Is There a Contribution of Structural Brain Phenotypes to the Variance in Resting Energy Expenditure before and after Weight Loss in Overweight Females?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Details of Study Populations
2.1.1. Study Group 1
2.1.2. Study Group 2
2.1.3. Study Group 3
2.2. Anthropometric Measurements and Detailed Body Composition Analysis
2.3. Resting Energy Expenditure (REE) Encephalic Measure (EM)
2.4. Formatting of Mathematical Components
2.5. Statistical Analyses
3. Results
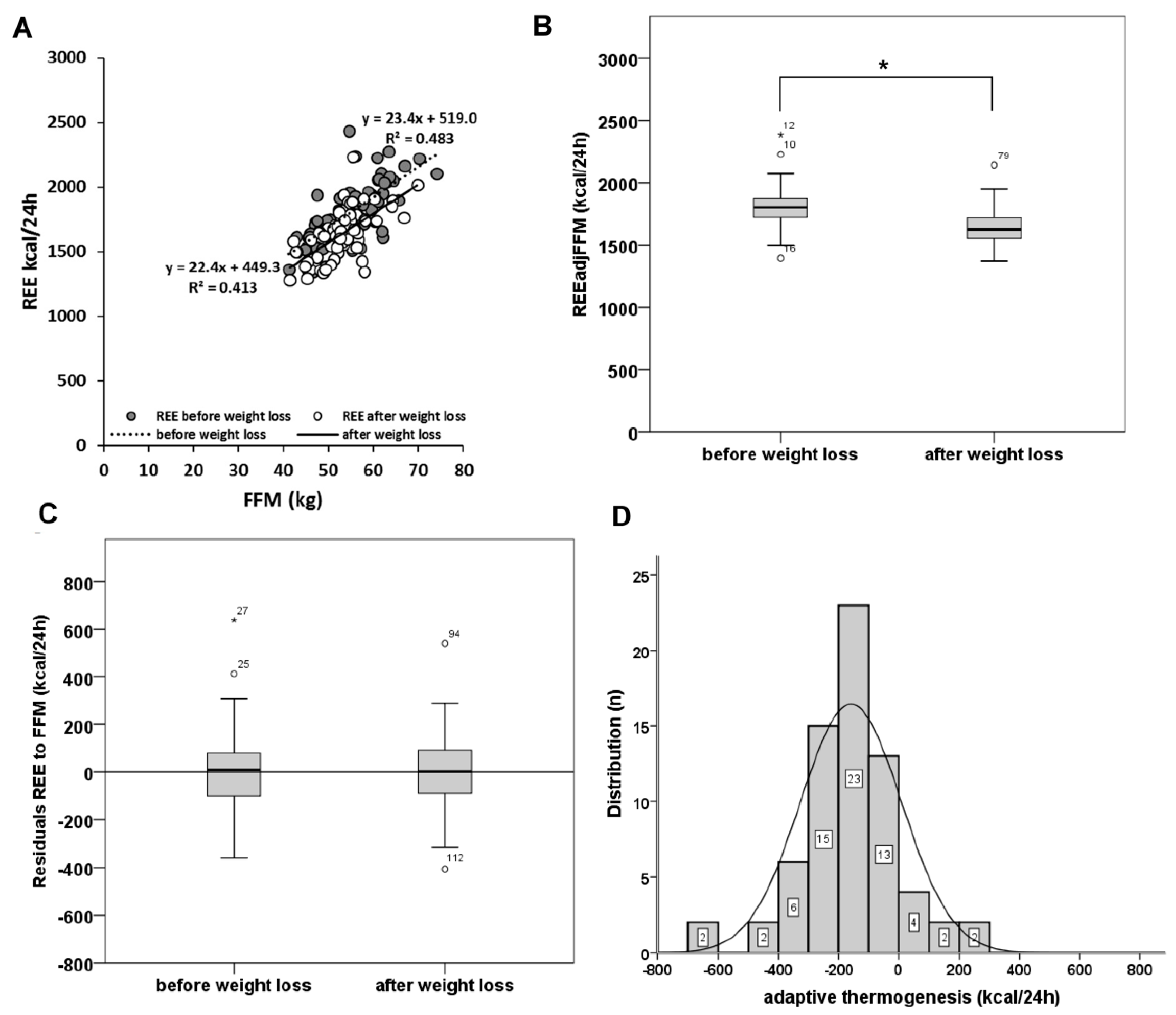
3.1. Is There an Effect of Weight Loss on Brain Mass, GM, WM and REE?
3.2. Contribution of GM and WM to the Variances in REE, REEadjFFM and REE on FFM Residuals before and after Weight Loss
4. Discussion
4.1. Is There an Effect of Weight Loss on Brain Mass and Brain Tissue Structure?
4.2. Strengths and Limitations
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Geisler, C.; Müller, M.J. Impact of Fat-Free Mass Quality and Detailed Body Composition on Changes of Resting Energy Expenditure with Age. Curr. Nutr. Rep. 2017, 6, 111–121. [Google Scholar] [CrossRef]
- Müller, M.J.; Wang, Z.; Heymsfield, S.B.; Schautz, B.; Bosy-Westphal, A. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Phelps, M.E.; Hoffman, E.J.; Sideris, K.; Selin, C.J.; Kuhl, D.E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am. J. Physiol. 1980, 238, E69–E82. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.; Hubers, M.; Granert, O.; Muller, M.J. Contribution of structural brain phenotypes to the variance in resting energy expenditure in healthy Caucasian subjects. J. Appl. Physiol. 2018, 125, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Bosy-Westphal, A.; Kubera, B.; Langemann, D.; Goele, K.; Later, W.; Heller, M.; Hubold, C.; Muller, M.J. Why doesn’t the brain lose weight, when obese people diet? Obes. Facts 2011, 4, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Hitze, B.; Langemann, D.; Bosy-Westphal, A.; Muller, M.J. Brain size, body size and longevity. Int. J. Obes. 2010, 34, 1349–1352. [Google Scholar] [CrossRef]
- Augustijn, M.; D’Hondt, E.; Leemans, A.; Van Acker, L.; De Guchtenaere, A.; Lenoir, M.; Deconinck, F.J.A.; Caeyenberghs, K. Weight loss, behavioral change, and structural neuroplasticity in children with obesity through a multidisciplinary treatment program. Hum. Brain Mapp. 2019, 40, 137–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Xu, M.; Cai, W.; Zhu, Q.; Qian, L.; Zhang, Y.E.; Yuan, K.; Liu, J.; Li, Q.; et al. Recovery of brain structural abnormalities in morbidly obese patients after bariatric surgery. Int. J. Obes. 2016, 40, 1558–1565. [Google Scholar] [CrossRef]
