Functional Expression of Choline Transporters in the Blood–Brain Barrier
Abstract
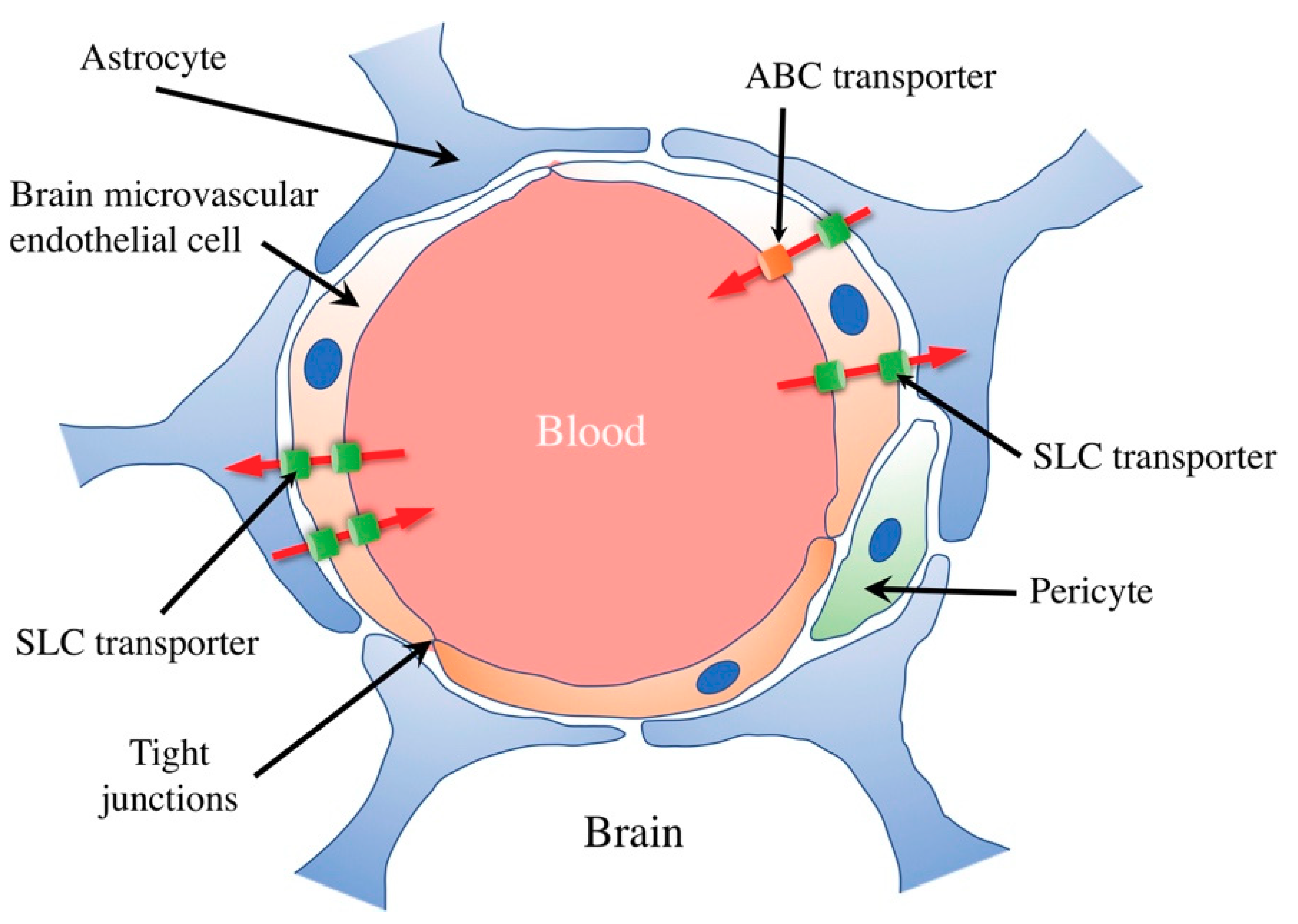
1. Transport at the Blood–Brain Barrier
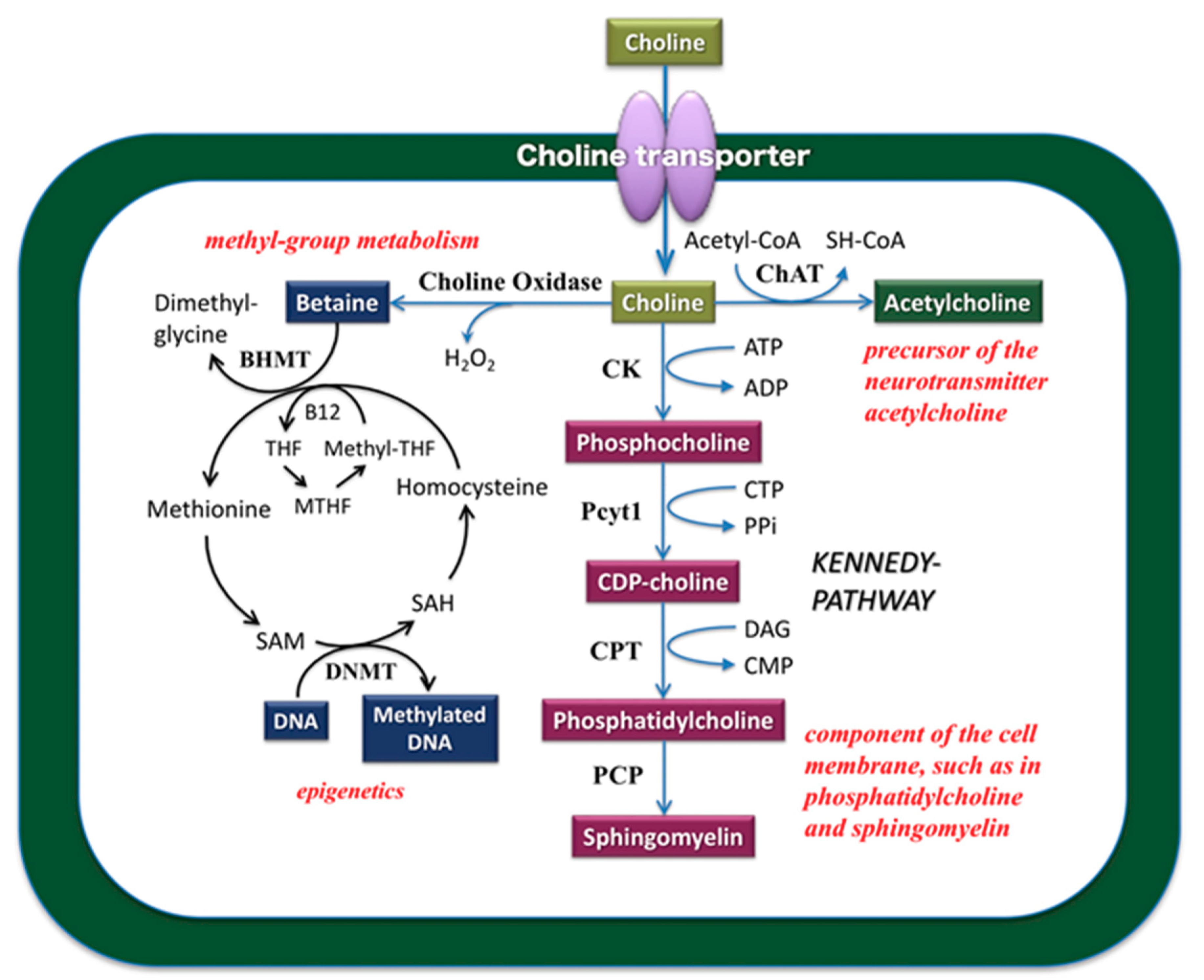
2. Role of Choline in the Central Nervous System
3. Characteristics of Choline Transporter Families
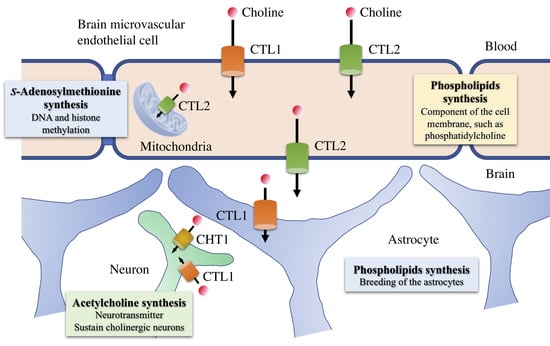
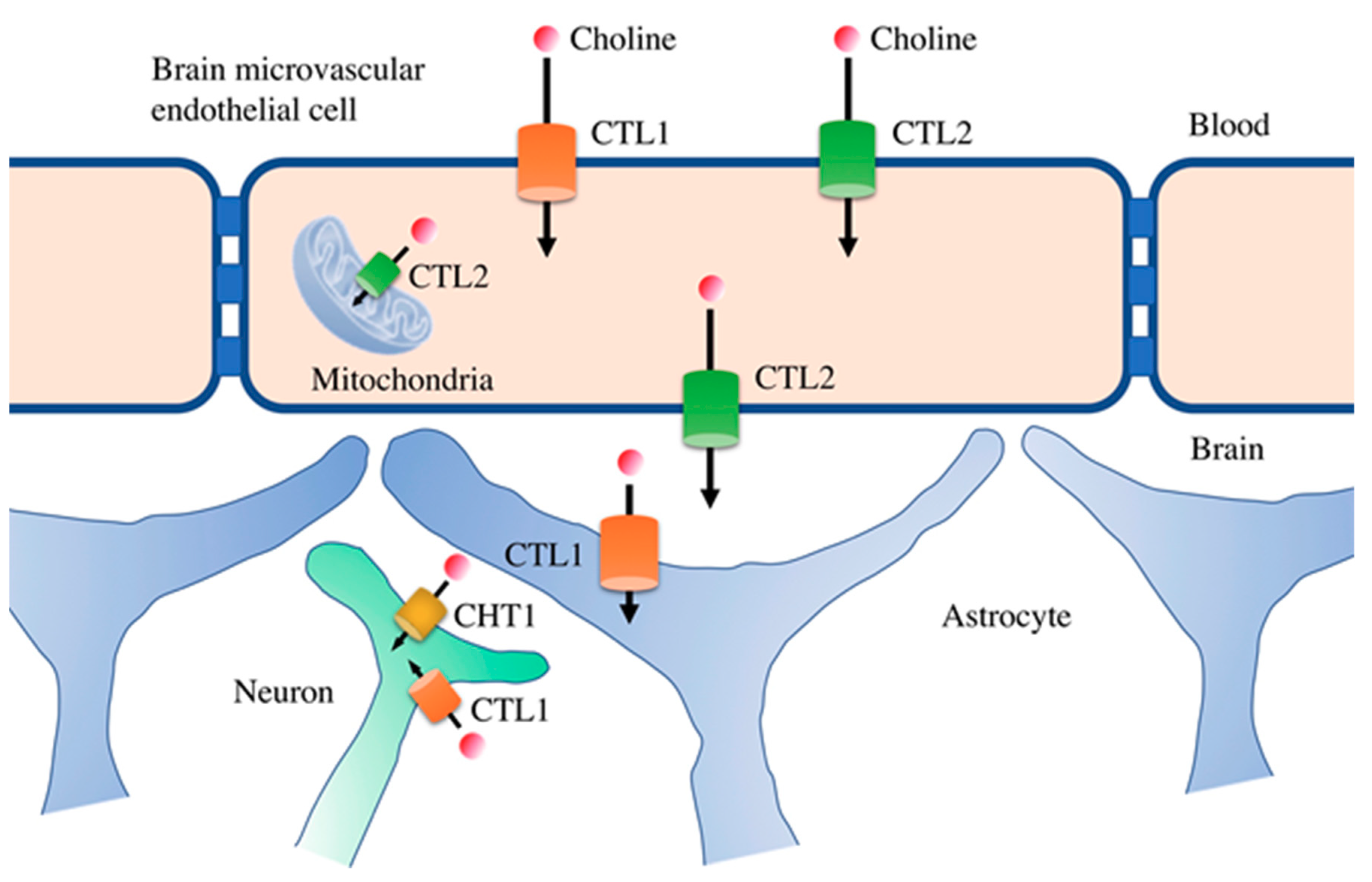
4. Functional Expression of Choline Transporters in Human Brain Microvascular Endothelial Cells
5. Potential of CTL1 and CTL2 as Drug Transporters
6. Role of Choline Transporters in the Central Nervous System
Funding
Conflicts of Interest
References
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Suzuki, Y.; Umegaki, H.; Ikari, H.; Tajima, T.; Endo, H.; Iguchi, A. Dietary restriction of choline reduces hippocampal acetylcholine release in rats: In vivo microdialysis study. Brain Res. Bull. 2001, 56, 593–597. [Google Scholar] [CrossRef]
- Zeisel, S.H. Gene response elements, genetic polymorphisms and epigenetics influence the human dietary requirement for choline. IUBMB Life 2007, 59, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef]
- Inazu, M. Choline transporter—Like proteins CTLs/SLC44 family as a novel molecular target for cancer therapy. Biopharm. Drug Dispos. 2014, 35, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Haga, T.; Kanai, Y.; Endou, H.; Ishihara, T.; Katsura, I. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 2000, 3, 120–125. [Google Scholar] [CrossRef]
- Haga, T. Molecular properties of the high-affinity choline transporter CHT1. J. Biochem. 2014, 156, 181–194. [Google Scholar] [CrossRef]
