Comparison of the Effects of Fasting Glucose, Hemoglobin A1c, and Triglyceride–Glucose Index on Cardiovascular Events in Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
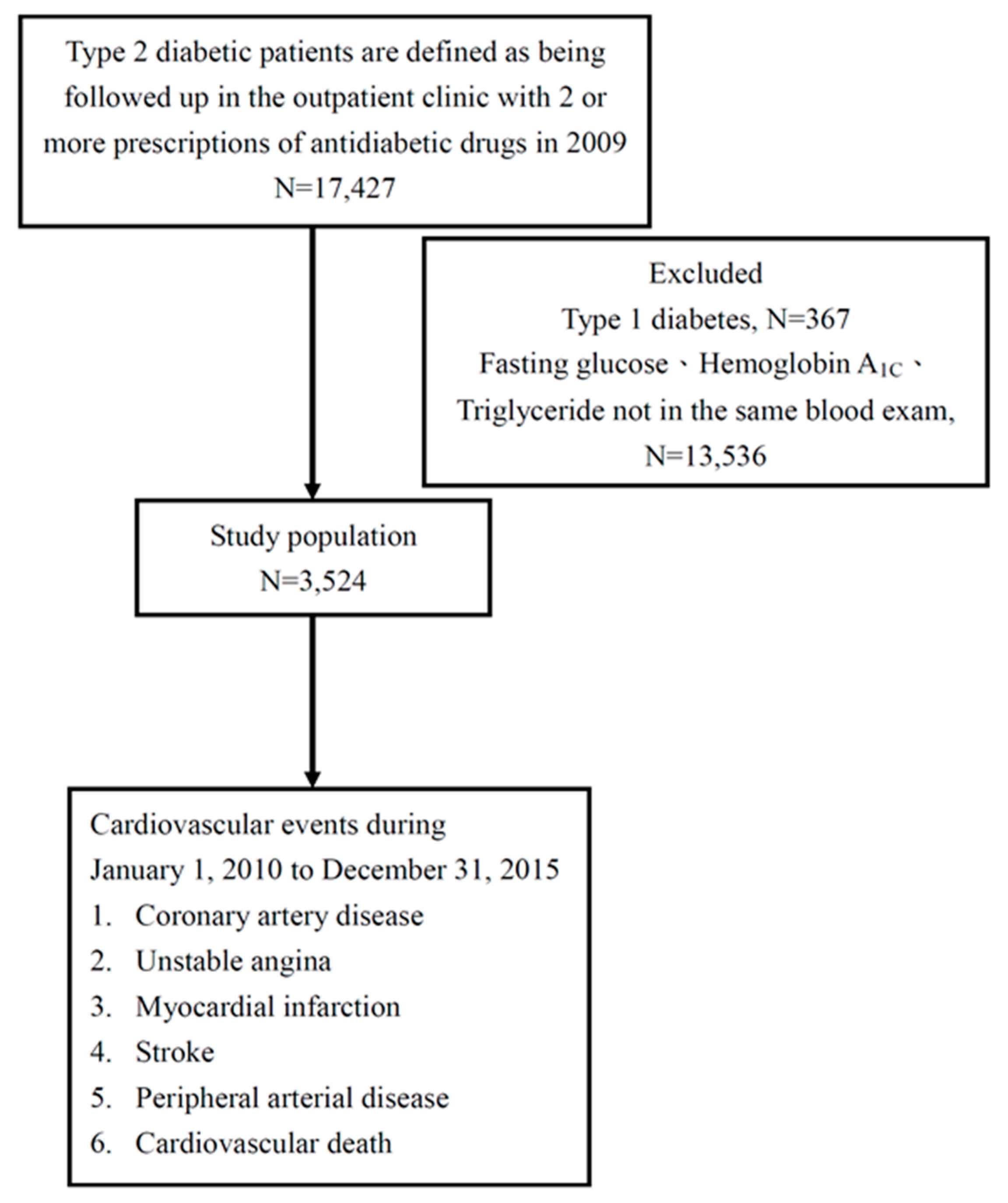
2.2. Study Population
2.3. Ethics Statement
2.4. Definition of Study Endpoint
2.5. TyG Index
2.6. Statistical Analysis
3. Results
3.1. Determinants of CV Events in the Study Patients
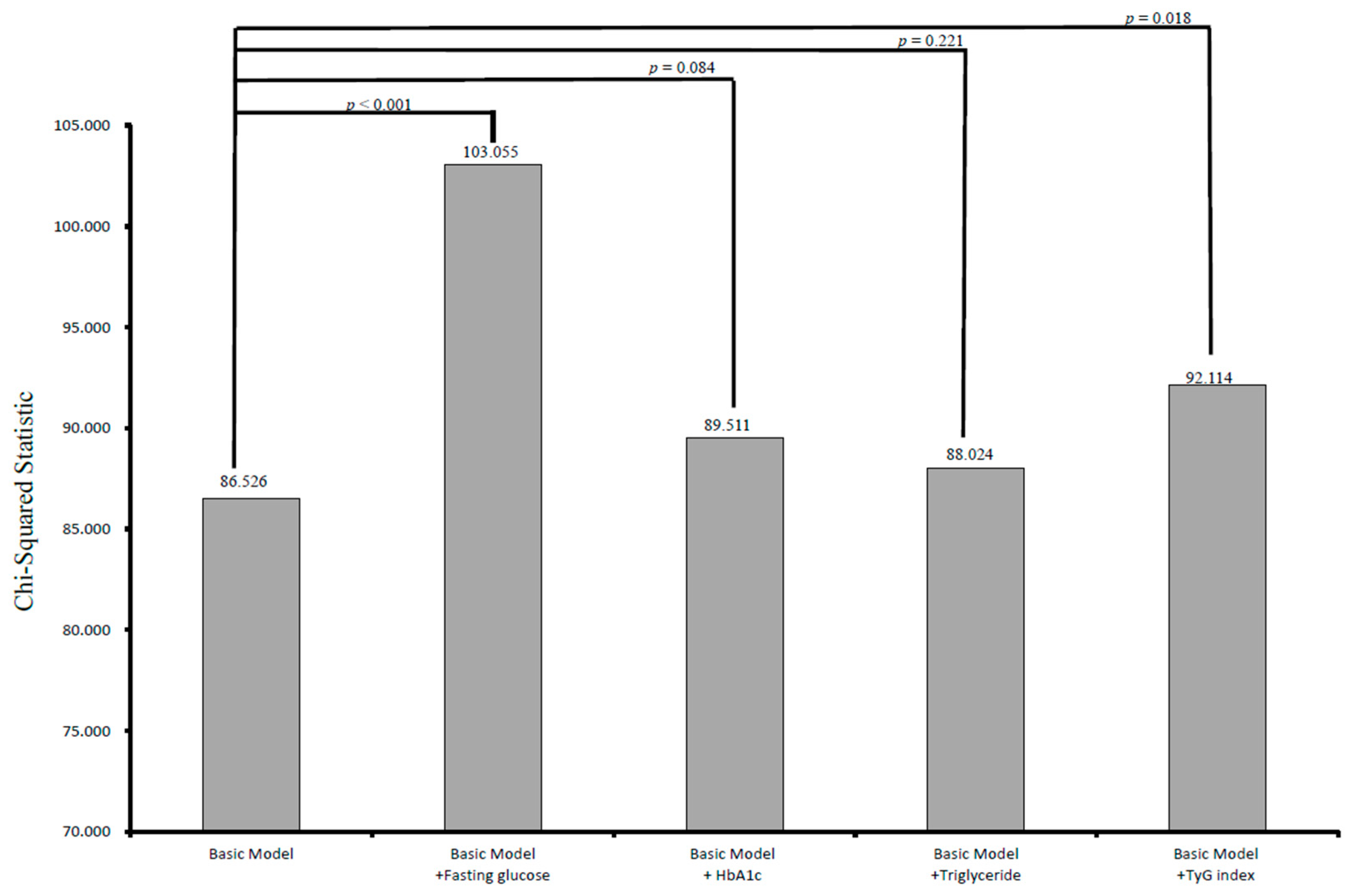
3.2. Incremental Values of Fasting Glucose, Hemoglobin A1c, Triglyceride, and the Tyg Index in Relation to CV Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Seshasai, S.R.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Emerging Risk Factors Collaboration, Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [PubMed]
- Anand, S.S.; Dagenais, G.R.; Mohan, V.; Diaz, R.; Probstfield, J.; Freeman, R.; Shaw, J.; Lanas, F.; Avezum, A.; Budaj, A.; et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: The EpiDREAM cohort study. Eur. J. Prev. Cardiol. 2012, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Rathmann, W.; Herder, C.; Thorand, B.; Wilsgaard, T.; Njølstad, I.; Siganos, G.; Mathiesen, E.B.; Saum, K.U.; Peasey, A.; et al. HbA1c levels in non-diabetic older adults—No J-shaped associations with primary cardiovascular events, cardiovascular and all-cause mortality after adjustment for confounders in a meta-analysis of individual participant data from six cohort studies. BMC Med. 2016, 14, 26. [Google Scholar] [CrossRef]
- Bots, S.H.; van der Graaf, Y.; Nathoe, H.M.; de Borst, G.J.; Kappelle, J.L.; Visseren, F.L.; Westerink, J.; SMART Study Group. The influence of baseline risk on the relation between HbA1c and risk for new cardiovascular events and mortality in patients with type 2 diabetes and symptomatic cardiovascular disease. Cardiovasc. Diabetol. 2016, 15, 101. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Unger, G.; Benozzi, S.F.; Perruzza, F.; Pennacchiotti, G.L. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 2014, 61, 533–540. [Google Scholar] [CrossRef]
