An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Background Diets
2.4. Dietary Treatments
2.5. Identification of Phenolics in Berries and Berry Juice by LC-MS/MS
2.6. Meal-Based Glucose Tolerance Test
2.7. Calculations and Statistics
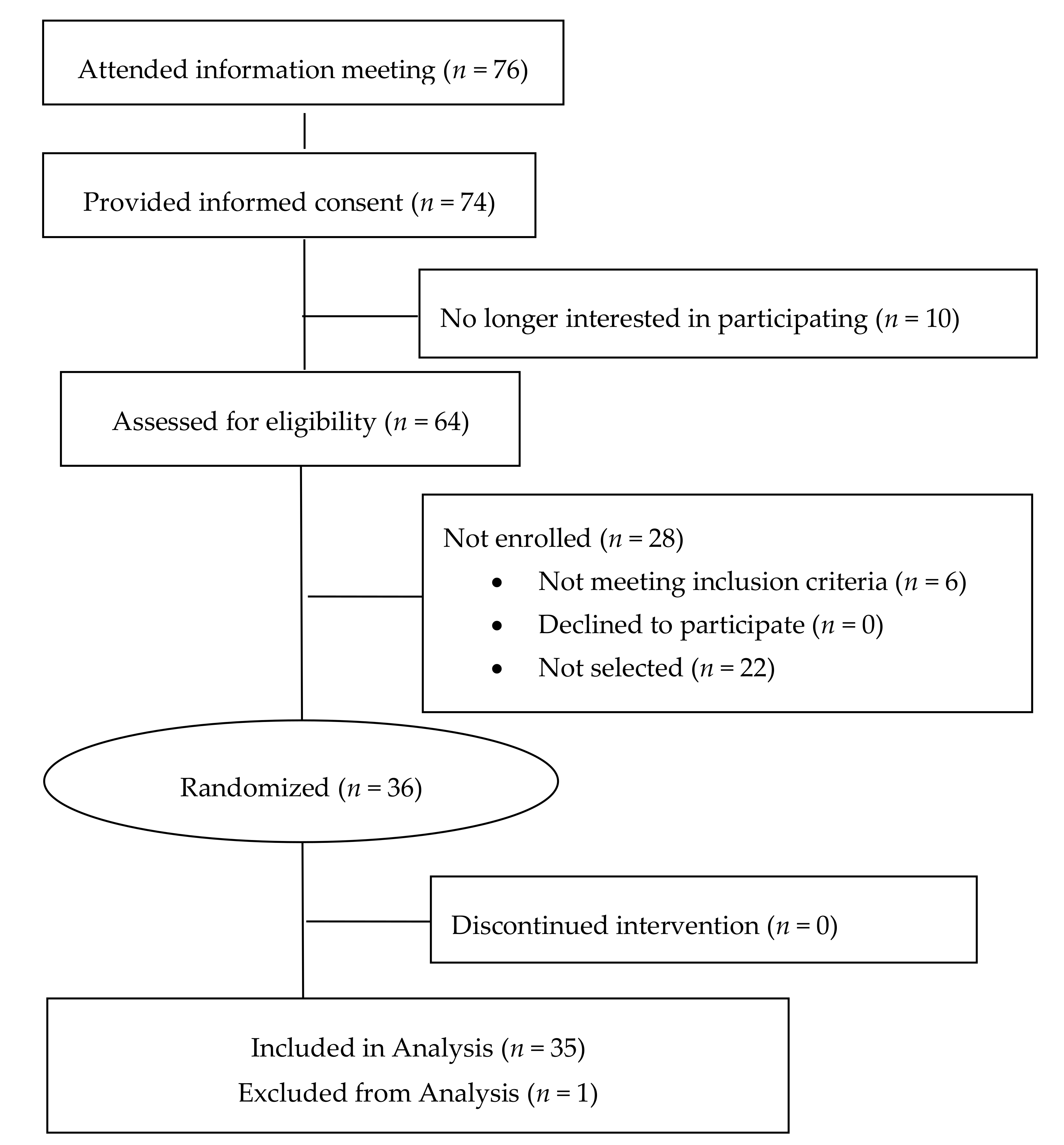
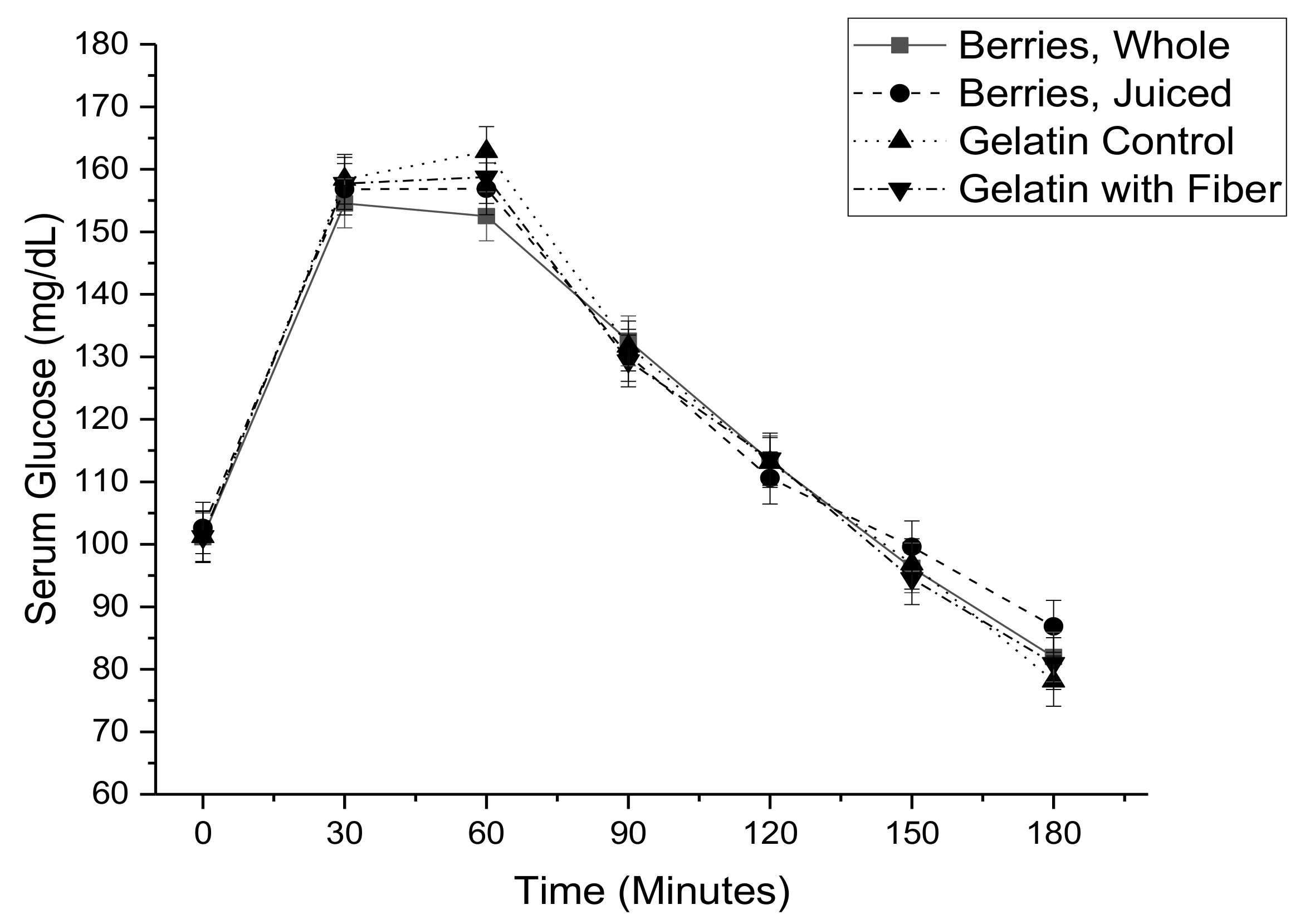
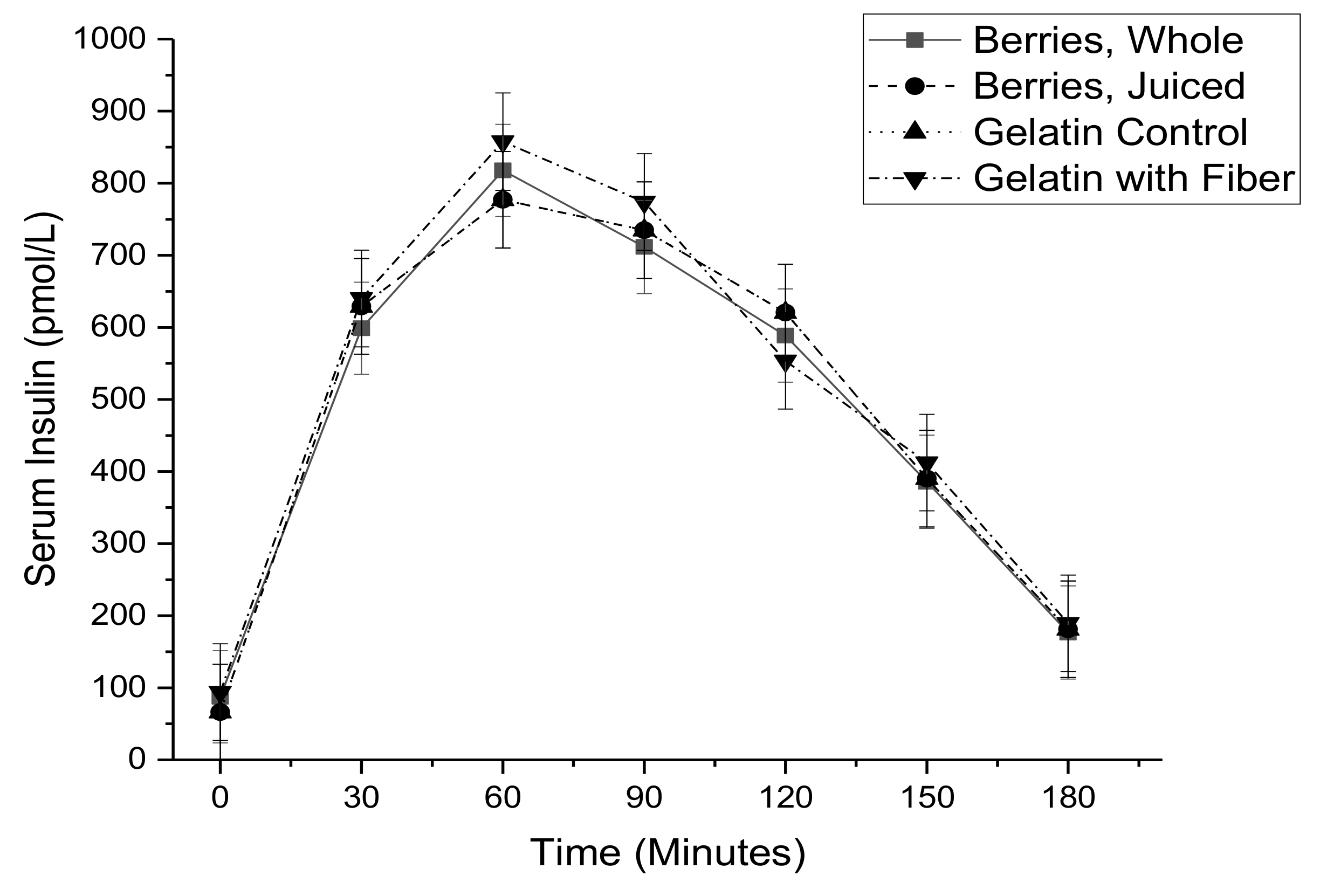
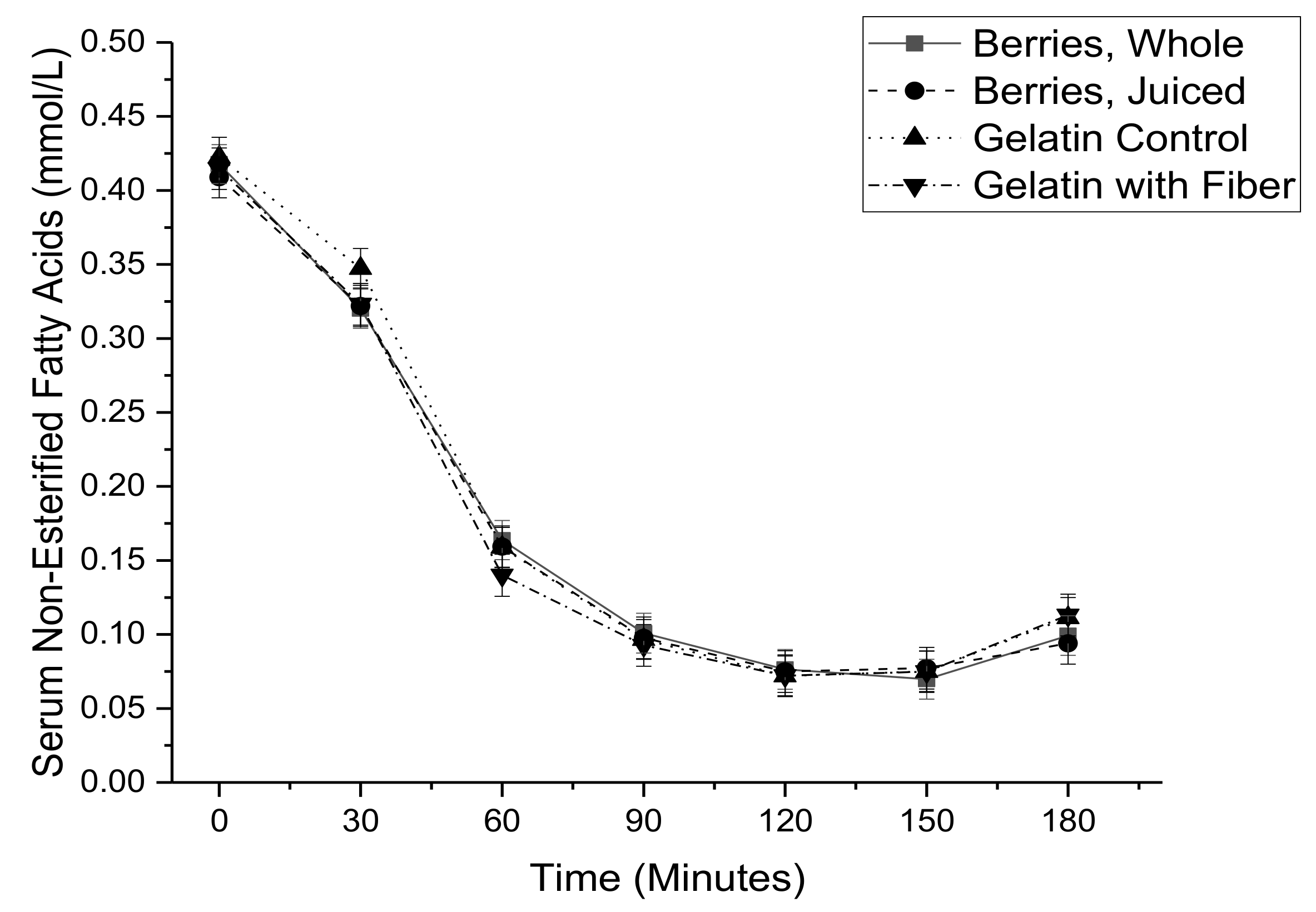
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balakumar, P.; Maung, U.K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharm. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of berry polyphenols—Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and blackcurrant polyphenol-rich drinks decrease postprandial glucose, insulin and incretin response to a high-carbohydrate meal in healthy men and women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Torronen, R.; Sarkkinen, E.; Niskanen, T.; Tapola, N.; Kilpi, K.; Niskanen, L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2012, 107, 1445–1451. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef]
- Torronen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkanen, H.; Niskanen, L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef]
- Torronen, R.; Kolehmainen, M.; Sarkkinen, E.; Mykkanen, H.; Niskanen, L. Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am. J. Clin. Nutr. 2012, 96, 527–533. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A study of glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.; Silva, J.E.S.; Cook, M.D.; Blacker, S.D. Beneficial effects on fasting insulin and postprandial responses through 7-day intake of new