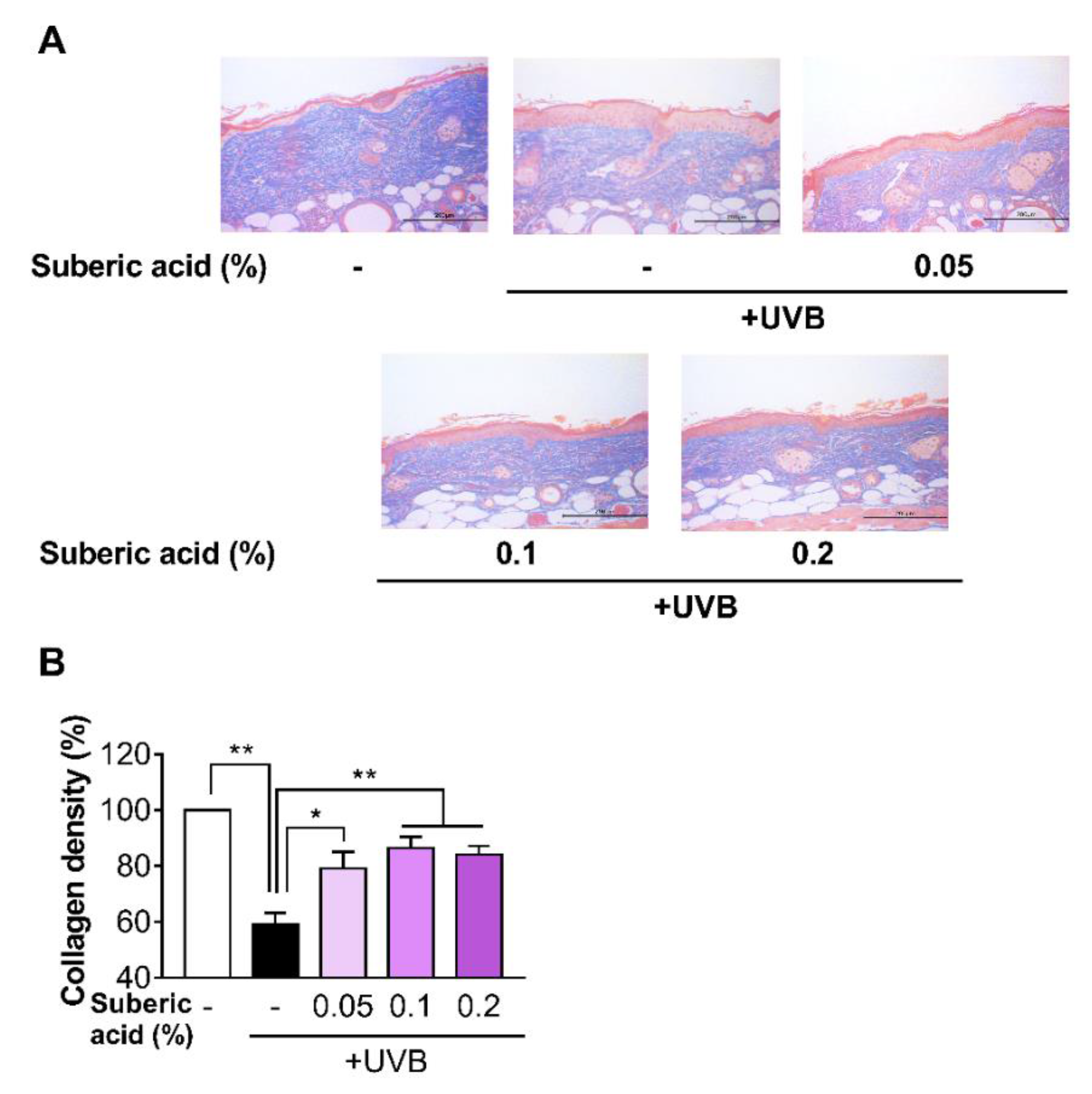
3.4. Suberic Acid Suppresses UVB-Induced Epidermal Thickness Increase and Prevents UVB-Induced Collagen Loss in Hairless Mouse Skin
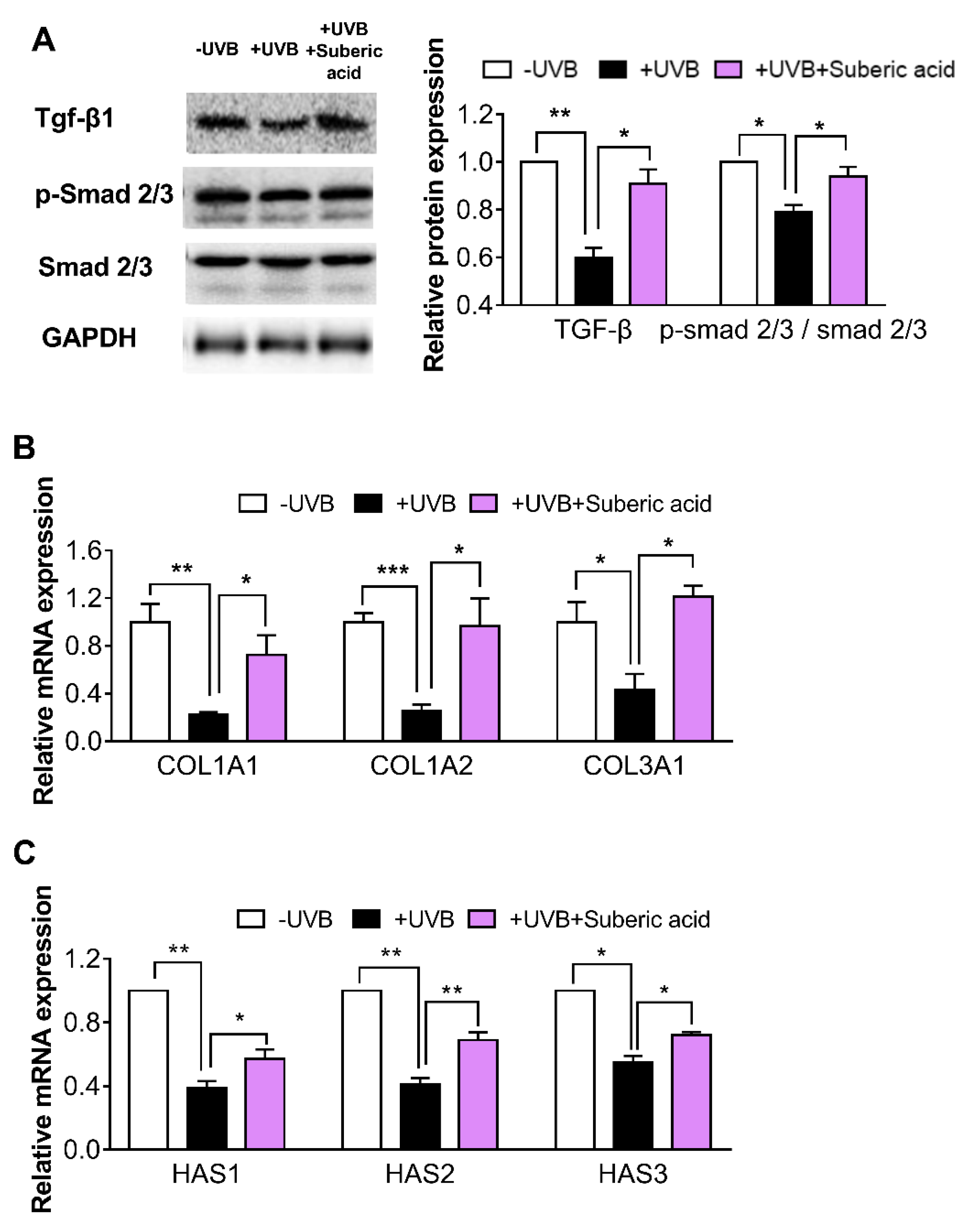
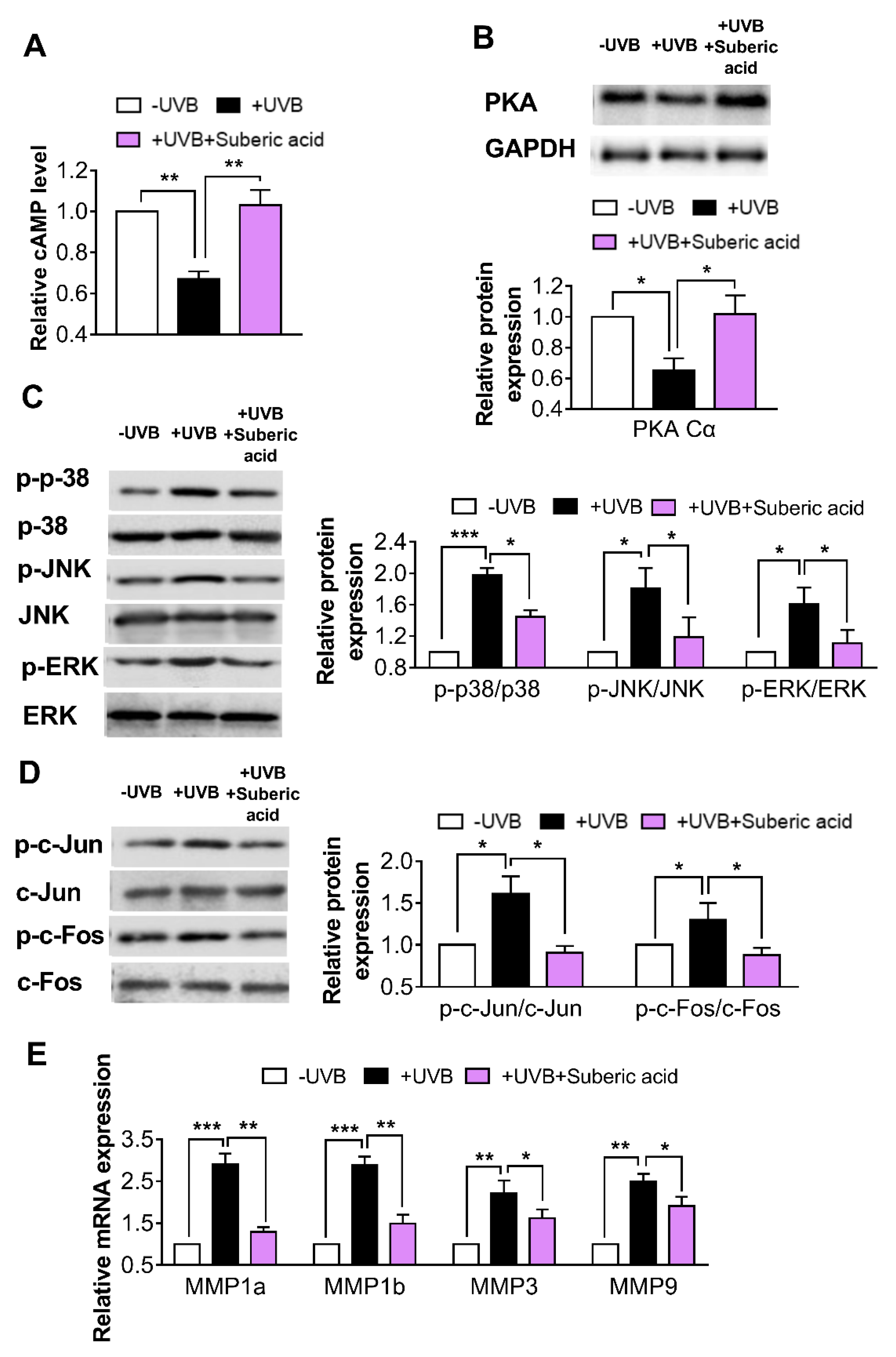
3.5. Suberic Acid Upregulates TGF-β Signaling and Downregulates MAPK Pathway Molecules in Hairless Mouse Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2009; Volume 14, pp. 20–24. [Google Scholar]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Huertas, A.C.M.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128, 163–173. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Hu, N.; Xiong, X.; Lei, Q.; Li, L. A novel promising therapy for skin aging: Dermal multipotent stem cells against photoaged skin by activation of tgf-β/smad and p38 mapk signaling pathway. Med Hypotheses 2011, 76, 343–346. [Google Scholar] [CrossRef]
- Tabibzadeh, S. Homeostasis of extracellular matrix by tgf-beta and lefty. Front. Biosci. 2002, 7, 46. [Google Scholar] [CrossRef]
- Taipale, J.; Miyazono, K.; Heldin, C.-H.; Keski-Oja, J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent tgf-beta binding protein. J. Cell Biol. 1994, 124, 171–181. [Google Scholar] [CrossRef]
- Martinez-Ferrer, M.; Afshar-Sherif, A.-R.; Uwamariya, C.; de Crombrugghe, B.; Davidson, J.M.; Bhowmick, N.A. Dermal transforming growth factor-β responsiveness mediates wound contraction and epithelial closure. Am. J. Pathol. 2010, 176, 98–107. [Google Scholar] [CrossRef]