- Navarro-González, D.; Sánchez-Íñigo, L.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martinez, J.A. Triglyceride–glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, J.S.; Ahn, Y. The Cut-off Values of Triglycerides and Glucose Index for Metabolic Syndrome in American and Korean Adolescents. J. Korean. Med. Sci. 2017, 32, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, B.; Liu, Y.; Sun, X.; Luo, X.; Wang, C.; Li, L.; Zhang, L.; Ren, Y.; Zhao, Y.; et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc. Diabetol. 2017, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Ahn, C.W.; Lee, B.K.; Kang, S.; Nam, J.S.; You, J.H.; Kim, M.J.; Kim, M.K.; Park, J.S. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc. Diabetol. 2018, 17, 41. [Google Scholar] [CrossRef]
- Kim, M.K.; Ahn, C.W.; Kang, S.; Nam, J.S.; Kim, K.R.; Park, J.S. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc. Diabetol. 2017, 16, 108. [Google Scholar] [CrossRef]
- Mao, Q.; Zhou, D.; Li, Y.; Wang, Y.; Xu, S.C.; Zhao, X.H. The Triglyceride-Glucose Index Predicts Coronary Artery Disease Severity and Cardiovascular Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Dis. Markers 2019, 2019, 6891537. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendia, L.E.; Gonzalez-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Quillard, T.; Franck, G.; Mawson, T.; Folco, E.; Libby, P. Mechanisms of erosion of atherosclerotic plaques. Curr. Opin. Lipidol. 2017, 28, 434–441. [Google Scholar] [CrossRef]
- Van Popele, N.M.; Elizabeth Hak, A.; Mattace-Raso, F.U.; Bots, M.L.; van der Kuip, D.A.; Reneman, R.S.; Hoeks, A.P.; Hofman, A.; Grobbee, D.E.; Witteman, J.C. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: The Rotterdam Study. J. Am. Geriatr. Soc. 2006, 54, 397–404. [Google Scholar] [CrossRef]
- Thomas, G.N.; Chook, P.; Qiao, M.; Huang, X.S.; Leong, H.C.; Celermajer, D.S.; Woo, K.S. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: The CATHAY study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 739–743. [Google Scholar] [CrossRef]
- Andreozzi, F.; Succurro, E.; Mancuso, M.R.; Perticone, M.; Sciacqua, A.; Perticone, F.; Sesti, G. Metabolic and cardiovascular risk factors in subjects with impaired fasting glucose: The 100 versus 110 mg/dL threshold. Diabetes Metab. Res. Rev. 2007, 23, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ahn, C.W.; Lee, S.B.; Kang, S.; Nam, J.S.; Lee, B.K.; Kim, J.H.; Park, J.S. Elevated TyG Index Predicts Progression of Coronary Artery Calcification. Diabetes Care 2019, 42, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Inigo, L.; Navarro-Gonzalez, D.; Fernandez-Montero, A.; Pastrana-Delgado, J.; Martinez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.L.; Barlow, C.E.; Grundy, S.M.; Leonard, D.; DeFina, L.F. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J. Investig. Med. 2014, 62, 345–349. [Google Scholar] [CrossRef]