- Tuulari, J.J.; Karlsson, H.K.; Antikainen, O.; Hirvonen, J.; Pham, T.; Salminen, P.; Helmio, M.; Parkkola, R.; Nuutila, P.; Nummenmaa, L. Bariatric Surgery Induces White and Grey Matter Density Recovery in the Morbidly Obese: A Voxel-Based Morphometric Study. Hum. Brain Mapp. 2016, 37, 3745–3756. [Google Scholar] [CrossRef]
- Haltia, L.T.; Viljanen, A.; Parkkola, R.; Kemppainen, N.; Rinne, J.O.; Nuutila, P.; Kaasinen, V. Brain white matter expansion in human obesity and the recovering effect of dieting. J. Clin. Endocrinol. Metab. 2007, 92, 3278–3284. [Google Scholar] [CrossRef]
- Prehn, K.; Jumpertz von Schwartzenberg, R.; Mai, K.; Zeitz, U.; Witte, A.V.; Hampel, D.; Szela, A.M.; Fabian, S.; Grittner, U.; Spranger, J.; et al. Caloric Restriction in Older Adults-Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cereb. Cortex 2017, 27, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Taki, Y.; Kinomura, S.; Sato, K.; Inoue, K.; Goto, R.; Okada, K.; Uchida, S.; Kawashima, R.; Fukuda, H. Relationship Between Body Mass Index and Gray Matter Volume in 1,428 Healthy Individuals. Obesity 2008, 16, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.; Beydoun, M.A.; An, Y.; Davatzikos, C.; Ferrucci, L.; Zonderman, A.B.; Resnick, S.M. Midlife obesity and trajectories of brain volume changes in older adults. Hum. Brain Mapp. 2012, 33, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Grieve, S.; Gordon, E. Relationship Between Body Mass Index and Brain Volume in Healthy Adults. Int. J. Neurosci. 2008, 118, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Pannacciulli, N.; Del Parigi, A.; Chen, K.; Le, D.S.N.T.; Reiman, E.M.; Tataranni, P.A. Brain abnormalities in human obesity: A voxel-based morphometric study. NeuroImage 2006, 31, 1419–1425. [Google Scholar] [CrossRef]
- Debette, S.; Wolf, C.; Lambert, J.-C.; Crivello, F.; Soumaré, A.; Zhu, Y.-C.; Schilling, S.; Dufouil, C.; Mazoyer, B.; Amouyel, P.; et al. Abdominal obesity and lower gray matter volume: A Mendelian randomization study. Neurobiol. Aging 2014, 35, 378–386. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Kossel, E.; Goele, K.; Later, W.; Hitze, B.; Settler, U.; Heller, M.; Gluer, C.C.; Heymsfield, S.B.; Muller, M.J. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am. J. Clin. Nutr. 2009, 90, 993–1001. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Muller, M.J.; Boschmann, M.; Klaus, S.; Kreymann, G.; Luhrmann, P.M.; Neuhauser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; et al. Grade of adiposity affects the impact of fat mass on resting energy expenditure in women. Br. J. Nutr. 2009, 101, 474–477. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Schautz, B.; Lagerpusch, M.; Pourhassan, M.; Braun, W.; Goele, K.; Heller, M.; Gluer, C.C.; Muller, M.J. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int. J. Obes. 2013, 37, 1371–1377. [Google Scholar] [CrossRef]
- Müller, M.J.; Bosy-Westphal, A. Adaptive thermogenesis with weight loss in humans. Obesity 2013, 21, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.J.; Bosy-Westphal, A.; Kutzner, D.; Heller, M. Metabolically active components of fat free mass (FFM) and resting energy expenditure (REE) in humans. Forum Nutr. 2003, 56, 301–303. [Google Scholar] [PubMed]
- Lagerpusch, M.; Bosy-Westphal, A.; Kehden, B.; Peters, A.; Muller, M.J. Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int. J. Obes. 2012, 36, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Lagerpusch, M.; Enderle, J.; Later, W.; Eggeling, B.; Pape, D.; Muller, M.J.; Bosy-Westphal, A. Impact of glycaemic index and dietary fibre on insulin sensitivity during the refeeding phase of a weight cycle in young healthy men. Br. J. Nutr. 2013, 109, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Siri, W.E. Body Composition from Fluid Spaces and Density: ANALYSIS of Methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar] [PubMed]
- Pourhassan, M.; Bosy-Westphal, A.; Schautz, B.; Braun, W.; Gluer, C.C.; Muller, M.J. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am. J. Clin. Nutr. 2014, 99, 779–791. [Google Scholar] [CrossRef]
- Snyder, W.S.; Cook, M.J.; Nasset, E.S.; Karhausen, L.R.; Howells, G.P.; Tipton, I.H. Report of the Task Group on Reference Man; Pergamon Press Oxford: Oxfordshire, UK, 1975. [Google Scholar]
- Kubera, B.; Bosy-Westphal, A.; Peters, A.; Braun, W.; Langemann, D.; Neugebohren, S.; Heller, M.; Muller, M.J. Energy allocation between brain and body during ontogenetic development. Am. J. Hum. Biol. 2013, 25, 725–732. [Google Scholar] [CrossRef]
- Sokoloff. Circulation & Energy Metabolism of the Brain; Raven Press: New York, NY, USA, 1989. [Google Scholar]
- Muller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Luhrmann, P.M.; Neuhauser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar] [CrossRef]
- Bader, N.; Bosy-Westphal, A.; Dilba, B.; Muller, M.J. Intra- and interindividual variability of resting energy expenditure in healthy male subjects—Biological and methodological variability of resting energy expenditure. Br. J. Nutr. 2005, 94, 843–849. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Eichhorn, C.; Kutzner, D.; Illner, K.; Heller, M.; Muller, M.J. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J. Nutr. 2003, 133, 2356–2362. [Google Scholar] [CrossRef]