- Muthusamy, V.; Piva, T.J. The uv response of the skin: A review of the mapk, nfκb and tnfα signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5. [Google Scholar] [CrossRef]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic acid inhibits induction of c-jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008, 20, 177. [Google Scholar] [PubMed]
- Johnson, R.W.; Pollock, C.M.; Cantrell, R.R. Dicarboxylic acids. Kirk-Othmer Encycl. Chem. Technol. 2000, 1–18. [Google Scholar]
- Ayorinde, F.O.; Osman, G.; Shepard, R.L.; Powers, F.T. Synthesis of azelaic acid and suberic acid fromvernonia galamensis oil. J. Am. Oil Chem. Soc. 1988, 65, 1774–1777. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, M.J.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Augmentation of uv-induced skin wrinkling by infrared irradiation in hairless mice. Mech. Ageing Dev. 2005, 126, 1170–1177. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. Anti-Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. Uv, stress and aging. Derm. Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef]
- Pfau, R.; HOOD, A.F.; Morison, W. Photoageing: The role of uvb, solar-simulated uvb, visible and psoralen uva radiation. Br. J. Dermatol. 1986, 114, 319–327. [Google Scholar] [CrossRef]
- Hiraku, Y.; Ito, K.; Hirakawa, K.; Kawanishi, S. Photosensitized DNA damage and its protection via a novel mechanism. Photochem. Photobiol. 2007, 83, 205–212. [Google Scholar] [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2005, 571, 3–17. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Manuskiatti, W.; Maibach, H.I. Hyaluronic acid and skin: Wound healing and aging. Int. J. Dermatol. 1996, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Röck, K.; Grandoch, M.; Majora, M.; Krutmann, J.; Fischer, J.W. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet b (uvb) new insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011, 286, 18268–18276. [Google Scholar] [CrossRef] [PubMed]