- Simental-Mendia, L.E.; Rodriguez-Moran, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting Compared With Nonfasting Triglycerides and Risk of Cardiovascular Events in Women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef]
- An, X.; Yu, D.; Zhang, R.; Zhu, J.; Du, R.; Shi, Y.; Xiong, X. Insulin resistance predicts progression of de novo atherosclerotic plaques in patients with coronary heart disease: A one-year follow-up study. Cardiovascu. Diabetol. 2012, 11, 71. [Google Scholar] [CrossRef]
- Bonora, E.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; Raffaelli, A.; et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002, 25, 1135–1141. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Arima, H.; Zoungas, S.; Ninomiya, T.; Cooper, M.; Hamet, P.; Mancia, G.; Poulter, N.; Harrap, S.; Woodward, M.; et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care 2014, 37, 2359–2365. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.; Bertoni, A.G.; Golden, S.H.; Mathioudakis, N.; Sears, D.D.; Szklo, M.; Anderson, C.A.M. Association of Low Fasting Glucose and HbA1c With Cardiovascular Disease and Mortality: The MESA Study. J. Endocr. Soc. 2019, 3, 892–901. [Google Scholar] [CrossRef]
- Arnold, L.W.; Wang, Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: A meta-analysis of observational studies. Rev. Diabet. Stud. 2014, 11, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Katzmarzyk, P.T.; Horswell, R.; Wang, Y.; Johnson, J.; Hu, G. HbA1c and all-cause mortality risk among patients with type 2 diabetes. Int. J. Cardiol. 2016, 202, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.J.; Schwenke, D.C.; Bahn, G.; Reaven, P. Glycemic Variation and Cardiovascular Risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2018, 41, 2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachin, J.M.; Genuth, S.; Nathan, D.M.; Zinman, B.; Rutledge, B.N. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—Revisited. Diabetes 2008, 57, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006, 29, 1486–1490. [Google Scholar] [CrossRef] [Green Version]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.H.; Tseng, C.H. The role of triglyceride in cardiovascular disease in asian patients with type 2 diabetes—A systematic review. Rev. Diabet. Stud. 2013, 10, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.; Best, J.; Forder, P.; Taskinen, M.R.; Simes, J.; Barter, P.; Keech, A.; FIELD Study Investigators. Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: Baseline characteristics and short-term effects of fenofibrate [ISRCTN64783481]. Cardiovasc. Diabetol. 2005, 4, 13. [Google Scholar]
- Gerstein, H.C.; Miller, M.E.; Genuth, S.; Ismail-Beigi, F.; Buse, J.B.; Goff, D.C., Jr.; Probstfield, J.L.; Cushman, W.C.; Ginsberg, H.N.; Bigger, J.T.; et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N. Engl. J. Med. 2011, 364, 818–828. [Google Scholar]
| Characteristics | All (n = 3524) |
|---|---|
| Age (year) | 61.68 ± 11.90 |
| Male gender (%) | 49.1 |
| Hypertension (%) | 69.7 |
| Dyslipidemia (%) | 69.8 |
| Retinopathy (%) | 5.4 |
| Neuropathy (%) | 13.4 |
| Nephropathy (%) | 5.0 |
| Coronary artery disease (%) | 1.8 |
| Stroke (%) | 0.4 |
| Peripheral artery disease (%) | 0.1 |
| DM duration > 5 years (%) | 87.5 |
| Hypoglycemia episode (%) | 3.6 |
| Laboratory parameters | |
| Fasting glucose (mg/dL) | 154.83 ± 58.53 |
| Hemoglobin A1c (%) | 7.85 ± 1.76 |
| Triglyceride (mg/dL) | 150.8 ± 217.81 |
| Total cholesterol (mg/dL) | 179.37 ± 44.00 |
| HDL-cholesterol (mg/dL) | 41.16 ± 13.04 |
| LDL-cholesterol (mg/dL) | 104.08 ± 33.86 |
| eGFR (mL/min/1.73 m2) | 87.66 ± 35.89 |
| TyG index | 9.09 ± 0.73 |
| UACR (mg/g) | 149.37 ± 531.69 |
| Medications | |
| ACEI and/or ARB use (%) | 65.6 |
| Anti-hypertensive drugs use (%) | 53.6 |
| Aspirin use (%) | 33.2 |
| Statin and/or fibrate use (%) | 70.7 |
| Sulfonyurea use (%) | 72.8 |
| Metformin use (%) | 82.2 |
| Meglitinides use (%) | 13.3 |
| Pioglitazone use (%) | 36.3 |
| Acarbose use (%) | 20.4 |
| DPP-4 inhibitor use (%) | 34.4 |
| Insulin use (%) | 25.9 |
| CV events (%) | 6.1 |
| Follow-up time (year) | 5.93 ± 1.14 |
| Parameters | Univariable | |
|---|---|---|
| HR (95% CI) | p | |
| Age (per 1 year) | 1.035 (1.022–1.047) | <0.001 |
