Poor Oral Health as a Determinant of Malnutrition and Sarcopenia
Abstract
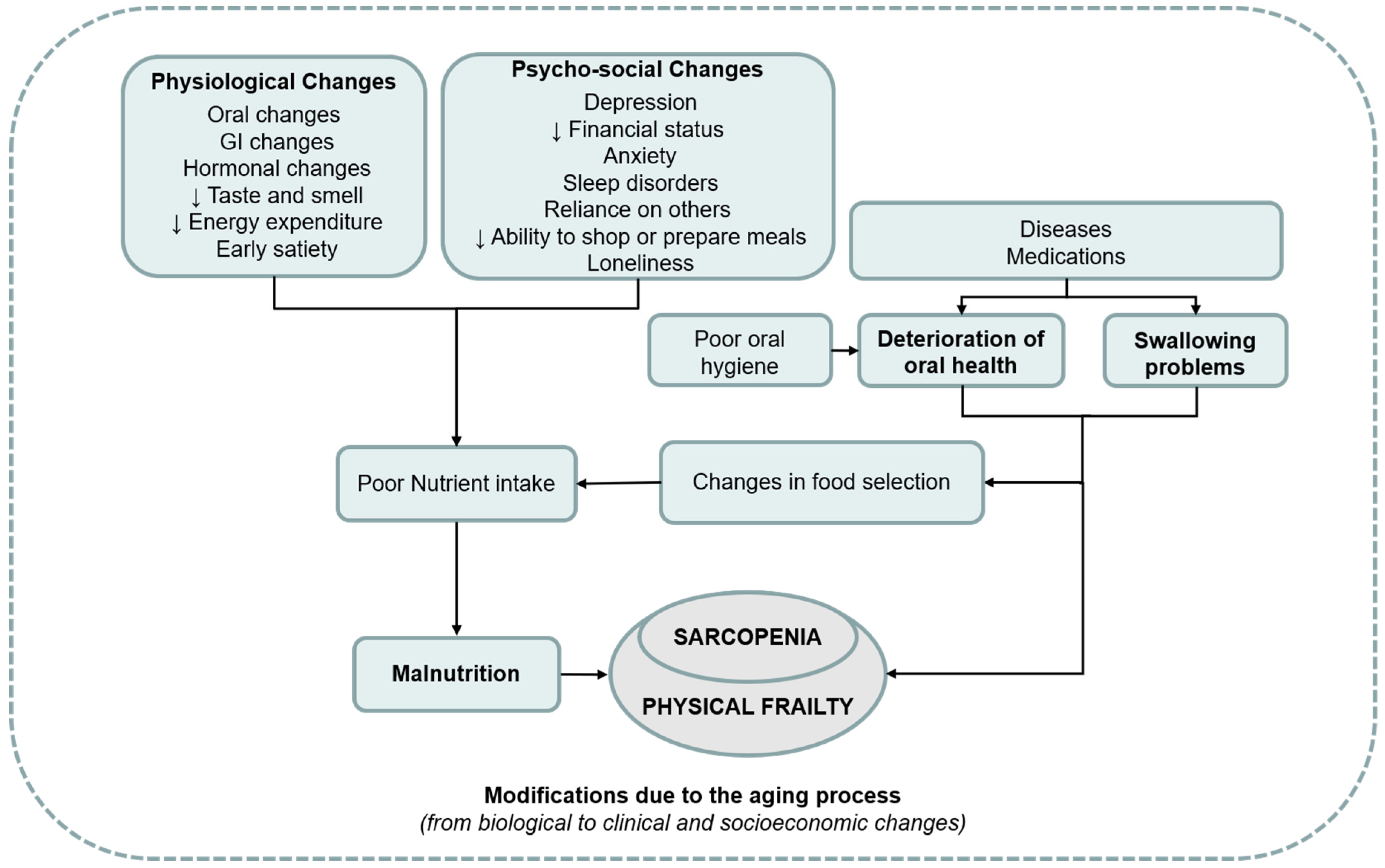
:1. Introduction
2. Oral Changes with Aging
2.1. Edentulism
2.2. Dry Mouth
2.3. Periodontal Disease
2.4. Dental Caries
2.5. Impact of Oral Health on Nutritional Status
3. Sarcopenia and Oral Status
4. Interventions
4.1. Oral Management
- (1)
- For the teeth affected by carious lesions, it must be recommended that prompt treatment be provided in order to prevent tooth loss. It would be equally appropriate for endodontic treatments for teeth with endodontic problems.
- (2)
- It is very important to monitor the periodontal status of the older patient and to provide a proper treatment plan, such as modification of general health-risk factors and oral health-specific risk factors, but professional hygiene or surgical procedures may also be necessary.