- Ferguson, S.M.; Bazalakova, M.; Savchenko, V.; Tapia, J.C.; Wright, J.; Blakely, R.D. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc. Natl. Acad. Sci. USA 2004, 101, 8762–8767. [Google Scholar] [CrossRef]
- Haberberger, R.V.; Pfeil, U.; Lips, K.S.; Kummer, W. Expression of the high-affinity choline transporter, CHT1, in the neuronal and non-neuronal cholinergic system of human and rat skin. J. Investing. Dermatol. 2002, 119, 943–948. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003, 74, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T. Basic and clinical aspects of non-neuronal acetylcholine: Overview of nonneuronal cholinergic systems and their biological significance. J. Pharmacol. Sci. 2008, 106, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Reconciling neuronally and nonneuronally derived acetylcholine in the regulation of immune function. Ann. N. Y. Acad. Sci. 2012, 1261, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Pfeil, U.; Lips, K.S.; Eberling, L.; Grau, V.; Haberberger, R.V.; Kummer, W. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am. J. Respir. Cell Mol. Biol. 2003, 28, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline transport for phospholipid synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef]
- Michel, V.; Bakovic, M. The ubiquitous choline transporter SLC44A1. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 70–81. [Google Scholar] [CrossRef]
- Okuda, M.; Saito, H.; Urakami, Y.; Takano, M.; Inui, K. cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem. Biophys. Res. Commun. 1996, 224, 500–507. [Google Scholar] [CrossRef]