| Male gender | 1.422 (1.084–1.864) | 0.011 |
| Hypertension | 2.435 (1.680–3.528) | <0.001 |
| Dyslipidemia | 1.057 (0.786–1.420) | 0.715 |
| Retinopathy | 1.615 (0.997–2.618) | 0.052 |
| Neuropathy | 1.425 (1.004–2.023) | 0.048 |
| Nephropathy | 1.924 (1.201–3.082) | 0.007 |
| Coronary artery disease | 8.524 (5.528–13.144) | <0.001 |
| Stroke | 2.924 (0.727–11.767) | 0.131 |
| Peripheral artery disease | 17.399 (5.566–54.390) | <0.001 |
| DM duration > 5 years | 1.508 (0.930–2.445) | 0.096 |
| Hypoglycemia episode | 2.238 (1.345–3.724) | 0.002 |
| Laboratory parameters | ||
| Fasting glucose (per 1 mg/dL) | 1.003 (1.001–1.005) | 0.001 |
| Hemoglobin A1c (per 1%) | 1.069 (0.996–1.146) | 0.064 |
| Triglyceride (log per 1 mg/dL) | 1.387 (1.125–1.709) | 0.002 |
| Total cholesterol (per 1 mg/dL) | 1.001 (0.999–1.004) | 0.310 |
| HDL-cholesterol (per 1 mg/dL) | 0.971 (0.958–0.983) | <0.001 |
| LDL-cholesterol (per 1 mg/dL) | 0.999 (0.995–1.004) | 0.776 |
| eGFR (per 1 mL/min/1.73 m2) | 0.979 (0.975–0.984) | <0.001 |
| TyG index (per 1) | 1.342 (1.136–1.586) | <0.001 |
| UACR (per 10 mg/g) | 1.005 (1.003–1.006) | <0.001 |
| Medications | ||
| ACEI and/or ARB use | 2.838 (1.967–4.094) | <0.001 |
| Anti-hypertensive drugs use | 3.774 (2.685–5.304) | <0.001 |
| Aspirin use | 3.066 (2.334–4.027) | <0.001 |
| Statin and/or fibrate use | 1.664 (1.191–2.325) | 0.003 |
| Sulfonyurea use | 0.891 (0.664–1.196) | 0.443 |
| Metformin use | 0.514 (0.382–0.690) | <0.001 |
| Meglitinides use | 1.921 (1.393–2.649) | <0.001 |
| Pioglitazone use | 0.995 (0.753–1.315) | 0.974 |
| Acarbose use | 1.683 (1.256–2.255) | <0.001 |
| DPP-4 inhibitor use | 0.792 (0.590–1.062) | 0.120 |
| Insulin use | 2.450 (1.872–3.207) | <0.001 |
| Model | Multivariable (Stepwise) | |
|---|---|---|
| HR (95% CI) | p | |
| Model 1 | ||
| Age (per 1 year) | 1.028 (1.004–1.053) | 0.021 |
| Coronary artery disease | 3.338 (1.084–10.273) | 0.036 |
| Peripheral artery disease | 12.362 (1.171–130.56) | 0.037 |
| Fasting glucose (per 1 mg/dL) | 1.007 (1.005–1.010) | <0.001 |
| eGFR (per 1 mL/min/1.73 m2) | 0.989 (0.981–0.998) | 0.012 |
| UACR (per 10 mg/g) | 1.003 (1.001–1.005) | 0.004 |
| Anti-hypertensive drugs use | 2.185 (1.177–4.056) | 0.013 |
| Aspirin use | 2.238 (1.418–3.532) | <0.001 |
| Model 2 | ||
| Age (per 1 year) | 1.040 (1.017–1.065) | <0.001 |
