Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of A Randomized Trial in Healthy Adults
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Ethics Statement
2.2. Subjects
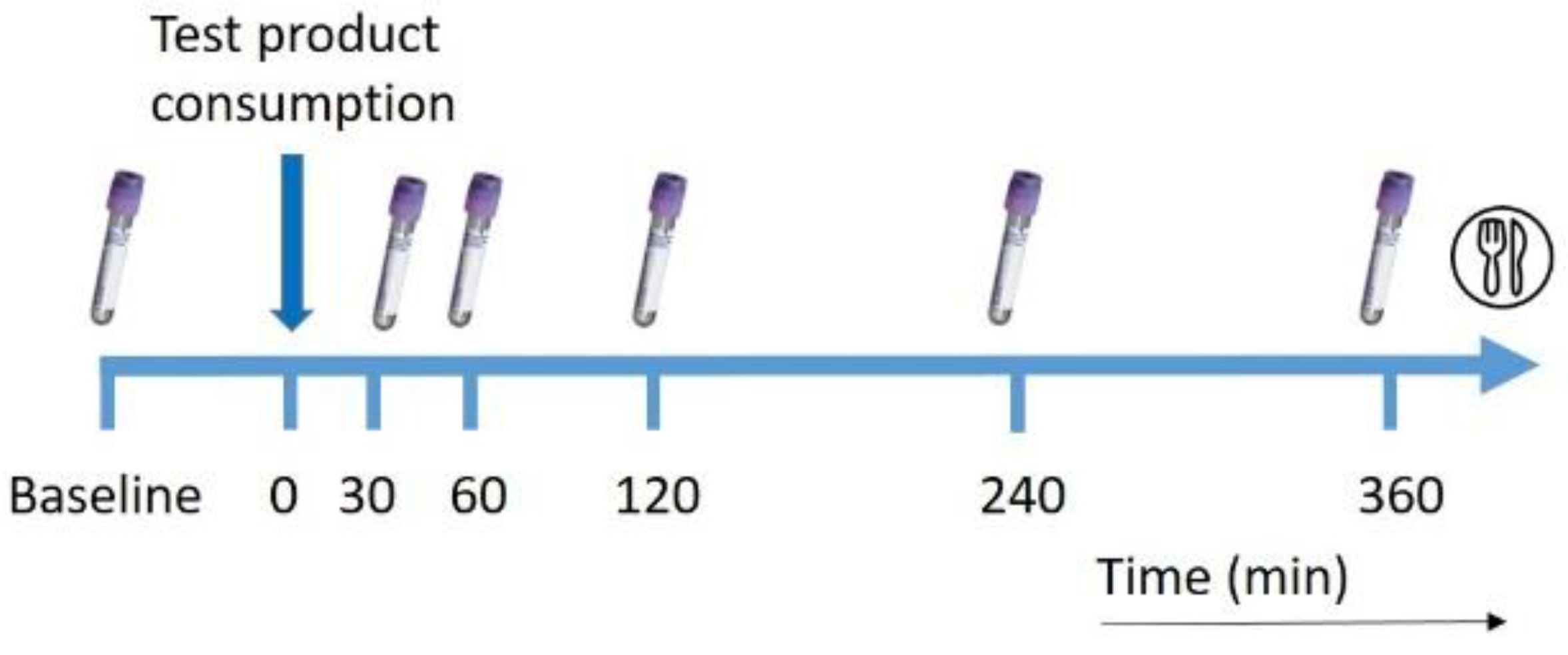
2.3. Study Design
2.4. Intervention Drinks
2.5. Quantification of Choline, Betaine and Dimethylglycine in Plasma
2.6. DHA in TG and Phospholipid Fraction
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
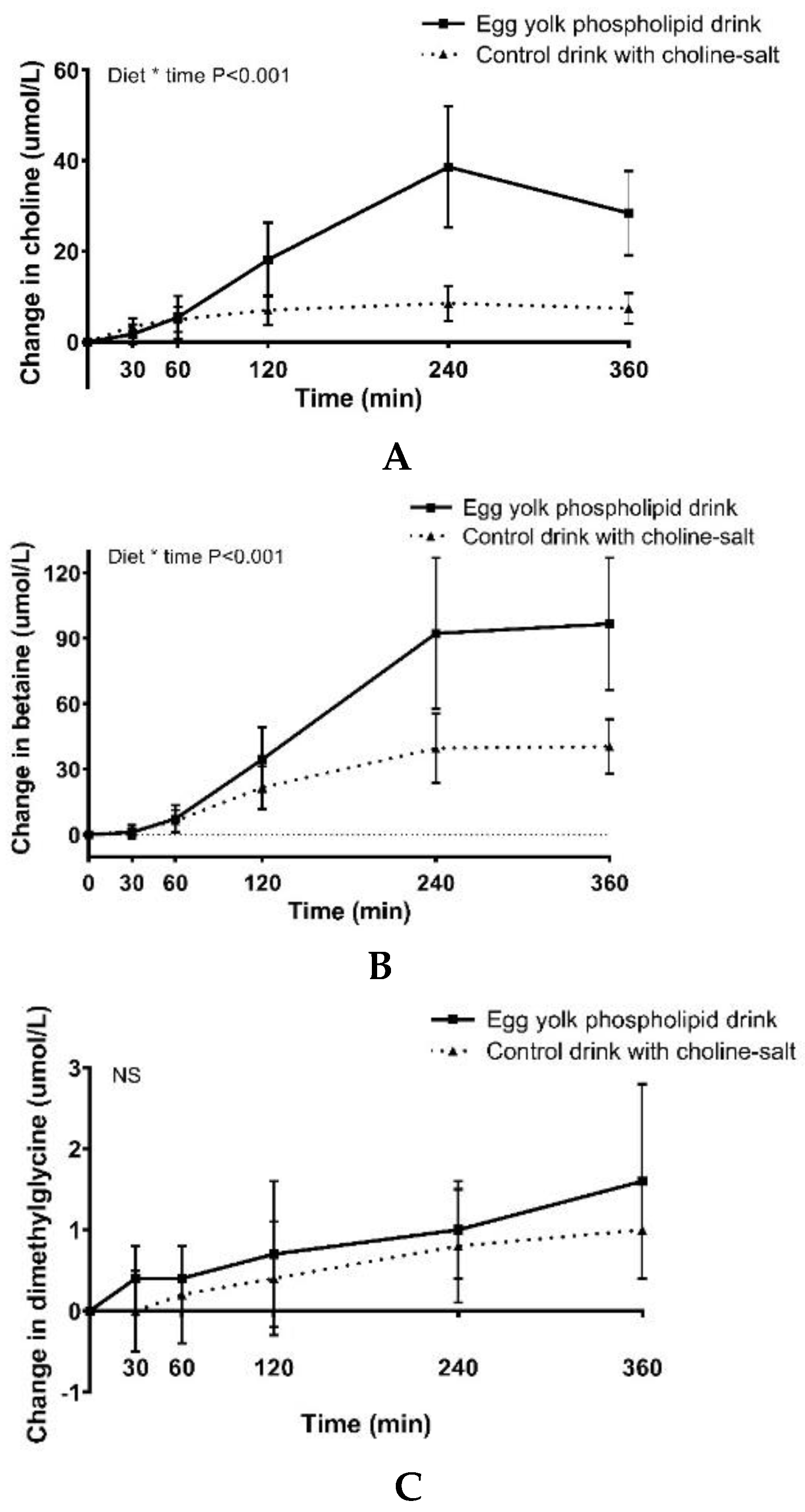
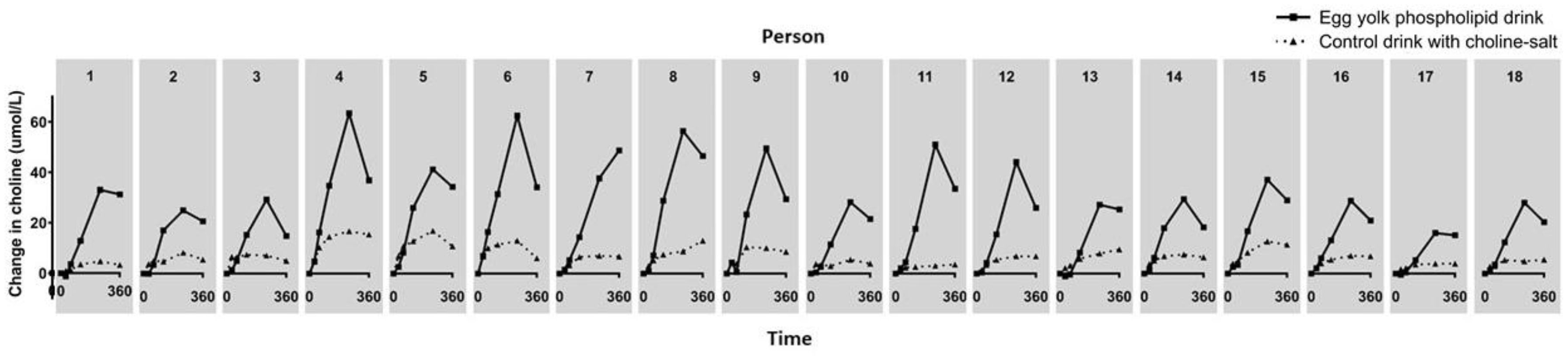
3.2. Choline, Betaine and Dimethylglycine Responses
3.3. Responses in Plasma DHA in TG and Phospholipid Fraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DHA | docosahexaenoic acids |
| TAG | triacylglycerol |
References
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Caudill, M.A. Pre- and postnatal health: Evidence of increased choline needs. J. Am. Diet. Assoc. 2010, 110, 1198–1206. [Google Scholar] [CrossRef]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med. 2005, 43, 1069–1075. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Friesen, R.W.; Novak, E.M.; Hasman, D.; Innis, S.M. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J. Nutr. 2007, 137, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Mills, J.L.; Cox, C.; Daly, S.F.; Conley, M.; Brody, L.C.; Kirke, P.N.; Scott, J.M.; Ueland, P.M. Choline and homocysteine interrelations in umbilical cord and maternal plasma at delivery. Am. J. Clin. Nutr. 2005, 82, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Allen, L.H. Analyzing B-vitamins in Human Milk: Methodological Approaches. Crit. Rev. Food Sci. Nutr. 2016, 56, 494–511. [Google Scholar] [CrossRef]
- Holmes, H.C.; Snodgrass, G.J.; Iles, R.A. Changes in the choline content of human breast milk in the first 3 weeks after birth. Eur. J. Pediatr. 2000, 159, 198–204. [Google Scholar] [CrossRef]