- (3)
- Prosthetic rehabilitation of the edentulous patient may help to prevent malnutrition [151] since it restores the chewing function.
- (4)
- In order to prevent problems related to the xerostomia and reduce exacerbation of carious lesions, it may be helpful to treat with saliva substitutes.
4.2. Nutritional Interventions
4.3. Exercise and Rehabilitative Strategies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bales, C.W.; Ritchie, C.S. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu. Rev. Nutr. 2002, 22, 309–323. [Google Scholar] [CrossRef]
- Palmer, K.; Onder, G.; Cesari, M. The geriatric condition of frailty. Eur. J. Intern. Med. 2018, 56, 1–2. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Leslie, W.; Hankey, C. Aging, Nutritional Status and Health. Healthcare 2015, 3, 648–658. [Google Scholar] [CrossRef]
- Roberts, H.C.; Lim, S.E.R.; Cox, N.J.; Ibrahim, K. The Challenge of Managing Undernutrition in Older People with Frailty. Nutrients 2019, 11, 808. [Google Scholar] [CrossRef]
- Hickson, M. Malnutrition and ageing. Postgrad. Med. J. 2006, 82, 2–8. [Google Scholar] [CrossRef]
- Schilp, J.; Wijnhoven, H.A.H.; Deeg, D.J.H.; Visser, M. Early determinants for the development of undernutrition in an older general population: Longitudinal Aging Study Amsterdam. Br. J. Nutr. 2011, 106, 708–717. [Google Scholar] [CrossRef]
- Locher, J.L.; Ritchie, C.S.; Roth, D.L.; Sen, B.; Vickers, K.S.; Vailas, L.I. Food choice among homebound older adults: Motivations and perceived barriers. J. Nutr. Health Aging 2009, 13, 659–664. [Google Scholar] [CrossRef]
- Bloom, I.; Lawrence, W.; Barker, M.; Baird, J.; Dennison, E.; Sayer, A.A.; Cooper, C.; Robinson, S. What influences diet quality in older people? A qualitative study among community-dwelling older adults from the Hertfordshire Cohort Study, UK. Public Health Nutr. 2017, 20, 2685–2693. [Google Scholar] [CrossRef]
- Cichero, J.A.Y. Age-Related Changes to Eating and Swallowing Impact Frailty: Aspiration, Choking Risk, Modified Food Texture and Autonomy of Choice. Geriatrics 2018, 3, 69. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38–53. [Google Scholar]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F. Sarcopenia. Clin. Med. 2014, 14, 183–186. [Google Scholar] [CrossRef]
- Komatsu, R.; Okazaki, T.; Ebihara, S.; Kobayashi, M.; Tsukita, Y.; Nihei, M.; Sugiura, H.; Niu, K.; Ebihara, T.; Ichinose, M. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J. Cachexia Sarcopenia Muscle 2018, 9, 643–653. [Google Scholar] [CrossRef]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Azzolino, D.; Damanti, S.; Bertagnoli, L.; Lucchi, T.; Cesari, M. Sarcopenia and swallowing disorders in older people. Aging. Clin. Exp. Res. 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Shiraishi, A.; Yoshimura, Y.; Wakabayashi, H.; Tsuji, Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clin. Nutr. 2018, 37, 204–207. [Google Scholar] [CrossRef]
- Iee Shin, H.; Kim, D.-K.; Seo, K.M.; Kang, S.H.; Lee, S.Y.; Son, S. Relation Between Respiratory Muscle Strength and Skeletal Muscle Mass and Hand Grip Strength in the Healthy Elderly. Ann. Rehabil. Med. 2017, 41, 686–692. [Google Scholar] [CrossRef]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef]
- Tamura, F.; Kikutani, T.; Tohara, T.; Yoshida, M.; Yaegaki, K. Tongue thickness relates to nutritional status in the elderly. Dysphagia 2012, 27, 556–561. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Pérez, R.C.; Jiménez-Corona, A.; Bernabé, E.; Villa-Romero, A.R.; Arrivé, E.; Dartigues, J.-F.; Gutiérrez-Robledo, L.M.; Borges-Yáñez, S.A. Oral Disease and 3-Year Incidence of Frailty in Mexican Older Adults. J. Gerontol. Ser. A 2017, 72, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Tong, C.; Yu, R. Chewing Difficulty Should be Included as a Geriatric Syndrome. Nutrients 2018, 10, 2019. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6315631/ (accessed on 11 October 2019). [CrossRef] [PubMed]
- Castrejón-Pérez, R.C.; Borges-Yáñez, S.A.; Gutiérrez-Robledo, L.M.; Avila-Funes, J.A. Oral health conditions and frailty in Mexican community-dwelling elderly: A cross sectional analysis. BMC Public Health 2012, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef]