- Hall, K.D.; Hallgreen, C.E. Increasing weight loss attenuates the preferential loss of visceral compared with subcutaneous fat: A predicted result of an allometric model. Int. J. Obes. 2008, 32, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J. Applied Linear Regression Models, 4th ed.; McGraw-Hill Irwin: New York, NY, USA, 2004. [Google Scholar]
- Steiger, J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980, 87, 245–251. [Google Scholar] [CrossRef]
- Heshka, S.; Lemos, T.; Astbury, N.M.; Widen, E.; Davidson, L.; Goodpaster, B.H.; DeLany, J.P.; Strain, G.W.; Pomp, A.; Courcoulas, A.P.; et al. Resting Energy Expenditure and Organ-Tissue Body Composition 5 Years After Bariatric Surgery. Obes. Surg. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gazdzinski, S.; Kornak, J.; Weiner, M.W.; Meyerhoff, D.J. Body mass index and magnetic resonance markers of brain integrity in adults. Ann. Neurol. 2008, 63, 652–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudheimer, K.D.; O’Hara, R.; Spiegel, D.; Powers, B.; Kraemer, H.C.; Neri, E.; Weiner, M.; Hardan, A.; Hallmayer, J.; Dhabhar, F.S. Cortisol, cytokines, and hippocampal volume interactions in the elderly. Front. Aging Neurosci. 2014, 6, 153. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.A.; Carlsson, C.M.; Trivedi, M.A.; Sager, M.A.; Johnson, S.C. The effect of body mass index on global brain volume in middle-aged adults: A cross sectional study. BMC Neurol. 2005, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Karbowski, J. Global and regional brain metabolic scaling and its functional consequences. BMC Biol. 2007, 5, 18. [Google Scholar] [CrossRef] [Green Version]

| n | Before Weight Loss | After Weight Loss | Δ | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Minimal | Maximal | Mean ± SD | Minimal | Maximal | Mean ± SD | ||
| Age (years) | 69 | 36.4 ± 9.4 | 19.4 | 68.8 | 36.72 ± 9.9 | 19.6 | 69.4 | |
| Height (m) | 69 | 1.68 ± 0.07 | 1.49 | 1.82 | 1.68 ± 0.07 | 1.49 | 1.83 | 0.00 ± 0.00 |
| Weight (kg) | 69 | 110.3 ± 23.2 | 72.6 | 159.0 | 95.7 ± 17.5 | 67.1 | 135.5 | −14.5 ± 11.9 * |
| BMI (kg/m2) | 69 | 38.9 ± 7.5 | 28.2 | 58.7 | 33.8 ± 5.0 | 25.4 | 44.5 | −5.2 ± 4.3 * |
| Body composition | ||||||||
| FM (%) | 69 | 49.2 ± 6.8 | 32.6 | 68.8 | 43.5 ± 7.4 | 19.80 | 56.4 | −5.7 ± 4.2 * |