- Urakami, Y.; Okuda, M.; Masuda, S.; Saito, H.; Inui, K. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J. Pharmacol. Exp. Ther. 1998, 287, 800–805. [Google Scholar]
- Kekuda, R.; Prasad, P.D.; Wu, X.; Wang, H.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V. Cloning and functional characterization of a potential sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J. Biol. Chem. 1998, 273, 15971–15979. [Google Scholar] [CrossRef]
- Wu, X.; Kekuda, R.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998, 273, 32776–32786. [Google Scholar] [CrossRef]
- O’Regan, S.; Traiffort, E.; Ruat, M.; Cha, N.; Compaore, D.; Meunier, F.M. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, S.; Meunier, F.M. Selection and characterization of the choline transport mutation suppressor from Torpedo electric lobe, CTL1. Neurochem. Res. 2003, 28, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Inazu, M.; Takeda, H.; Matsumiya, T. Molecular and functional characterization of a Na+-independent choline transporter in rat astrocytes. J. Neurochem. 2005, 94, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Inazu, M.; Tajima, H.; Matsumiya, T. Functional expression of choline transporter-like protein 1 (CTL1) in human neuroblastoma cells and its link to acetylcholine synthesis. Neurochem. Int. 2011, 58, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Inazu, M.; Saiki, I.; Yara, M.; Hara, N.; Yamanaka, T.; Uchino, H. Functional analysis of [methyl-(3)H]choline uptake in glioblastoma cells: Influence of anti-cancer and central nervous system drugs. Biochem. Pharmacol. 2014, 88, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, M.; Inazu, M.; Yamada, T.; Tajima, H.; Matsumiya, T. Molecular and functional characterization of choline transporter in rat renal tubule epithelial NRK-52E cells. Arch. Biochem. Biophys. 2009, 485, 88–96. [Google Scholar] [CrossRef]