zealand blackcurrant powder. Funct. Foods Health Dis. 2017, 7, 483–493. [Google Scholar]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Burton, J.; Heiman, M.; Greenway, F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J. Diabetes Complicat. 2015, 29, 1272–1276. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef]
- Udani, J.K.; Singh, B.B.; Singh, V.J.; Barrett, M.L. Effects of acai (Euterpe oleracea mart.) berry preparation on metabolic parameters in a healthy overweight population: A pilot study. Nutr. J. 2011, 10, 45. [Google Scholar] [CrossRef]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry feeding increases fat oxidation and improves insulin sensitivity in overweight and obese males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larque, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonne, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Stote, K.; Corkum, A.; Sweeney, M.; Shakerley, N.; Kean, T.; Gottschall-Pass, K. Postprandial effects of blueberry (Vaccinium angustifolium) consumption on glucose metabolism, gastrointestinal hormone response, and perceived appetite in healthy adults: A randomized, placebo-controlled crossover trial. Nutrients 2019, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Lambert, J.D.; Proctor, D.N.; Kris-Etherton, P.M. Incorporating freeze-dried strawberry powder into a high-fat meal does not alter postprandial vascular function or blood markers of cardiovascular disease risk: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef]
- Kaneko, T.; Wang, P.Y.; Tawata, M.; Sato, A. Low carbohydrate intake before oral glucose-tolerance tests. Lancet 1998, 352, 289. [Google Scholar] [CrossRef]