| FM (kg) | 69 | 55.5 ± 18.2 | 26.2 | 104.7 | 42.7 ± 13.9 | 14.2 | 70.4 | −12.9 ± 9.8 * |
| FFM (kg) | 69 | 54.68 ± 7.01 | 41.2 | 74.1 | 52.9 ± 5.5 | 41.4 | 69.7 | −1.7 ± 4.8 * |
| Gray matter (mL) | 69 | 638.9 ± 74.7 | 340.9 | 773.1 | 638.3 ± 71.8 | 390.6 | 779.3 | −0.6 ± 36.9 |
| Gray matter (ratio to ICV) | 69 | 0.50 ± 0.04 | 0.31 | 0.58 | 0.49 ± 0.04 | 0.35 | 0.59 | −0.005 ± 0.03 |
| White matter (mL) | 69 | 430.3 ± 65.6 | 282.2 | 608.6 | 435.9 ± 53.9 | 305.2 | 606.8 | 5.5 ± 43.3 |
| White matter (ratio to ICV) | 69 | 0.34 ± 0.05 | 0.24 | 0.55 | 0.34 ± 0.04 | 0.25 | 0.51 | 0.001 ± 0.03 |
| Cerebrospinal Fluid (mL) | 69 | 202.1 ± 54.6 | 115.4 | 402.4 | 211.2 ± 54.3 | 119.3 | 408.6 | 9.0 ± 20.2 * |
| Cerebrospinal Fluid (ratio to ICV) | 69 | 0.16 ± 0.04 | 0.09 | 0.29 | 0.16 ± 0.04 | 0.10 | 0.29 | 0.004 ± 0.02 * |
| Intracranial Volume (mL) | 69 | 1271.4 ± 94.1 | 1099.7 | 1477.7 | 1285.3 ± 97.4 | 1100.4 | 1485.6 | 13.9 ± 55.3 * |
| Brain parenchymal fraction (mL) | 69 | 1069.3 ± 92.0 | 868.6 | 1241.5 | 1074.2 ± 80.5 | 915.0 | 1241.4 | 4.9 ± 53.1 |
| Brain parenchymal fraction (ratio to ICV) | 69 | 0.84 ± 0.04 | 0.71 | 0.91 | 0.84 ± 0.04 | 0.71 | 0.90 | −0.004 ± 0.01 * |
| Encephalic measure | 69 | 4.25 ± 0.61 | 2.90 | 5.40 | 4.68 ± 0.58 | 3.42 | 6.29 | 0.43 ± 0.50 * |
| Ratio of body metabolism to brain metabolism | 69 | 6.68 ± 0.99 | 5.18 | 9.56 | 6.05 ± 0.72 | 4.47 | 8.10 | −0.63 ± 0.76 * |
| Energy expenditure | ||||||||
| REE (kcal/24 h) | 69 | 1799 ± 236 | 1346 | 2433 | 1640 ± 191 | 1282 | 2234 | −159 ± 191 * |
| REEadjFFM (kcal/24 h) | 69 | 1799 ± 169 | 1082 | 3070 | 1640 ± 146 | 942 | 2773 | −159 ± 177 * |
| REE on FFM residuals | 69 | 0.00 ± 169 | −360 | 638 | 0.00 ± 146 | −405 | 540 | 0.00 ± 177 |
| Regression Coefficient B | SE | ß-Coefficient | p-Value | Regression Coefficient B | SE | ß-Coefficient | p-Value | |
|---|---|---|---|---|---|---|---|---|
| REE Before weight loss | REE after weight loss | |||||||
| Model 1 | ||||||||
| Constant | 545.9 | 293.8 | 0.068 | 520.29 | 260.3 | 0.050 | ||
| FFM (kg) | 14.13 | 3.39 | 0.419 | 0.001 | 14.27 | 3.48 | 0.407 | 0.001 |
| FM (kg) | 5.83 | 1.31 | 0.449 | 0.001 | 5.94 | 1.38 | 0.433 | 0.001 |
| Age | −1.04 | 2.02 | −0.043 | 0.611 | −1.75 | 1.67 | −0.091 | 0.296 |
| ICV (mL) | 0.17 | 0.20 | 0.066 | 0.419 | 0.14 | 0.17 | 0.075 | 0.377 |
| R² total | 0.611 | 0.550 | ||||||
| Model 2 | ||||||||
| Constant | 720.9 | 146.6 | 0.001 | 274.9 | 211.3 | 0.198 | ||
| FFM (kg) | 14.15 | 3.33 | 0.420 | 0.001 | 15.37 | 3.32 | 0.438 | 0.001 |
| FM (kg) | 5.76 | 1.28 | 0.444 | 0.001 | 5.73 | 1.29 | 0.417 | 0.001 |
| Age | ||||||||