- Hämäläinen, P.; Rantanen, T.; Keskinen, M.; Meurman, J.H. Oral health status and change in handgrip strength over a 5-year period in 80-year-old people. Gerodontology 2004, 21, 155–160. [Google Scholar] [CrossRef]
- Lamster, I.B.; Asadourian, L.; Del Carmen, T.; Friedman, P.K. The aging mouth: Differentiating normal aging from disease. Periodontol 2000 2016, 72, 96–107. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Chen, Y.; Yao, Y. Tooth wear in aging people: An investigation of the prevalence and the influential factors of incisal/occlusal tooth wear in northwest China. BMC Oral Health 2014, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.R. Oral soft tissue changes in geriatric patients. Bull. N. Y. Acad. Med. 1980, 56, 721–727. [Google Scholar] [PubMed]
- MacEntee, M.I.; Donnelly, L.R. Oral health and the frailty syndrome. Periodontology 2000 2016, 72, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Razak, P.A.; Richard, K.M.J.; Thankachan, R.P.; Hafiz, K.A.A.; Kumar, K.N.; Sameer, K.M. Geriatric Oral Health: A Review Article. J. Int. Oral Health 2014, 6, 110–116. [Google Scholar] [PubMed]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl S18), S39–S51. [Google Scholar] [CrossRef] [Green Version]
- Hirotomi, T.; Yoshihara, A.; Yano, M.; Ando, Y.; Miyazaki, H. Longitudinal study on periodontal conditions in healthy elderly people in Japan. Community Dent. Oral Epidemiol. 2002, 30, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, B.A.; Ismail, A.I.; Morrison, E.C.; Beltran, E.D. Risk factors for tooth loss over a 28-year period. J. Dent. Res. 1990, 69, 1126–1130. [Google Scholar] [CrossRef]
- Bahrami, G.; Vaeth, M.; Kirkevang, L.-L.; Wenzel, A.; Isidor, F. Risk factors for tooth loss in an adult population: A radiographic study. J. Clin. Periodontol. 2008, 35, 1059–1065. [Google Scholar] [CrossRef]
- Singh, K.A.; Brennan, D.S. Chewing disability in older adults attributable to tooth loss and other oral conditions. Gerodontology 2012, 29, 106–110. [Google Scholar] [CrossRef]
- Zhang, Q.; Witter, D.J.; Bronkhorst, E.M.; Creugers, N.H. Chewing ability in an urban and rural population over 40 years in Shandong Province, China. Clin. Oral Investig. 2013, 17, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Gil-Montoya, J.A.; Ferreira de Mello, A.L.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral health in the elderly patient and its impact on general well-being: A nonsystematic review. Clin. Interv. Aging 2015, 10, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musacchio, E.; Perissinotto, E.; Binotto, P.; Sartori, L.; Silva-Netto, F.; Zambon, S.; Manzato, E.; Corti, M.C.; Baggio, G.; Crepaldi, G. Tooth loss in the elderly and its association with nutritional status, socio-economic and lifestyle factors. Acta Odontol. Scand. 2007, 65, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Felton, D.A. Complete Edentulism and Comorbid Diseases: An Update. J. Prosthodont. 2016, 25, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Nagler, R.M. Salivary glands and the aging process: Mechanistic aspects, health-status and medicinal-efficacy monitoring. Biogerontology 2004, 5, 223–233. [Google Scholar] [CrossRef]
- Scully, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef]
- Singh, M.L.; Papas, A. Oral implications of polypharmacy in the elderly. Dent. Clin. 2014, 58, 783–796. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M.; Movahhedian, A.; Mohammadi, M.; Khodadoustan, A. Xerostomia Due to Systemic Disease: A Review of 20 Conditions and Mechanisms. Ann. Med. Health Sci. Res. 2014, 4, 503–510. [Google Scholar]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019. Available online: https://www.hindawi.com/journals/cjgh/2019/6757524/ (accessed on 11 October 2019). [CrossRef] [Green Version]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Kossioni, A.E. The Association of Poor Oral Health Parameters with Malnutrition in Older Adults: A Review Considering the Potential Implications for Cognitive Impairment. Nutrients 2018, 10, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.; Marawar, P.P.; Shete, S.; Saini, S. Periodontitis, a true infection. J. Glob. Infect. Dis. 2009, 1, 149–150. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Oral Health Country Area Profile Programe. Available online: https://www.who.int/oral_health/databases/malmo/en/ (accessed on 11 October 2019).