- Russell, W.R.; Baka, A.; Bjorck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P.; Mikhailidis, D.P.; Toth, P.P.; Ray, K.K.; Banach, M. Dietary food patterns and glucose/insulin homeostasis: A cross-sectional study involving 24,182 adult americans. Lipids Health Dis. 2017, 16, 192. [Google Scholar] [CrossRef]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-o-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of beneficial metabolic effects of berries in high-fat fed c57bl/6j mice. J. Nutr. Metab. 2014, 2014, 403041. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.L.; Hager, T.J.; Hager, A.; Howard, L. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.L.; Howard, L.R. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.L.; Gu, L.W.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the c57bl/6j mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Singer, A.A.M.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of amp-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef]
- Mason, B.S.; Slover, H.T. A gas chromatographic method for the determination of sugars in foods. J. Agric. Food Chem. 1971, 19, 551–554. [Google Scholar] [CrossRef]
- Redan, B.W.; Albaugh, G.P.; Charron, C.S.; Novotny, J.A.; Ferruzzi, M.G. Adaptation in caco-2 human intestinal cell differentiation and phenolic transport with chronic exposure to blackberry (Rubus sp.) extract. J. Agric. Food Chem. 2017, 65, 2694–2701. [Google Scholar] [CrossRef]
- Moazen, S.; Amani, R.; Homayouni Rad, A.; Shahbazian, H.; Ahmadi, K.; Taha Jalali, M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Ann. Nutr. Metab. 2013, 63, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Nguyen, A.; Newman, E.D.; Fu, D.; Lyons, T.J. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J. Nutr. 2014, 144, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Flores, C.; Hidalgo, M.A.; Perez, M.; Yanez, A.; Quinones, L.; Caceres, D.D.; Burgos, R.A. Delphinol(r) standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva Med. 2014, 56, 1–7. [Google Scholar] [PubMed]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Dose effects of new zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef]
- Mortensen, M.W.; Spagner, C.; Cuparencu, C.; Astrup, A.; Raben, A.; Dragsted, L.O. Sea buckthorn decreases and delays insulin response and improves glycaemic profile following a sucrose-containing berry meal: A randomised, controlled, crossover study of danish sea buckthorn and strawberries in overweight and obese male subjects. Eur. J. Nutr. 2018, 57, 2827–2837. [Google Scholar] [CrossRef]
- de Mello, V.D.; Lankinen, M.A.; Lindstrom, J.; Puupponen-Pimia, R.; Laaksonen, D.E.; Pihlajamaki, J.; Lehtonen, M.; Uusitupa, M.; Tuomilehto, J.; Kolehmainen, M.; et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
| Mean ± SE, n = 36 (18M/18F) | |
|---|---|
| Age (years) | 58.7 ± 1.9 |
| Weight (kg) | 88.6 ± 2.6 |
| Body mass index (kg/m2) | 30.8 ± 0.6 |
| Fasting glucose (mg/dL) | 94.2 ± 1.3 |
| Fasting insulin (pmol/L) | 80.0 ± 7.4 |
| Fasting triglycerides (mg/dL) | 122.1 ± 7.7 |
| Systolic blood pressure (mm Hg) | 133.3 ± 2.4 |
| Diastolic blood pressure (mm Hg) | 79.7 ± 2.0 |
| Gelatin Control | Whole Berries | Berry Juice | Gelatin + Fiber | |
