Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Glucose Tolerance Tests
2.3. Serum Analysis
2.4. Haematoxylin and Eosin (H&E) Staining
2.5. PCR Real-Time Quantitative PCR (RT-qPCR)
2.6. Enzyme Activity Evaluation
2.7. 16S rRNA Sequencing with Illumina MiSeq Sequencing
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results
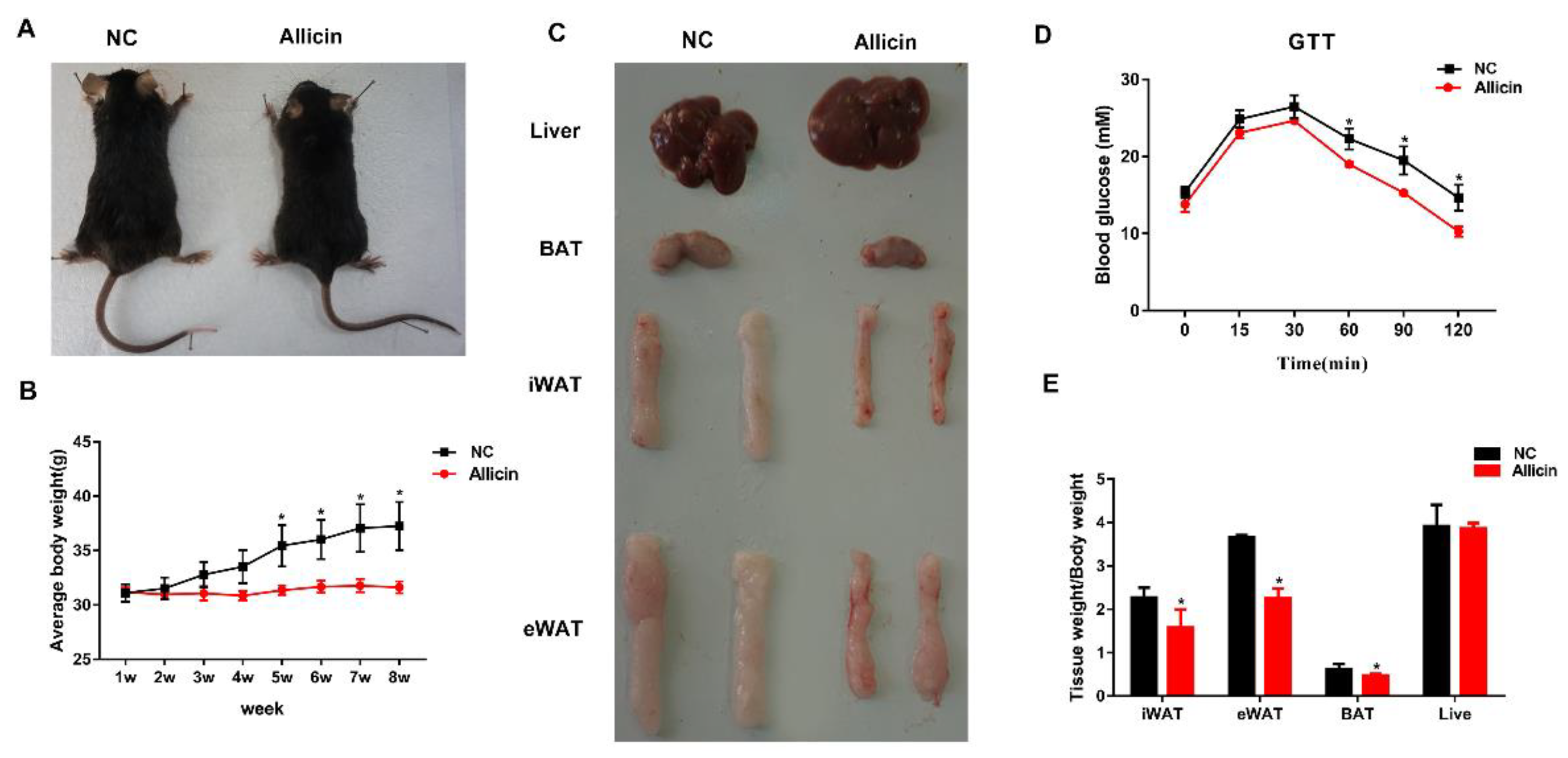
3.1. Allicin Reduces Body Weight Gain and Fat Deposition in a Mouse High-Fat Diet-Induced Obesity Model
3.2. Allicin Ameliorates Blood Metabolic Parameters in Mice with High-Fat Diet-Induced Obesity
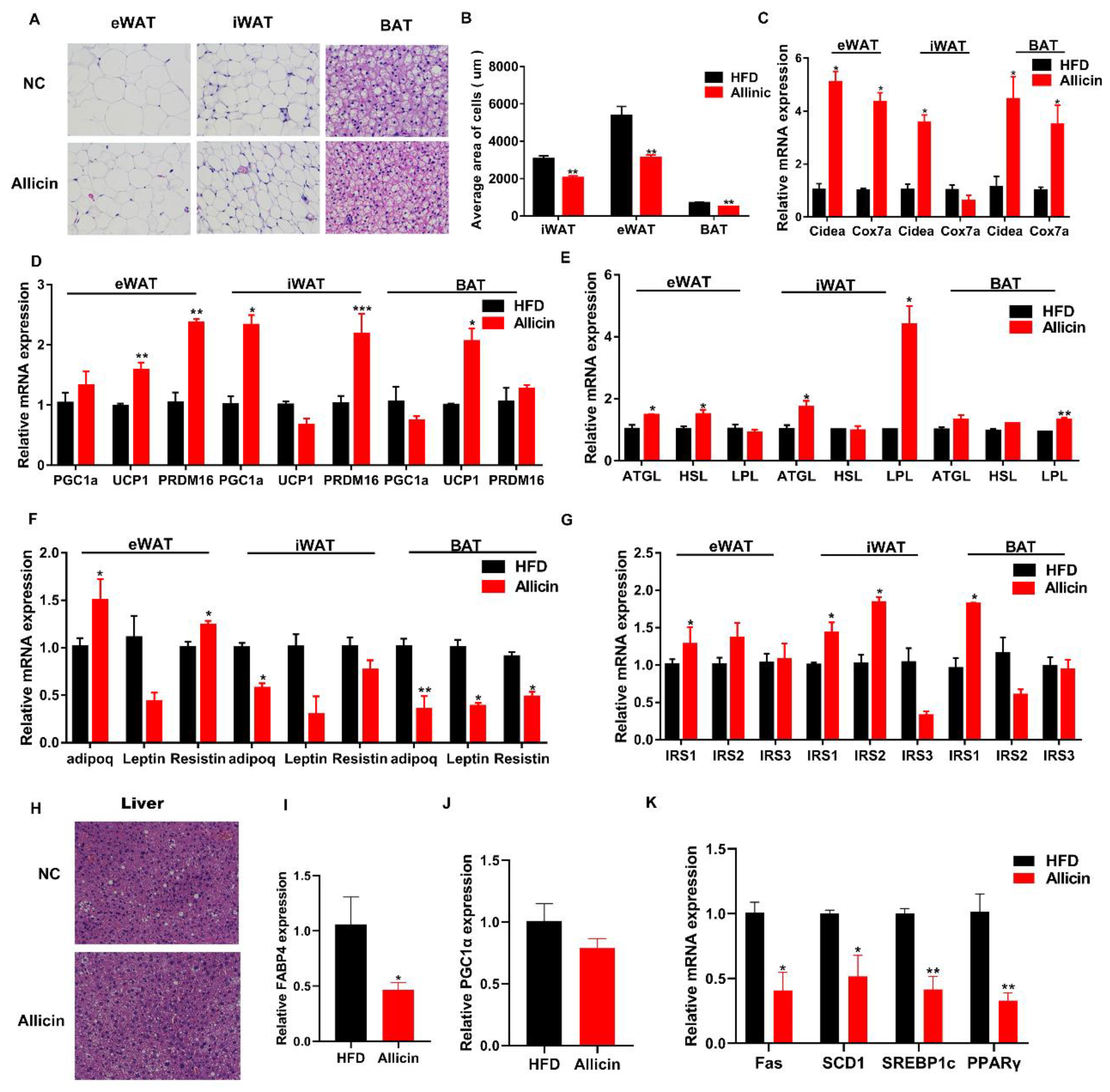
3.3. Allicin Reduced Lipid Droplets and Increased the Expression of Genes Involved in Lipid Metabolism in Obese Mice Induced by High-Fat Diet
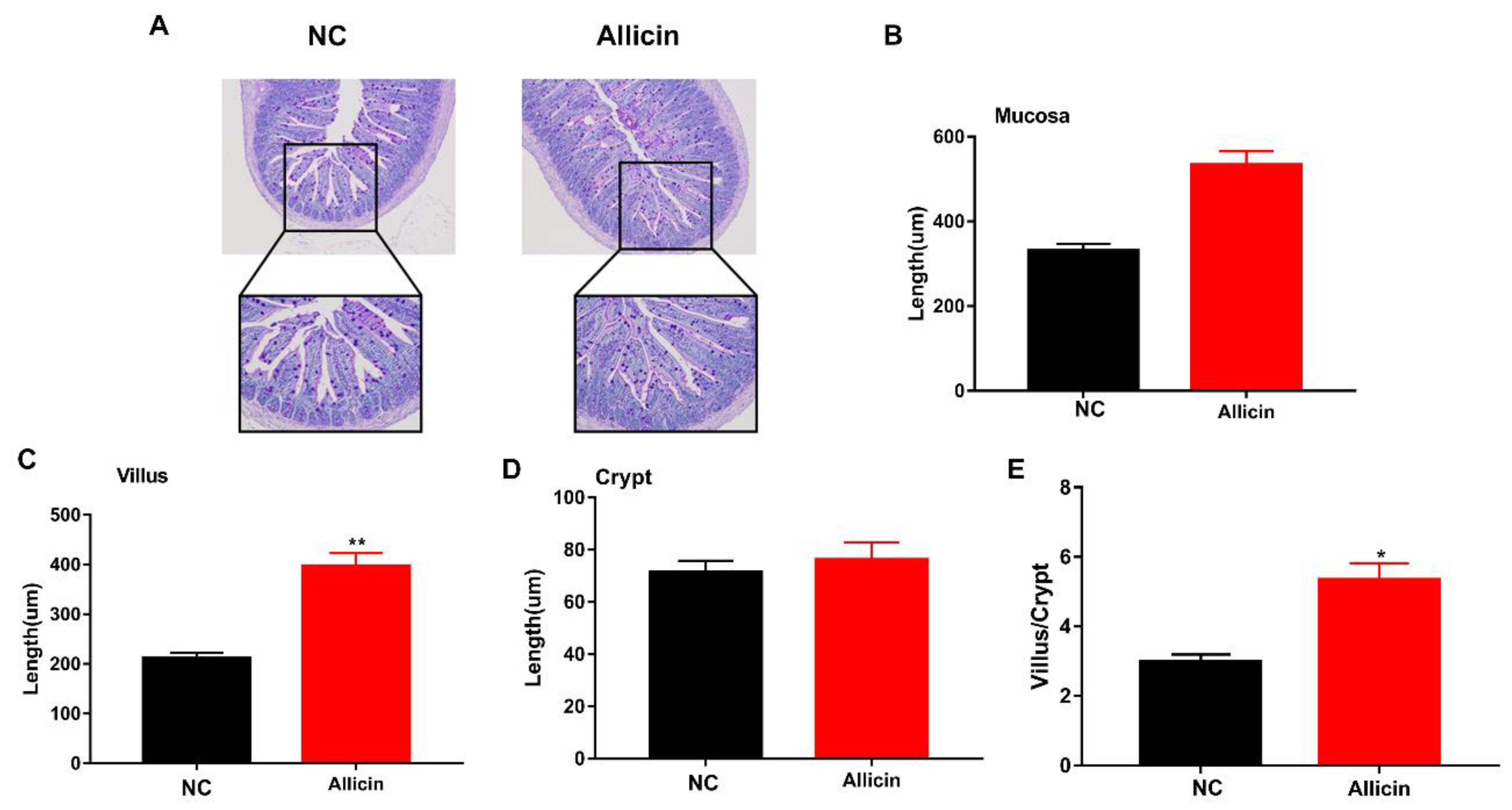
3.4. Allicin Improves the Intestinal Morphology of Obese Mice Induced by High-Fat Diet
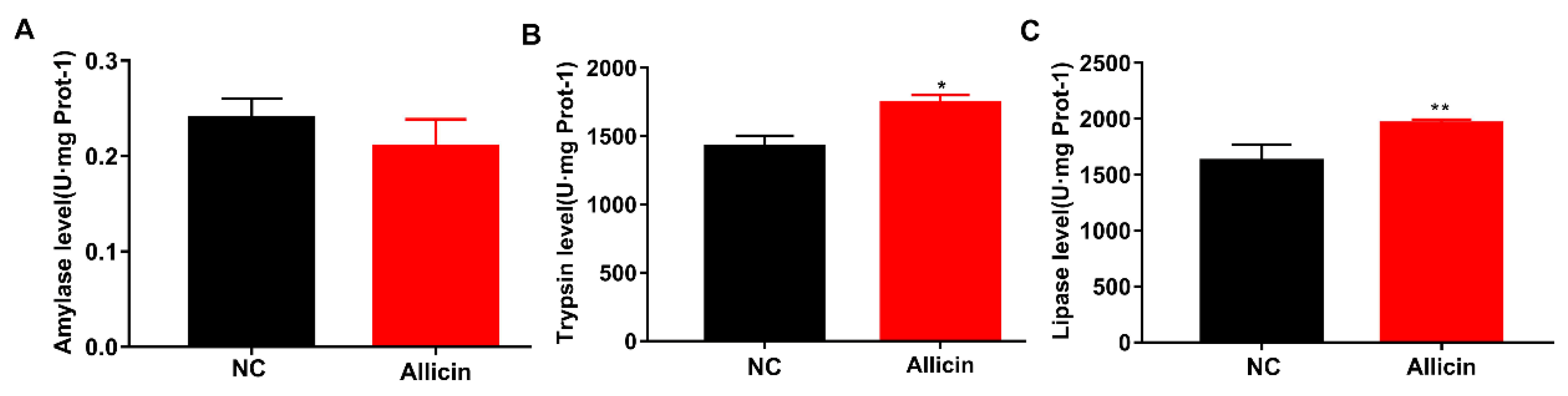