- Nishiyama, R.; Nagashima, F.; Iwao, B.; Kawai, Y.; Inoue, K.; Midori, A.; Yamanaka, T.; Uchino, H.; Inazu, M. Identification and functional analysis of choline transporter in tongue cancer: A novel molecular target for tongue cancer therapy. J. Pharmacol. Sci. 2016, 131, 101–109. [Google Scholar] [CrossRef][Green Version]
- Seki, M.; Kawai, Y.; Ishii, C.; Yamanaka, T.; Odawara, M.; Inazu, M. Functional analysis of choline transporters in rheumatoid arthritis synovial fibroblasts. Mod. Rheumatol. 2017, 27, 995–1003. [Google Scholar] [CrossRef]
- Nagashima, F.; Nishiyama, R.; Iwao, B.; Kawai, Y.; Ishii, C.; Yamanaka, T.; Uchino, H.; Inazu, M. Molecular and Functional Characterization of Choline Transporter-Like Proteins in Esophageal Cancer Cells and Potential Therapeutic Targets. Biomol. Ther. (Seoul) 2018, 26, 399–408. [Google Scholar] [CrossRef]
- Machová, E.; O’Regan, S.; Newcombe, J.; Meunier, F.M.; Prentice, J.; Dove, R.; Lisá, V.; Dolezal, V. Detection of choline transporter-like 1 protein CTL1 in neuroblastoma x glioma cells and in the CNS, and its role in choline uptake. J. Neurochem. 2009, 110, 1297–1309. [Google Scholar] [CrossRef]
- Traiffort, E.; Ruat, M.; O’Regan, S.; Meunier, F.M. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J. Neurochem. 2005, 92, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Nair, T.S.; Kozma, K.E.; Hoefling, N.L.; Kommareddi, P.K.; Ueda, Y.; Gong, T.W.; Lomax, M.I.; Lansford, C.D.; Telian, S.A.; Satar, B.; et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J. Neurosci. 