- Ashimoto, A.; Chen, C.; Bakker, I.; Slots, J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 1996, 11, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A. Activation and Resolution of Periodontal Inflammation and Its Systemic Impact. Periodontol 2000 2015, 69, 255–273. [Google Scholar] [CrossRef] [PubMed]
- O′Connor, J.-L.P.; Milledge, K.L.; O′Leary, F.; Cumming, R.; Eberhard, J.; Hirani, V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: A systematic literature review. Nutr. Rev. 2019. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5037517/ (accessed on 11 October 2019). [CrossRef]
- Kunin, A.A.; Evdokimova, A.Y.; Moiseeva, N.S. Age-related differences of tooth enamel morphochemistry in health and dental caries. EPMA J. 2015, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Avlund, K.; Holm-Pedersen, P.; Morse, D.E.; Viitanen, M.; Winblad, B. Tooth loss and caries prevalence in very old Swedish people: The relationship to cognitive function and functional ability. Gerodontology 2004, 21, 17–26. [Google Scholar] [CrossRef]
- Banting, D.W.; Ellen, R.P.; Fillery, E.D. Prevalence of root surface caries among institutionalized older persons. Community Dent. Oral Epidemiol. 1980, 8, 84–88. [Google Scholar] [CrossRef]
- Ellefsen, B.; Holm-Pedersen, P.; Morse, D.E.; Schroll, M.; Andersen, B.B.; Waldemar, G. Caries prevalence in older persons with and without dementia. J. Am. Geriatr. Soc. 2008, 56, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Fure, S.; Zickert, I. Prevalence of root surface caries in 55, 65, and 75-year-old Swedish individuals. Community Dent. Oral Epidemiol. 1990, 18, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.N.; Osterberg, T.; Steen, B.; Birkhed, D. Prevalence and incidence of dental caries and related risk factors in 70- to 76-year-olds. Acta Odontol. Scand. 2009, 67, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wiktorsson, A.M.; Martinsson, T.; Zimmerman, M. Salivary levels of lactobacilli, buffer capacity and salivary flow rate related to caries activity among adults in communities with optimal and low water fluoride concentrations. Swed. Dent. J. 1992, 16, 231–237. [Google Scholar]
- Lundberg, J.O. Nitrate transport in salivary glands with implications for NO homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13144–13145. [Google Scholar] [CrossRef] [Green Version]
- Guivante-Nabet, C.; Berenholc, C.; Berdal, A. Caries activity and associated risk factors in elderly hospitalised population – 15-months follow-up in French institutions. Gerodontology 1999, 16, 47–58. [Google Scholar] [CrossRef]
- Fure, S. Ten-year cross-sectional and incidence study of coronal and root caries and some related factors in elderly Swedish individuals. Gerodontology 2004, 21, 130–140. [Google Scholar] [CrossRef]
- Su, N.; Marek, C.L.; Ching, V.; Grushka, M. Caries prevention for patients with dry mouth. J. Can. Dent. Assoc. 2011, 77, 1–8. [Google Scholar]
- Morley, J.E. Anorexia of ageing: A key component in the pathogenesis of both sarcopenia and cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol 2000 2007, 44, 127–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnoutsos, K.; Papastergiou, P.; Stefanidis, S.; Vakaloudi, A. Periodontitis as a risk factor for cardiovascular disease: The role of anti-phosphorylcholine and anti-cardiolipin antibodies. Hippokratia 2008, 12, 144. [Google Scholar]
- Syrjälä, A.-M.H.; Ylöstalo, P.; Hartikainen, S.; Sulkava, R.; Knuuttila, M. Number of teeth and selected cardiovascular risk factors among elderly people. Gerodontology 2010, 27, 189–192. [Google Scholar] [CrossRef]
- Touger-Decker, R. Diet, cardiovascular disease and oral health: Promoting health and reducing risk. J. Am. Dent. Assoc. 2010, 141, 167–170. [Google Scholar] [CrossRef]
- Helkimo, E.; Carlsson, G.E.; Helkimo, M. Chewing efficiency and state of dentition. A methodologic study. Acta Odontol. Scand. 1978, 36, 33–41. [Google Scholar] [CrossRef]
- Akeel, R.; Nilner, M.; Nilner, K. Masticatory efficiency in individuals with natural dentition. Swed. Dent. J. 1992, 16, 191–198. [Google Scholar]
- Naka, O.; Anastassiadou, V.; Pissiotis, A. Association between functional tooth units and chewing ability in older adults: A systematic review. Gerodontology 2014, 31, 166–177. [Google Scholar] [CrossRef]
- Tate, G.S.; Throckmorton, G.S.; Ellis, E.; Sinn, D.P. Masticatory performance, muscle activity, and occlusal force in preorthognathic surgery patients. J. Oral Maxillofac. Surg. 1994, 52, 476–482; discussion 482. [Google Scholar] [CrossRef]