| GM (mL) | 487.4 | 208.2 | 0.190 | 0.022 | ||||
| WM (mL) | ||||||||
| R² total | 0.603 | 0.574 | ||||||
| REEadjFFM before weight loss | REEadjFFM after weight loss | |||||||
| Model 3 | ||||||||
| Constant | 1515.5 | 289.1 | 0.001 | 1351.6 | 277.5 | 0.001 | ||
| FM (kg) | 3.54 | 1.05 | 0.379 | 0.001 | 4.23 | 1.22 | 0.402 | 0.001 |
| Age | −1.68 | 2.10 | −0.098 | 0.427 | −1.14 | 1.71 | −0.077 | 0.508 |
| ICV (mL) | 0.09 | 0.212 | 0.048 | 0.628 | 0.097 | 0.171 | 0.065 | 0.572 |
| R² total | 0.157 | 0.166 | ||||||
| Model 4 | ||||||||
| Constant | 1576.8 | 61.6 | 0.001 | 1109.3 | 153.2 | 0.001 | ||
| FM (kg) | 3.54 | 1.05 | 0.379 | 0.001 | 4.28 | 1.15 | 0.407 | 0.001 |
| Age | ||||||||
| GM (mL) | 487.3 | 214.0 | 0.248 | 0.026 | ||||
| WM (mL) | ||||||||
| R² total | 0.144 | 0.217 | ||||||
| REE on FFM residuals before weight loss | REE on FFM residuals after weight loss | |||||||
| Model 3 | ||||||||
| Constant | −227.6 | 289.0 | 0.340 | −218.5 | 227.4 | 0.220 | ||
| FM (kg) | 3.55 | 1.05 | 0.380 | 0.001 | 4.24 | 1.22 | 0.403 | 0.001 |
| Age | −1.77 | 2.10 | −0.103 | 0.402 | −1.22 | 1.71 | −0.082 | 0.479 |
| ICV (mL) | 0.11 | 0.21 | 0.058 | 0.623 | 0.113 | 0.17 | 0.075 | 0.512 |
| R² total | 0.160 | 0.168 | ||||||
| Model 4 | ||||||||
| Constant | −197.2 | 67.7 | 0.002 | −518.9 | 152.9 | 0.001 | ||
| FM (kg) | 3.55 | 1.05 | 0.380 | 0.001 | 4.29 | 1.14 | 0.408 | 0.001 |
| Age | ||||||||
| GM (mL) | 506.4 | 213.7 | 0.258 | 0.021 | ||||
| WM (mL) | ||||||||
| R² total | 0.163 | 0.222 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geisler, C.; Müller, M.J. Is There a Contribution of Structural Brain Phenotypes to the Variance in Resting Energy Expenditure before and after Weight Loss in Overweight Females? Nutrients 2019, 11, 2759. https://doi.org/10.3390/nu11112759
Geisler C, Müller MJ. Is There a Contribution of Structural Brain Phenotypes to the Variance in Resting Energy Expenditure before and after Weight Loss in Overweight Females? Nutrients. 2019; 11(11):2759. https://doi.org/10.3390/nu11112759
Chicago/Turabian StyleGeisler, Corinna, and Manfred J. Müller. 2019. "Is There a Contribution of Structural Brain Phenotypes to the Variance in Resting Energy Expenditure before and after Weight Loss in Overweight Females?" Nutrients 11, no. 11: 2759. https://doi.org/10.3390/nu11112759
APA StyleGeisler, C., & Müller, M. J. (2019). Is There a Contribution of Structural Brain Phenotypes to the Variance in Resting Energy Expenditure before and after Weight Loss in Overweight Females? Nutrients, 11(11), 2759. https://doi.org/10.3390/nu11112759