- Lagendijk, A.K.; Goumans, M.J.; Burkhard, S.B.; Bakkers, J. Microrna-23 restricts cardiac valve formation by inhibiting has2 and extracellular hyaluronic acid production. Circ. Res. 2011, 109, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Docampo, M.J.; Zanna, G.; Fondevila, D.; Cabrera, J.; López-Iglesias, C.; Carvalho, A.; Cerrato, S.; Ferrer, L.; Bassols, A. Increased has2-driven hyaluronic acid synthesis in shar-pei dogs with hereditary cutaneous hyaluronosis (mucinosis). Vet. Dermatol. 2011, 22, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, J.D. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 2006, 6, 532. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Ginty, D.D.; Greenberg, M.E. Coupling of the ras-mapk pathway to gene activation by rsk2, a growth factor-regulated creb kinase. Science 1996, 273, 959–963. [Google Scholar] [CrossRef]
- Naor, Z.; Benard, O.; Seger, R. Activation of mapk cascades by g-protein-coupled receptors: The case of gonadotropin-releasing hormone receptor. Trends Endocrinol. Metab. 2000, 11, 91–99. [Google Scholar] [CrossRef]
- Choi, W.-S.; Eom, D.-S.; Han, B.S.; Kim, W.K.; Han, B.H.; Choi, E.-J.; Oh, T.H.; Markelonis, G.J.; Cho, J.W.; Oh, Y.J. Phosphorylation of p38 mapk induced by oxidative stress is linked to activation of both caspase-8-and-9-mediated apoptotic pathways in dopaminergic neurons. J. Biol. Chem. 2004, 279, 20451–20460. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Yi, B.-R.; Choi, K.-C. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef]