- Holmes-McNary, M.Q.; Cheng, W.L.; Mar, M.H.; Fussell, S.; Zeisel, S.H. Choline and choline esters in human and rat milk and in infant formulas. Am. J. Clin. Nutr. 1996, 64, 572–576. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. The fetal origins of memory: The role of dietary choline in optimal brain development. J. Pediatr. 2006, 149, S131–S136. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Niculescu, M.D. Perinatal choline influences brain structure and function. Nutr. Rev. 2006, 64, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Nutrition in pregnancy: The argument for including a source of choline. Int. J. Womens Health 2013, 5, 193–199. [Google Scholar] [CrossRef]
- FDA. TITLE 21—Food and Drugs. In Chapter I Subchapter B—Food for Human Consumption; FDA: White Oak, Maryland, 2018. [Google Scholar]
- EU Commission. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. OJ 2016, 59, 1–29. [Google Scholar]
- Hirsch, M.J.; Growdon, J.H.; Wurtman, R.J. Relations between dietary choline or lecithin intake, serum choline levels, and various metabolic indices. Metabolism 1978, 27, 953–960. [Google Scholar] [CrossRef]
- Sheard, N.F.; Zeisel, S.H. An in vitro study of choline uptake by intestine from neonatal and adult rats. Pediatr. Res. 1986, 20, 768–772. [Google Scholar] [CrossRef]
- de Veth, M.J.; Artegoitia, V.M.; Campagna, S.R.; Lapierre, H.; Harte, F.; Girard, C.L. Choline absorption and evaluation of bioavailability markers when supplementing choline to lactating dairy cows. J. Dairy Sci. 2016, 99, 9732–9744. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Lien, E.L.; Richard, C.; Hoffman, D.R. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 26–40. [Google Scholar] [CrossRef]
- Carnielli, V.P.; Verlato, G.; Pederzini, F.; Luijendijk, I.; Boerlage, A.; Pedrotti, D.; Sauer, P.J. Intestinal absorption of long-chain polyunsaturated fatty acids in preterm infants fed breast milk or formula. Am. J. Clin. Nutr. 1998, 67, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr. Res. 2009, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef]