- Fontijn-Tekamp, F.A.; Slagter, A.P.; Van Der Bilt, A.; Van ′T Hof, M.A.; Witter, D.J.; Kalk, W.; Jansen, J.A. Biting and chewing in overdentures, full dentures, and natural dentitions. J. Dent. Res. 2000, 79, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Kapur, K.K.; Soman, S.D. Masticatory performance and efficiency in denture wearers. J. Prosthet. Dent. 2006, 95, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Evans, R.D. Functional occlusion: I. A review. J. Orthod. 2001, 28, 76–81. [Google Scholar] [CrossRef]
- Furuta, M.; Yamashita, Y. Oral Health and Swallowing Problems. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Mishellany, A.; Woda, A.; Labas, R.; Peyron, M.-A. The challenge of mastication: Preparing a bolus suitable for deglutition. Dysphagia 2006, 21, 87–94. [Google Scholar] [CrossRef]
- Hutton, B.; Feine, J.; Morais, J. Is there an association between edentulism and nutritional state? J. Can. Dent. Assoc. 2002, 68, 182–187. [Google Scholar]
- Fontijn-Tekamp, F.A.; van ′t Hof, M.A.; Slagter, A.P.; van Waas, M.A. The state of dentition in relation to nutrition in elderly Europeans in the SENECA Study of 1993. Eur. J. Clin. Nutr. 1996, 50 (Suppl. S2), S117–S122. [Google Scholar]
- Greksa, L.P.; Parraga, I.M.; Clark, C.A. The dietary adequacy of edentulous older adults. J. Prosthet. Dent. 1995, 73, 142–145. [Google Scholar] [CrossRef]
- Hung, H.-C.; Colditz, G.; Joshipura, K.J. The association between tooth loss and the self-reported intake of selected CVD-related nutrients and foods among US women. Community Dent. Oral Epidemiol. 2005, 33, 167–173. [Google Scholar] [CrossRef]
- Takahashi, M.; Maeda, K.; Wakabayashi, H. Prevalence of sarcopenia and association with oral health-related quality of life and oral health status in older dental clinic outpatients. Geriatr. Gerontol. Int. 2018, 18, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.O. Nutrition, tooth development, and dental caries. Am. J. Clin. Nutr. 1995, 61, 410S–416S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enwonwu, C.O.; Phillips, R.S.; Falkler, W.A. Nutrition and oral infectious diseases: State of the science. Compend. Contin. Educ. Dent. 2002, 23, 431–434. [Google Scholar] [PubMed]
- Friedlander, A.H.; Weinreb, J.; Friedlander, I.; Yagiela, J.A. Metabolic syndrome: Pathogenesis, medical care and dental implications. J. Am. Dent. Assoc. 2007, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5831951/ (accessed on 11 October 2019). [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. Available online: http://link.springer.com/10.1007/s11882-013-0404-6 (accessed on 7 June 2019). [CrossRef] [Green Version]
- Kang, J.; Smith, S.; Pavitt, S.; Wu, J. Association between central obesity and tooth loss in the non-obese people: Results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2012. J. Clin. Periodontol. 2019, 46, 430–437. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Valls-Bellés, V.; Arilla-Codoñer, A.; Alonso-Iglesias, E. Oxidant mechanisms in childhood obesity: The link between inflammation and oxidative stress. Transl. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Lingström, P. Nutrition, dental caries and periodontal disease: A narrative review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S79–S84. [Google Scholar] [CrossRef] [Green Version]
- Moynihan, P.J. The role of diet and nutrition in the etiology and prevention of oral diseases. Bull. World Health Org. 2005, 83, 694–699. [Google Scholar]
- Sheiham, A.; Steele, J.G.; Marcenes, W.; Lowe, C.; Finch, S.; Bates, C.J.; Prentice, A.; Walls, A.W. The relationship among dental status, nutrient intake, and nutritional status in older people. J. Dent. Res. 2001, 80, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Nowjack-Raymer, R.E.; Sheiham, A. Numbers of natural teeth, diet, and nutritional status in US adults. J. Dent. Res. 2007, 86, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hollis, J.H. Tooth loss and its association with dietary intake and diet quality in American adults. J. Dent. 2014, 42, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.-P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, T.M.; Jaffe, D. Head and neck disorders affecting swallowing. GI Motil. Online 2006. Available online: https://www.nature.com/gimo/contents/pt1/full/gimo36.html (accessed on 11 October 2019).
- Wirth, R.; Dziewas, R.; Beck, A.M.; Clavé, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.; Leischker, A.H.; Martino, R.; Pluschinski, P.; et al. Oropharyngeal dysphagia in older persons—From pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin. Interv. Aging 2016, 11, 189–208. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.; Bridges, A.D.; Taylor, A. Oral, pharyngeal and esophageal motor function in aging. GI Motil. Online 2006. Available online: https://www.nature.com/gimo/contents/pt1/full/gimo39.html (accessed on 11 October 2019).