- Cooper, S.; Bowden, G. Ultraviolet b regulation of transcription factor families: Roles of nuclear factor-kappa b (nf-κb) and activator protein-1 (ap-1) in uvb-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Abaffy, T.; Matsunami, H.; Luetje, C.W. Functional analysis of a mammalian odorant receptor subfamily. J. Neurochem. 2006, 97, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Bahk, Y.Y.; Lee, N.; Jae, Y.; Cho, Y.H.; Ku, C.R.; Byun, Y.; Lee, E.J.; Kim, M.-S.; Koo, J. Olfactory receptor olfr544 responding to azelaic acid regulates glucagon secretion in α-cells of mouse pancreatic islets. Biochem. Biophys. Res. Commun. 2015, 460, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hwang, S.H.; Jia, Y.; Choi, J.; Kim, Y.-J.; Choi, D.; Pathiraja, D.; Choi, I.-G.; Koo, S.-H.; Lee, S.-J. Olfactory receptor 544 reduces adiposity by steering fuel preference toward fats. J. Clin. Investig. 2017, 127, 4118–4123. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2a receptor promotes collagen production by human fibroblasts via pathways involving cyclic amp and akt but independent of smad2/3. FASEB J. 2014, 28, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Moon, Y.; Shin, C.M.; Chung, J.H. Cyclic amp suppresses matrix metalloproteinase-1 expression through inhibition of mapk and gsk-3β. J. Investig. Dermatol. 2010, 130, 2049–2056. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
| Type | Gene Description | Sequences (5’→3) |
|---|---|---|
| Mouse | Collagen type I alpha 1 chain (COL1A1) | F: GGCAACAGTCGCTTCACCTA |
| R: AGTCCGAATTCCTGGTCTGG | ||