- Cook, C.M.; Hallaråker, H.; Sæbø, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Berger, A.; Maki, K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 17–24. [Google Scholar] [CrossRef]
- Ehr, I.J.; Persia, M.E.; Bobeck, E.A. Comparative omega-3 fatty acid enrichment of egg yolks from first-cycle laying hens fed flaxseed oil or ground flaxseed. Poult. Sci. 2017, 96, 1791–1799. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Herrmann, W.; Rabagny, Y.; Obeid, R. Quantification of acetylcholine, choline, betaine, and dimethylglycine in human plasma and urine using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 3338–3344. [Google Scholar] [CrossRef]
- Glatz, J.F.; Soffers, A.E.; Katan, M.B. Fatty acid composition of serum cholesteryl esters and erythrocyte membranes as indicators of linoleic acid intake in man. Am. J. Clin. Nutr. 1989, 49, 269–276. [Google Scholar] [CrossRef]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef]
- Collins, P.J.; Houghton, L.A.; Read, N.W.; Horowitz, M.; Chatterton, B.E.; Heddle, R.; Dent, J. Role of the proximal and distal stomach in mixed solid and liquid meal emptying. Gut 1991, 32, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zierenberg, O.; Grundy, S.M. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982, 23, 1136–1142. [Google Scholar] [PubMed]
- Cohn, J.S.; Wat, E.; Kamili, A.; Tandy, S. Dietary phospholipids, hepatic lipid metabolism and cardiovascular disease. Curr. Opin. Lipidol. 2008, 19, 257–262. [Google Scholar] [CrossRef]
- Zeisel, S.H. Dietary choline: Biochemistry, physiology, and pharmacology. Annu. Rev. Nutr. 1981, 1, 95–121. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, Y.; Zhou, R.F.; Chen, X.L.; Wang, C.; Tan, X.Y.; Wang, L.J.; Zheng, R.D.; Zhang, H.W.; Ling, W.H.; et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Chrysant, S.G.; Chrysant, G.S. The current status of homocysteine as a risk factor for cardiovascular disease: A mini review. Expert Rev. Cardiovasc. Ther. 2018, 16, 559–565. [Google Scholar] [CrossRef]