- Feng, X.; Todd, T.; Lintzenich, C.R.; Ding, J.; Carr, J.J.; Ge, Y.; Browne, J.D.; Kritchevsky, S.B.; Butler, S.G. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J. Gerontol. Ser. A 2013, 68, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.P.; Yemm, R.; Abel, R.W.; Menhinick, S. Changes in human jaw muscles with age and dental state. Gerodontology 1993, 10, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Takahashi, R.; Watanabe, N.; Oritsu, H.; Shimizu, Y. Prevalence of skeletal muscle mass loss and its association with swallowing function after cardiovascular surgery. Nutrition 2017, 38, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Machida, N.; Tohara, H.; Hara, K.; Kumakura, A.; Wakasugi, Y.; Nakane, A.; Minakuchi, S. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr. Gerontol. Int. 2017, 17, 295–301. [Google Scholar] [CrossRef]
- Sakai, K.; Nakayama, E.; Tohara, H.; Kodama, K.; Takehisa, T.; Takehisa, Y.; Ueda, K. Relationship between tongue strength, lip strength, and nutrition-related sarcopenia in older rehabilitation inpatients: A cross-sectional study. Clin. Interv. Aging 2017, 12, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Sporns, P.B.; Muhle, P.; Hanning, U.; Suntrup-Krueger, S.; Schwindt, W.; Eversmann, J.; Warnecke, T.; Wirth, R.; Zimmer, S.; Dziewas, R. Atrophy of Swallowing Muscles Is Associated with Severity of Dysphagia and Age in Patients with Acute Stroke. J. Am. Med. Dir. Assoc. 2017, 18, 635.e1–635.e7. [Google Scholar] [CrossRef]
- Maeda, K.; Akagi, J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia 2015, 30, 80–87. [Google Scholar] [CrossRef]
- Butler, S.G.; Stuart, A.; Leng, X.; Wilhelm, E.; Rees, C.; Williamson, J.; Kritchevsky, S.B. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J. Gerontol. Ser. A 2011, 66, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Buehring, B.; Hind, J.; Fidler, E.; Krueger, D.; Binkley, N.; Robbins, J. Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J. Am. Geriatr. Soc. 2013, 61, 418–422. [Google Scholar] [CrossRef]
- Tsuga, K.; Yoshikawa, M.; Oue, H.; Okazaki, Y.; Tsuchioka, H.; Maruyama, M.; Yoshida, M.; Akagawa, Y. Maximal voluntary tongue pressure is decreased in Japanese frail elderly persons. Gerodontology 2012, 29, e1078–e1085. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, C.; Aydogdu, I. Neurophysiology of swallowing. Clin. Neurophysiol. 2003, 114, 2226–2244. [Google Scholar] [CrossRef]
- Sakai, K.; Sakuma, K. Sarcopenic Dysphagia as a New Concept. Frailty Sarcopenia Onset Dev. Clin. Chall. 2017. Available online: https://www.intechopen.com/books/frailty-and-sarcopenia-onset-development-and-clinical-challenges/sarcopenic-dysphagia-as-a-new-concept (accessed on 11 October 2019).
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [PubMed]
- Rowlerson, A.; Raoul, G.; Daniel, Y.; Close, J.; Maurage, C.-A.; Ferri, J.; Sciote, J.J. Fiber-type differences in masseter muscle associated with different facial morphologies. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Yao, X.; Li, H.; Leng, S.X. Inflammation and immune system alterations in frailty. Clin. Geriatr. Med. 2011, 27, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Napa, K.; Baeder, A.C.; Witt, J.E.; Rayburn, S.T.; Miller, M.G.; Dallon, B.W.; Gibbs, J.L.; Wilcox, S.H.; Winden, D.R.; Smith, J.H.; et al. LPS from, P. gingivalis Negatively Alters Gingival Cell Mitochondrial Bioenergetics. Int. J. Dent. 2017, 2017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5448046/ (accessed on 11 October 2019). [CrossRef]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M.; Knowler, W.C.; Pettitt, D.J. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J. Periodontol. 1996, 67 (Suppl. S10), 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Chee, B.; Park, B.; Bartold, P.M. Periodontitis and type II diabetes: A two-way relationship. Int. J. Evid. Based Healthc. 2013, 11, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Smith, T.O.; Kaul, A.; Sofat, N. Hand to Mouth: A Systematic Review and Meta-Analysis of the Association between Rheumatoid Arthritis and Periodontitis. Front. Immunol. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, J.D.; Offenbacher, S.; Williams, R.; Gibbs, P.; Garcia, R. Periodontitis: A risk factor for coronary heart disease? Ann. Periodontol. 1998, 3, 127–141. [Google Scholar] [CrossRef] [PubMed]