|---|---|---|---|---|
| Daily dose (g) | 400–800 (523) | 400–800 (523) | 384–768 (502) | 400–800 (523) |
| Total sugar (g) | 28.8–57.6 (37.6) | 28.8–57.6 (37.6) | 28.6–57.3 (37.4) | 28.8-57.6 (37.6) |
| Glucose (g) | 15.0–30.0 (19.6) | 15.0–30.0 (19.6) | 15.0–30.0 (19.6) | 15.0–30.0 (19.6) |
| Fructose (g) | 13.8–27.6 (18.0) | 13.8–27.6 (18.0) | 13.6–27.3 (17.8) | 13.8–27.6 (18.0) |
| Soluble fiber (g) | 0 | 0 | 0 | 0 |
| Insoluble fiber (g) | 0 | 7.2–14.5 (9.5) | 0 | 7.2–14.5 (9.5) |
| Anthocyanins (mg) 2 | 0 | 109.0–218.0 (143.5) | 77.3–154.6 (101.1) | 0 |
| Flavonols (mg) 2 | 0 | 11.7–23.4 (15.3) | 4.9–9.8 (6.4) | 0 |
| Flavan-3-ols (mg) 2 | 0 | 2.2–4.5 (2.9) | 0.5–0.9 ((0.7) | 0 |
| Phenolic acids (mg) 2 | 0 | 29.2–58.4 (38.2) | 4.7–9.4 (6.1) | 0 |
| mg per 100 g Fresh Weight | Whole Berries | Pressed Berry Juice | |||||
|---|---|---|---|---|---|---|---|
| Mixed Berries, Whole | Pressed Juice | Polyphenols in mg per 400 g 2 | Polyphenols in mg per 800 g 3 | Polyphenols in mg per 384 g 4 | Polyphenols in mg per 768 g 5 | ||
| Anthocyanins | Cyanidin-3-Gal | 1.70 ± 0.08 | 3.19 ± 0.05 | 6.81 | 13.6 | 12.3 | 24.5 |
| Cyanidin-3-Glu | 14.05 ± 0.96 | 6.57 ± 0.20 | 56.2 | 112.4 | 25.2 | 50.4 | |
| Cyanidin-3-Ara | 0.906 ± 0.168 | 2.01 ± 0.05 | 3.63 | 7.25 | 7.70 | 15.4 | |
| Delphinidin-3-Gal | 0.874 ± 0.103 | 0.101 ± 0.002 | 3.50 | 6.99 | 0.39 | 0.78 | |
| Delphinidin-3-Glu | ND | trace | ND | ND | ND | ND | |
| Delphinidin-3-Ara | 0.830 ± 0.081 | 0.053 ± 0.002 | 3.32 | 6.64 | 0.21 | 0.41 | |
| Malvidin-3-Gal | 2.54 ± 0.15 | 1.05 ± 0.06 | 10.2 | 20.3 | 4.04 | 8.07 | |
| Malvidin-3-Glu | 0.500 ± 0.060 | 0.012 ± 0.003 | 2.00 | 4.00 | 0.05 | 0.09 | |