- Köhler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects—A randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S.; Varvel, S.A.; Pottala, J.V.; Warnick, G.R.; McConnell, J.P. Comparative effects of an acute dose of fish oil on omega-3 fatty acid levels in red blood cells versus plasma: Implications for clinical utility. J. Clin. Lipidol. 2013, 7, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Harzer, G.; Haug, M.; Dieterich, I.; Gentner, P.R. Changing patterns of human milk lipids in the course of the lactation and during the day. Am. J. Clin. Nutr. 1983, 37, 612–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheatham, C.L.; Sheppard, K.W. Synergistic Effects of Human Milk Nutrients in the Support of Infant Recognition Memory: An Observational Study. Nutrients 2015, 7, 9079–9095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhard, W.; Böckmann, K.; Maas, C.; Mathes, M.; Hövelmann, J.; Shunova, A.; Hund, V.; Schleicher, E.; Poets, C.F.; Franz, A.R. Combined choline and DHA supplementation: A randomized controlled trial. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, E.; Yabuki, Y.; Izumi, H.; Shinoda, Y.; Watanabe, F.; Hishida, Y.; Kamimura, A.; Fukunaga, K. Combined citicoline and docosahexaenoic acid treatment improves cognitive dysfunction following transient brain ischemia. J. Pharmacol. Sci. 2019, 139, 319–324. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies. Dietary Reference Values for choline. EFSA J. 2016, 14, 4484. [Google Scholar]
| ELIP (wt%) | |
|---|---|
| Phospholipids | 75 |
| Phosphatidylcholine | 59 |
| Phosphatidylinositol | 1 |
| Sphingomyelin | 2 |
| Phosphatidylethanolamine | 11 |
| Phosphatidylserine | 0 |
| Egg Yolk Phospholipid Drink | Control Drink Choline-Bitartrate | |