- D′Aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246. [Google Scholar] [CrossRef] [Green Version]
- Borges, I.; Moreira, E.A.M.; Filho, D.W.; de Oliveira, T.B.; da Silva, M.B.S.; Fröde, T.S. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediat. Inflamm. 2007, 2007, 45794. [Google Scholar] [CrossRef]
- Horton, A.L.; Boggess, K.A.; Moss, K.L.; Beck, J.; Offenbacher, S. Periodontal disease, oxidative stress, and risk for preeclampsia. J. Periodontol. 2010, 81, 199–204. [Google Scholar] [CrossRef]
- Bullon, P.; Cordero, M.D.; Quiles, J.L.; del Carmen Ramirez-Tortosa, M.; Gonzalez-Alonso, A.; Alfonsi, S.; García-Marín, R.; de Miguel, M.; Battino, M. Autophagy in periodontitis patients and gingival fibroblasts: Unraveling the link between chronic diseases and inflammation. BMC Med. 2012, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Bullon, P.; Cordero, M.D.; Quiles, J.L.; Morillo, J.M.; del Carmen Ramirez-Tortosa, M.; Battino, M. Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic. Biol. Med. 2011, 50, 1336–1343. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-L.; Ohura, K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit. Rev. Oral Biol. Med. 2002, 13, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Andersson, P.; Hallberg, I.R.; Lorefält, B.; Unosson, M.; Renvert, S. Oral health problems in elderly rehabilitation patients. Int. J. Dent. Hyg. 2004, 2, 70–77. [Google Scholar] [CrossRef]
- Hanne, K.; Ingelise, T.; Linda, C.; Ulrich, P.P. Oral status and the need for oral health care among patients hospitalised with acute medical conditions. J. Clin. Nurs. 2012, 21, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and Physical Frailty: Two Sides of the Same Coin. Front. Aging Neurosci 2014, 6, 192. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4112807/ (accessed on 5 November 2019). [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Cherubini, A.; Cesari, M.; Calvani, R.; Tosato, M.; Sisto, A.; Martone, A.M.; Bernabei, R.; Marzetti, E. Sarcopenia and frailty: From theoretical approach into clinical practice. Eur. Geriatr. Med. 2016, 7, 197–200. Available online: https://moh-it.pure.elsevier.com/en/publications/sarcopenia-and-frailty-from-theoretical-approach-into-clinical-pr (accessed on 11 October 2019). [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Ellis, G.; Whitehead, M.A.; O′Neill, D.; Langhorne, P.; Robinson, D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane. Database. Syst. Rev. 2011, 7, CD006211. [Google Scholar]
- Bollero, P.; Passarelli, P.C.; D′Addona, A.; Pasquantonio, G.; Mancini, M.; Condò, R.; Cerroni, L. Oral management of adult patients undergoing hematopoietic stem cell transplantation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 876–887. [Google Scholar]
- Tonetti, M.S.; Bottenberg, P.; Conrads, G.; Eickholz, P.; Heasman, P.; Huysmans, M.-C.; López, R.; Madianos, P.; Müller, F.; Needleman, I.; et al. Dental caries and periodontal diseases in the ageing population: Call to action to protect and enhance oral health and well-being as an essential component of healthy ageing—Consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S135–S144. [Google Scholar]
- Andreas Zenthöfer, A.; Rammelsberg, P.; Cabrera, T.; Hassel, A. Prosthetic rehabilitation of edentulism prevents malnutrition in nursing home residents. Int. J. Prosthodont. 2015, 28, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft , A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.A.; Kimlin, M.G. Vitamin D, aging, and the 2005 Dietary Guidelines for Americans. Nutr. Rev. 2006, 64, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.H.; Lips, P. Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Ney, D.M.; Weiss, J.M.; Kind, A.J.H.; Robbins, J. Senescent Swallowing: Impact, Strategies, and Interventions. Nutr. Clin. Pract. 2009, 24, 395–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sura, L.; Madhavan, A.; Carnaby, G.; Crary, M.A. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The Atherosclerosis Risk in Communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Guideline: Sugars Intake for Adults and Children. Available online: https://apps.who.int/iris/handle/10665/149782 (accessed on 22 October 2019).