3.5. Allicin Improves Intestinal Enzymatic Activity in Obese Mice Induced by High-Fat Diet
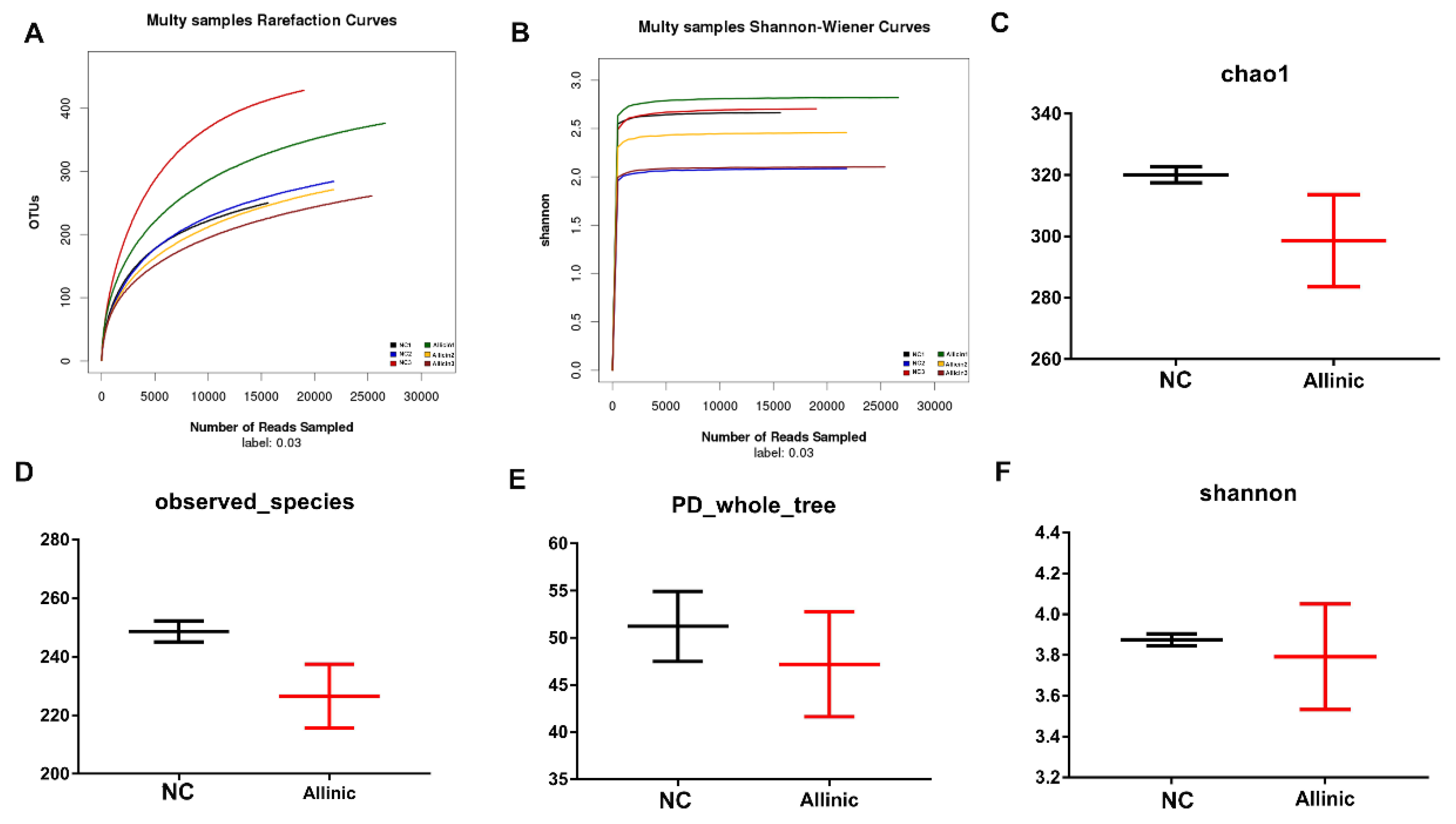
3.6. Allicin Does Not Affect Intestinal Microbial Structure in Obese Mice Induced by High-Fat Diet
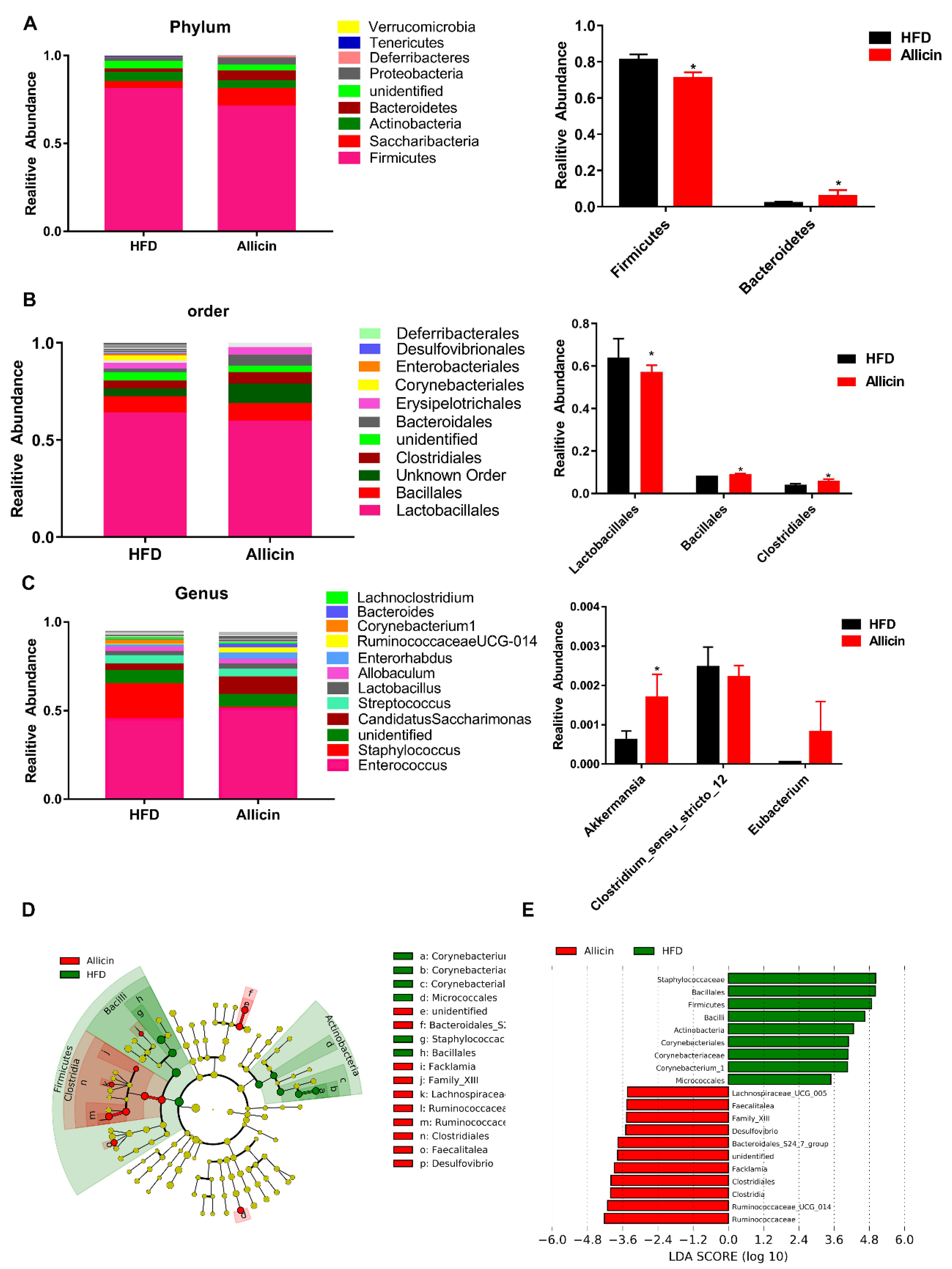
3.7. Allicin Alters the Gut Microbiota in Mice with High-Fat Diet-Induced Obesity
4. Discussion
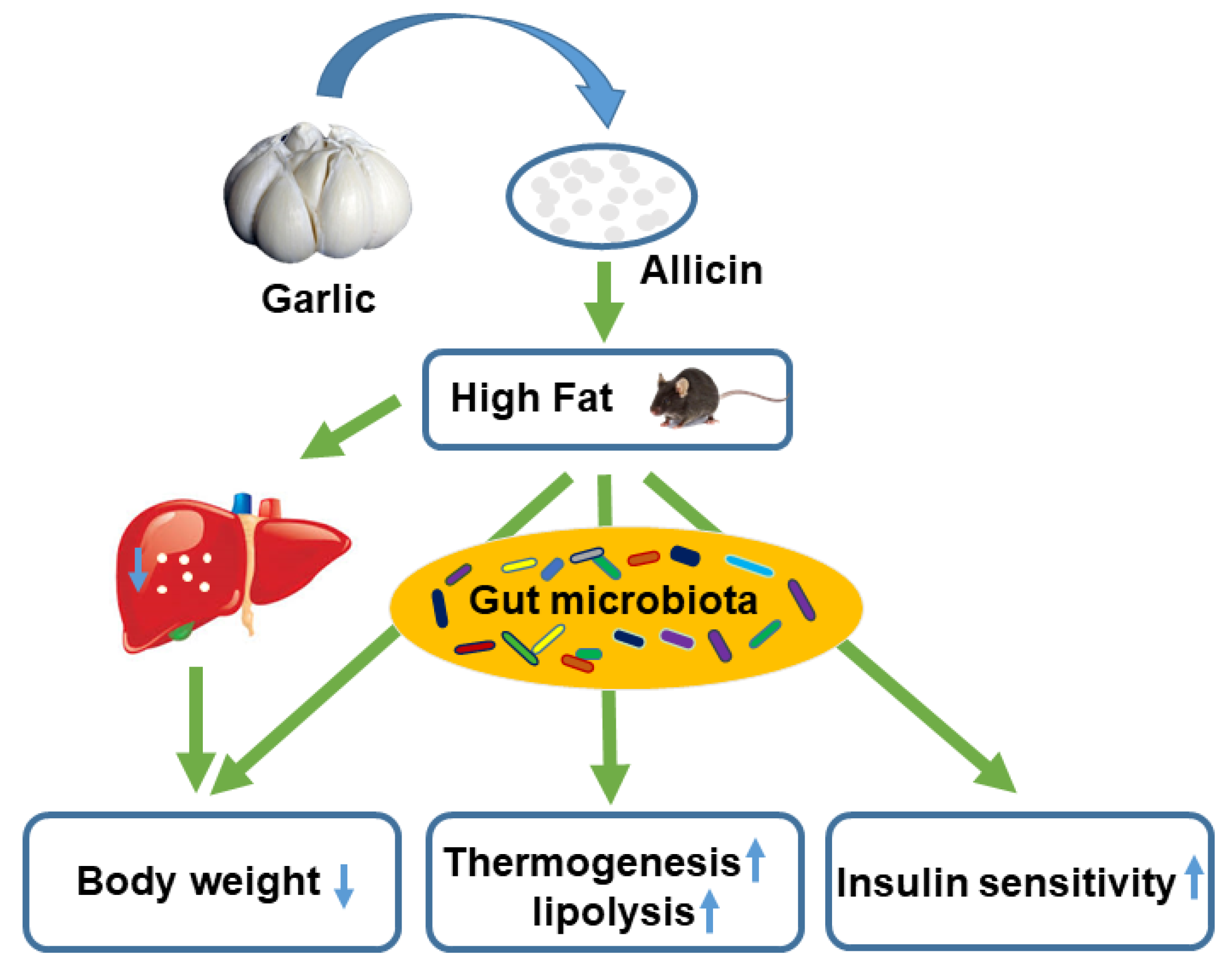
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Quach, D.; Britton, R.A. Gut Microbiota and Bone Health. Adv. Exp. Med. Biol. 2017, 1033, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- An, Y.; Li, Y.; Wang, X.; Chen, Z.; Xu, H.; Wu, L.; Li, S.; Wang, C.; Luan, W.; Wang, X.; et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats. Lipids Health Dis. 2018, 17, 276. [Google Scholar] [CrossRef]
- Gruhlke, M.C.; Nicco, C.; Batteux, F.; Slusarenko, A.J. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants (Basel) 2016, 6, 1. [Google Scholar] [CrossRef]