| Malvidin-3-Ara | 3.05 ± 0.17 | 0.805 ± 0.017 | 12.2 | 24.4 | 3.09 | 6.18 | |
| Peonidin-3-Gal | 0.793 ± 0.102 | 3.43 ± 0.10 | 3.17 | 6.34 | 13.2 | 26.4 | |
| Peonidin-3-Glu | 0.070 ± 0.012 | 0.17 ± 0.02 | 0.28 | 0.56 | 0.64 | 1.27 | |
| Peonidin-3-Ara | 0.588 ± 0.075 | 2.53 ± 0.06 | 2.35 | 4.71 | 9.72 | 19.44 | |
| Petunidin-3-Gal | 0.920 ± 0.039 | 0.159 ± 0.003 | 3.68 | 7.36 | 0.61 | 1.22 | |
| Petunidin-3-Glu | 0.340 ± 0.011 | 0.016 ± 0.004 | 1.36 | 2.72 | 0.06 | 0.12 | |
| Petunidin-3-Ara | 0.076 ± 0.066 | 0.057 ± 0.002 | 0.30 | 0.60 | 0.22 | 0.44 | |
| Total | Anthocyanins | 27.2 ± 1.9 | 20.1 ± 0.4 | 109.0 | 218.0 | 77.3 | 154.6 |
| Phenolic acids | Chlorogenic acid | 7.09 ± 0.27 | 1.08 ± 0.02 | 28.4 | 56.7 | 4.14 | 8.28 |
| Caffeic acid | 0.211 ± 0.013 | 0.032 ± 0.002 | 0.84 | 1.69 | 0.12 | 0.24 | |
| Gallic acid | ND | 0.116 ± 0.004 | ND | ND | 0.44 | 0.89 | |
| Total | Phenolic acids | 7.30 ± 0.28 | 1.23 ± 0.02 | 29.2 | 58.4 | 4.71 | 9.42 |
| Flavonols | Quercetin-3-Gal | 2.01 ± 0.64 | 1.00 ± 0.01 | 8.06 | 16.1 | 3.84 | 7.68 |
| Quercetin-3-Glu | 0.448 ± 0.001 | 0.088 ± 0.001 | 1.79 | 3.58 | 0.34 | 0.67 | |
| Kaempferol-3-Glu | 0.466 ± 0.042 | 0.186 ± 0.003 | 1.86 | 3.73 | 0.71 | 1.43 | |
| Total | Flavonols | 2.93 ± 0.67 | 1.27 ± 0.01 | 11.7 | 23.4 | 4.89 | 9.78 |
| Flavan-3-ols | Catechin | 0.226 ± 0.026 | 0.079 ± 0.003 | 0.90 | 1.81 | 0.30 | 0.60 |
| Epicatechin | 0.330 ± 0.016 | 0.039 ± 0.001 | 1.32 | 2.64 | 0.15 | 0.30 | |
| Total | Flavan-3-ols | 0.556 ± 0.041 | 0.118 ± 0.004 | 2.22 | 4.45 | 0.45 | 0.90 |
| Gelatin Control 1 | Whole Berries 1 | Berry Juice 1 | Gelatin + Fiber 1 | p-Value 2 | |