| Coronary artery disease | 3.143 (1.024–9.648) | 0.045 |
| Stroke | 6.189 (1.448–26.457) | 0.014 |
| Peripheral artery disease | 22.958 (2.317–227.45) | 0.007 |
| TyG index (per 1) | 1.521 (1.141–2.027) | 0.004 |
| UACR (per 10 mg/g) | 1.003 (1.002–1.005) | <0.001 |
| Anti-hypertensive drugs use | 2.358 (1.272–4.372) | 0.007 |
| Aspirin use | 2.022 (1.276–3.206) | 0.003 |
| Insulin use | 2.031 (1.273–3.239) | 0.003 |
| Model | Multivariable (Stepwise) | |
|---|---|---|
| HR (95% CI) | p | |
| Model 1 | ||
| Fasting glucose (per 1 mg/dL) | 1.003 (1.001–1.005) | 0.004 |
| eGFR (per 1 mL/min/1.73 m2) | 0.983 (0.978–0.988) | <0.001 |
| Anti-hypertensive drugs use | 1.992 (1.378–2.880) | <0.001 |
| Aspirin use | 2.382 (1.769–3.209) | <0.001 |
| Model 2 | ||
| eGFR (per 1 mL/min/1.73 m2) | 0.985 (0.980–0.990) | <0.001 |
| TyG index (per 1) | 1.228 (1.015–1.486) | 0.035 |
| Anti-hypertensive drugs use | 1.911 (1.324–2.759) | <0.001 |
| Aspirin use | 1.800 (1.329–2.437) | <0.001 |
| Insulin use | 2.306 (1.711–3.107) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-Y.; Chen, S.-C.; Huang, Y.-T.; Huang, J.-C.; Wu, P.-Y.; Hsu, W.-H.; Lee, M.-Y. Comparison of the Effects of Fasting Glucose, Hemoglobin A1c, and Triglyceride–Glucose Index on Cardiovascular Events in Type 2 Diabetes Mellitus. Nutrients 2019, 11, 2838. https://doi.org/10.3390/nu11112838
Su W-Y, Chen S-C, Huang Y-T, Huang J-C, Wu P-Y, Hsu W-H, Lee M-Y. Comparison of the Effects of Fasting Glucose, Hemoglobin A1c, and Triglyceride–Glucose Index on Cardiovascular Events in Type 2 Diabetes Mellitus. Nutrients. 2019; 11(11):2838. https://doi.org/10.3390/nu11112838
Chicago/Turabian StyleSu, Wei-Yu, Szu-Chia Chen, Yu-Ting Huang, Jiun-Chi Huang, Pei-Yu Wu, Wei-Hao Hsu, and Mei-Yueh Lee. 2019. "Comparison of the Effects of Fasting Glucose, Hemoglobin A1c, and Triglyceride–Glucose Index on Cardiovascular Events in Type 2 Diabetes Mellitus" Nutrients 11, no. 11: 2838. https://doi.org/10.3390/nu11112838
APA StyleSu, W.-Y., Chen, S.-C., Huang, Y.-T., Huang, J.-C., Wu, P.-Y., Hsu, W.-H., & Lee, M.-Y. (2019). Comparison of the Effects of Fasting Glucose, Hemoglobin A1c, and Triglyceride–Glucose Index on Cardiovascular Events in Type 2 Diabetes Mellitus. Nutrients, 11(11), 2838. https://doi.org/10.3390/nu11112838