- Gutteridge, J.M.C.; Halliwell, B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010, 393, 561–564. [Google Scholar] [CrossRef]
- Welch, A.A.; MacGregor, A.J.; Minihane, A.-M.; Skinner, J.; Valdes, A.A.; Spector, T.D.; Cassidy, A. Dietary fat and fatty acid profile are associated with indices of skeletal muscle mass in women aged 18-79 years. J. Nutr. 2014, 144, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Dupont, J.; Dedeyne, L.; Dalle, S.; Koppo, K.; Gielen, E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin. Exp. Res. 2019, 31, 825–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damanti, S.; Azzolino, D.; Roncaglione, C.; Arosio, B.; Rossi, P.; Cesari, M. Efficacy of Nutritional Interventions as Stand-Alone or Synergistic Treatments with Exercise for the Management of Sarcopenia. Nutrients 2019, 11, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, J.M.; Volpi, E.; Rasmussen, B.B. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc. Sport Sci. Rev. 2013, 41, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Glover, E.I.; Phillips, S.M.; Oates, B.R.; Tang, J.E.; Tarnopolsky, M.A.; Selby, A.; Smith, K.; Rennie, M.J. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J. Physiol. 2008, 586, 6049–6061. [Google Scholar] [CrossRef]
- Bawadi, H.A.; Khader, Y.S.; Haroun, T.F.; Al-Omari, M.; Tayyem, R.F. The association between periodontal disease, physical activity and healthy diet among adults in Jordan. J. Periodont. Res. 2011, 46, 74–81. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Sugiyama, T.; Ohkubo, M.; Honda, Y.; Tasaka, A.; Nagasawa, K.; Ishida, R.; Sakurai, K. Effect of swallowing exercises in independent elderly. Bull. Tokyo. Dent. Coll. 2013, 54, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Argolo, N.; Sampaio, M.; Pinho, P.; Melo, A.; Nóbrega, A.C. Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson′s disease? NeuroRehabilitation 2013, 32, 949–955. [Google Scholar]
- Kim, H.-J.; Lee, J.-Y.; Lee, E.-S.; Jung, H.-J.; Ahn, H.-J.; Kim, B.-I. Improvements in oral functions of elderly after simple oral exercise. Clin. Interv. Aging 2019, 14, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Oh, D.H.; Chang, M.Y.; Kim, K.M. Effects of expiratory muscle strength training on oropharyngeal dysphagia in subacute stroke patients: A randomised controlled trial. J. Oral Rehabil. 2016, 43, 364–372. [Google Scholar] [CrossRef]
- Park, J.-S.; Oh, D.-H.; Chang, M.-Y. Effect of expiratory muscle strength training on swallowing-related muscle strength in community-dwelling elderly individuals: A randomized controlled trial. Gerodontology 2017, 34, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Hwang, N.K.; Oh, D.H.; Chang, M.Y. Effect of head lift exercise on kinematic motion of the hyolaryngeal complex and aspiration in patients with dysphagic stroke. J. Oral Rehabil. 2017, 44, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Antunes, E.B.; Lunet, N. Effects of the head lift exercise on the swallow function: A systematic review. Gerodontology 2012, 29, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Bayley, M.T.; Peladeau-Pigeon, M.; Nagy, A.; Namasivayam, A.M.; Stokely, S.L.; Wolkin, T. A Randomized Trial Comparing Two Tongue-Pressure Resistance Training Protocols for Post-Stroke Dysphagia. Dysphagia 2016, 31, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Choi, J.B.; Yoo, S.J.; Chang, M.Y.; Lee, S.W.; Park, J.S. Tongue-to-palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagia. J. Oral Rehabil. 2017, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Yeates, E.M.; Molfenter, S.M.; Steele, C.M. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: Three case reports. Clin. Interv. Aging 2008, 3, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.; Gangnon, R.E.; Theis, S.M.; Kays, S.A.; Hewitt, A.L.; Hind, J.A. The effects of lingual exercise on swallowing in older adults. J. Am. Geriatr. Soc. 2005, 53, 1483–1489. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzolino, D.; Passarelli, P.C.; De Angelis, P.; Piccirillo, G.B.; D’Addona, A.; Cesari, M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients 2019, 11, 2898. https://doi.org/10.3390/nu11122898
Azzolino D, Passarelli PC, De Angelis P, Piccirillo GB, D’Addona A, Cesari M. Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients. 2019; 11(12):2898. https://doi.org/10.3390/nu11122898
Chicago/Turabian StyleAzzolino, Domenico, Pier Carmine Passarelli, Paolo De Angelis, Giovan Battista Piccirillo, Antonio D’Addona, and Matteo Cesari. 2019. "Poor Oral Health as a Determinant of Malnutrition and Sarcopenia" Nutrients 11, no. 12: 2898. https://doi.org/10.3390/nu11122898
APA StyleAzzolino, D., Passarelli, P. C., De Angelis, P., Piccirillo, G. B., D’Addona, A., & Cesari, M. (2019). Poor Oral Health as a Determinant of Malnutrition and Sarcopenia. Nutrients, 11(12), 2898. https://doi.org/10.3390/nu11122898