| Collagen type I alpha 2 chain (COL1A2) | F: CCCAGAGTGGAACAGCGATT | |
| R: ATGAGTTCTTCGCTGGGGTG | ||
| Collagen type III alpha 1 chain (COL3A1) | F: TAACCAAGGCTGCAAGATGG | |
| R: ACCAGTGCTTACGTGGGACA | ||
| Matrix metalloproteinase 1a (MMP1a) | F: AGTACTACAACTGACAACCCAAGA | |
| R: CCTGTTCCTGTTTTCAGAGCC | ||
| Matrix metalloproteinase 1b (MMP1b) | F: CCTTCCCCAAATCCCATCCA | |
| R: CACATCGATCAAAGGTTCTGGC | ||
| Matrix metalloproteinase 3 (MMP3) | F: ACTCCCTGGGACTCTACCAC | |
| R: GGTACCACGAGGACATCAGG | ||
| Matrix metalloproteinase 9 (MMP9) | F: GTGGACCATGAGGTGAACCA | |
| R: ACTGCACGGTTGAAGCAAAG | ||
| Hyaluronic acid synthase 1 (HAS1) | F: CTATGCTACCAAGTATACCTCG | |
| R: TCTCGGAAGTAAGATTTGGAC | ||
| Hyaluronic acid synthase 2 (HAS2) | F: CGGTCGTCTCAAATTCATCTG | |
| R: ACAATGCATCTTGTTCAGCTC | ||
| Hyaluronic acid synthase 3 (HAS3) | F: GATGTCCAAATCCTCAACAAG | |
| R: CCCACTAATACATTGCACAC | ||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: GTGATGGCATGGACTGTGGT | |
| R: GGAGCCAAAAGGGTCATCAT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Choi, D.; Park, T. Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients 2019, 11, 2948. https://doi.org/10.3390/nu11122948
Kang W, Choi D, Park T. Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients. 2019; 11(12):2948. https://doi.org/10.3390/nu11122948
Chicago/Turabian StyleKang, Wesuk, Dabin Choi, and Taesun Park. 2019. "Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice" Nutrients 11, no. 12: 2948. https://doi.org/10.3390/nu11122948
APA StyleKang, W., Choi, D., & Park, T. (2019). Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients, 11(12), 2948. https://doi.org/10.3390/nu11122948