- Jacob, C.; Anwar, A. The chemistry behind redox regulation with a focus on sulphur redox systems. Physiol. Plant 2008, 133, 469–480. [Google Scholar] [CrossRef]
- Gruhlke, M.C.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Warshafsky, S.; Kamer, R.S.; Sivak, S.L. Effect of garlic on total serum cholesterol. A meta-analysis. Ann. Intern. Med. 1993, 119, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Rhee, D.K.; Kim, B.O.; Um, S.H.; Pyo, S. Allicin induces beige-like adipocytes via KLF15 signal cascade. J. Nutr. Biochem. 2019, 64, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Kilany, O.E.; Khalifa, H.A.; Ahmed, A.A.M. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol. 2017, 80, 745–753. [Google Scholar] [CrossRef]
- Dubey, H.; Singh, A.; Patole, A.M.; Tenpe, C.R. Antihypertensive effect of allicin in dexamethasone-induced hypertensive rats. Integr. Med. Res. 2017, 6, 60–65. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen Male Study. Arter. Thromb Vasc. Biol. 1997, 17, 1114–1120. [Google Scholar] [CrossRef]
- Casirola, D.M.; Ferraris, R.P. Role of the small intestine in postpartum weight retention in mice. Am. J. Clin. Nutr. 2003, 78, 1178–1187. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Li, J.; Li, Y.; Huang, P.; Ding, X.; Yin, J.; He, S.; Yang, H.; Yin, Y. Dietary vitamin E affects small intestinal histomorphology, digestive enzyme activity, and the expression of nutrient transporters by inhibiting proliferation of intestinal epithelial cells within jejunum in weaned piglets1. J. Anim. Sci. 2019, 97, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Metabolism in 2013: The gut microbiota manages host metabolism. Nat. Rev. Endocrinol. 2014, 10, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.T.; Augusti, K.T. Studies on the effect of allicin (diallyl disulphide-oxide) on alloxan diabetes. I. Hypoglycaemic action and enhancement of serum insulin effect and glycogen synthesis. Indian J. Biochem. Biophys. 1973, 10, 209–212. [Google Scholar] [PubMed]