|---|---|---|---|---|---|
| Glucose iAUC 3 (ln mg·min/dL) | 3.38 ± 0.05 | 3.32 ± 0.05 | 3.34 ± 0.05 | 3.31 ± 0.05 | 0.19 |
| Insulin iAUC 4 (ln pmol·min/L) | 11.40 ± 0.08 | 11.31 ± 0.08 | 11.32 ± 0.08 | 11.39 ± 0.8 | 0.13 |
| NEFA AUC 5 (ln mmol·min/L) | 9.03 ± 0.08 | 8.91 ± 0.08 | 8.90 ± 0.08 | 8.96 ± 0.08 | 0.65 |
| Difference between Gelatin Treatments vs. Berry Treatments 1 | p-Value 2 | |
|---|---|---|
| Glucose iAUC (ln mg·min/dL) | 0.169 ± 0.093 | 0.074 |
| Insulin iAUC (ln pmol·min/mL) | 0.153 ± 0.066 | 0.023 |
| NEFA AUC (ln mmol·min/L) | 0.028 ± 0.062 | 0.648 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solverson, P.M.; Henderson, T.R.; Debelo, H.; Ferruzzi, M.G.; Baer, D.J.; Novotny, J.A. An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults. Nutrients 2019, 11, 2876. https://doi.org/10.3390/nu11122876
Solverson PM, Henderson TR, Debelo H, Ferruzzi MG, Baer DJ, Novotny JA. An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults. Nutrients. 2019; 11(12):2876. https://doi.org/10.3390/nu11122876
Chicago/Turabian StyleSolverson, Patrick M., Theresa R. Henderson, Hawi Debelo, Mario G. Ferruzzi, David J. Baer, and Janet A. Novotny. 2019. "An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults" Nutrients 11, no. 12: 2876. https://doi.org/10.3390/nu11122876
APA StyleSolverson, P. M., Henderson, T. R., Debelo, H., Ferruzzi, M. G., Baer, D. J., & Novotny, J. A. (2019). An Anthocyanin-Rich Mixed-Berry Intervention May Improve Insulin Sensitivity in a Randomized Trial of Overweight and Obese Adults. Nutrients, 11(12), 2876. https://doi.org/10.3390/nu11122876