|---|---|---|
| Energy (kcal) | 712 | 714 |
| Fat (g) | 41 | 41 |
| Fat (E%) | 52 | 52 |
| Carbohydrates (g) | 67 | 67 |
| Carbohydrates (E%) | 38 | 38 |
| Protein (g) | 19 | 19 |
| Protein (E%) | 10 | 10 |
| Choline (g) | 3.0 | 3.0 |
| DHA (mg) | 595 | 787 |
| (n = 18) | |
|---|---|
| Male/female, n (%) | 7/11 |
| Age (years), Mean ± SD | 62.3 ± 7.2 |
| BMI (kg/m2), Mean ± SD | 22.8 ± 2.0 |
| Intervention | |||
|---|---|---|---|
| Egg Yolk Phospholipid Drink | Control Drink with Choline Bitartrate | p Value | |
| Choline (µmol/L/min) | 8020 ± 2967 | 2424 ± 1112 | <0.01 |
| Betaine (µmol/L/min) | 20351 ± 7350 | 9455 ± 3603 | <0.01 |
| Dimethylglycine (µmol/L/min) | 309 ± 196 | 215 ± 161 | 0.03 |
| DHA-PL (mg/ml/min) * | 5 ± 6 | 4 ± 5 | 0.63 |
| DHA-TG (mg/ml/min) * | 4 ± 1 | 6 ± 2 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolders, L.; de Wit, N.J.W.; Balvers, M.G.J.; Obeid, R.; Vissers, M.M.M.; Esser, D. Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of A Randomized Trial in Healthy Adults. Nutrients 2019, 11, 2758. https://doi.org/10.3390/nu11112758
Smolders L, de Wit NJW, Balvers MGJ, Obeid R, Vissers MMM, Esser D. Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of A Randomized Trial in Healthy Adults. Nutrients. 2019; 11(11):2758. https://doi.org/10.3390/nu11112758
Chicago/Turabian StyleSmolders, Lotte, Nicole J.W. de Wit, Michiel G.J. Balvers, Rima Obeid, Marc M.M. Vissers, and Diederik Esser. 2019. "Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of A Randomized Trial in Healthy Adults" Nutrients 11, no. 11: 2758. https://doi.org/10.3390/nu11112758
APA StyleSmolders, L., de Wit, N. J. W., Balvers, M. G. J., Obeid, R., Vissers, M. M. M., & Esser, D. (2019). Natural Choline from Egg Yolk Phospholipids Is More Efficiently Absorbed Compared with Choline Bitartrate; Outcomes of A Randomized Trial in Healthy Adults. Nutrients, 11(11), 2758. https://doi.org/10.3390/nu11112758