- Hosseini, A.; Hosseinzadeh, H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J. Endocrinol. Investig. 2015, 38, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, I.H.; Kim, C.T.; Kim, Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J. Nutr. 2011, 141, 1947–1953. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef]
- Mathew, B.C.; Daniel, R.S.; Augusti, K.T. Hypolipidemic effect of garlic protein substituted for casein in diet of rats compared to those of garlic oil. Indian J. Exp. Biol. 1996, 34, 337–340. [Google Scholar]
- Dong, L.; Zhong, X.; He, J.; Zhang, L.; Bai, K.; Xu, W.; Wang, T.; Huang, X. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin. Nutr. 2016, 35, 399–407. [Google Scholar] [CrossRef]
- Hu, L.; Peng, X.; Chen, H.; Yan, C.; Liu, Y.; Xu, Q.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur. J. Nutr. 2017, 56, 1753–1765. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, S.; Li, X.; Yan, T.; Duan, Y.; Yang, X.; Duan, Y.; Sun, Q.; Yang, X. Simultaneous Supplementation of Bacillus subtilis and Antibiotic Growth Promoters by Stages Improved Intestinal Function of Pullets by Altering Gut Microbiota. Front. Microbiol. 2018, 9, 2328. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin Alleviates High Fat Diet-Induced Obesity and Improves the Gut Microbiota Composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Wostmann, B.S.; Larkin, C.; Moriarty, A.; Bruckner-Kardoss, E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab. Anim. Sci. 1983, 33, 46–50. [Google Scholar]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; Garcia-Valdes, L.; Segura, M.T.; Martin-Lagos, J.A.; Anjos, T.; Marti-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef]
- Shen, F.; Feng, J.; Wang, X.; Qi, Z.; Shi, X.; An, Y.; Zhang, Q.; Wang, C.; Liu, M.; Liu, B.; et al. Vinegar Treatment Prevents the Development of Murine Experimental Colitis via Inhibition of Inflammation and Apoptosis. J. Agric. Food Chem. 2016, 64, 1111–1121. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Blachier, F.; Darcy-Vrillon, B.; de Coppet, P.; Bourreille, A.; Segain, J.P. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm. Bowel. Dis. 2010, 16, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, J.; Shi, X.; Hu, S.; Zhao, L. Allicin alleviated learning and memory deficits caused by lead exposure at developmental stage. Life Sci. 2019, 231, 116532. [Google Scholar] [CrossRef]
- Su, A.; Yang, W.; Zhao, L.; Pei, F.; Yuan, B.; Zhong, L.; Ma, G.; Hu, Q. Flammulina velutipes polysaccharides improve scopolamine-induced learning and memory impairment in mice by modulating gut microbiota composition. Food Funct. 2018, 9, 1424–1432. [Google Scholar] [CrossRef]
| Parameter | NC | Allicin | p-Value |
|---|---|---|---|
| AST, U/L | 246.64 ± 139.77 | 196.62 ± 34.18 | 0.4593 |
| ALT, U/L | 43.86 ± 17.58 | 50.12 ± 11.34 | 0.5223 |
| TC, mmol/L | 2.59 ± 0.45 | 3.67 ± 0.51 | 0.0076 |
| TG, mmol/L | 0.82 ± 0. 20 | 0.91 ± 0.11 | 0.4077 |
| HDL-C, mmol/L | 1.40 ± 0.16 | 2.07 ± 0.18 | 0.0003 |
| LDL-C, mmol/L | 0.33 ± 0.05 | 0.26 ± 0.06 | 0.0808 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. https://doi.org/10.3390/nu11122909
Shi X, Zhou X, Chu X, Wang J, Xie B, Ge J, Guo Y, Li X, Yang G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients. 2019; 11(12):2909. https://doi.org/10.3390/nu11122909
Chicago/Turabian StyleShi, Xin’e, Xiaomin Zhou, Xinyi Chu, Jie Wang, Baocai Xie, Jing Ge, Yuan Guo, Xiao Li, and Gongshe Yang. 2019. "Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota" Nutrients 11, no. 12: 2909. https://doi.org/10.3390/nu11122909
APA StyleShi, X., Zhou, X., Chu, X., Wang, J., Xie, B., Ge, J., Guo, Y., Li, X., & Yang, G. (2019). Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients, 11(12), 2909. https://doi.org/10.3390/nu11122909