2004, 24, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Berthold, T.; Wesche, J.; Kuhnert, K.; Fürll, B.; Hippe, H.; Hoppen, J.; Reil, A.; Muschter, S.; Bux, J.; Greinacher, A. Epitope mapping of antibodies directed against the human neutrophil alloantigen 3a. Transfusion 2011, 51, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Nabokina, S.M.; Inoue, K.; Subramanian, V.S.; Valle, J.E.; Yuasa, H.; Said, H.M. Molecular identification and functional characterization of the human colonic thiamine pyrophosphate transporter. J. Biol. Chem. 2014, 289, 4405–4416. [Google Scholar] [CrossRef] [PubMed]
- Nabokina, S.M.; Ramos, M.B.; Valle, J.E.; Said, H.M. Regulation of basal promoter activity of the human thiamine pyrophosphate transporter SLC44A4 in human intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2015, 308, C750–C757. [Google Scholar] [CrossRef] [PubMed]
- Iwao, B.; Yara, M.; Hara, N.; Kawai, Y.; Yamanaka, T.; Nishihara, H.; Inoue, T.; Inazu, M. Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem. Int. 2016, 93, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef]
- Inazu, M.; Yamada, T.; Kubota, N.; Yamanaka, T. Functional expression of choline transporter-like protein 1 (CTL1) in small cell lung carcinoma cells: A target molecule for lung cancer therapy. Pharmacol. Res. 2013, 76, 119–131. [Google Scholar] [CrossRef]
- Okura, T.; Hattori, A.; Takano, Y.; Sato, T.; Hammarlund-Udenaes, M.; Terasaki, T.; Deguchi, Y. Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab. Dispos. 2008, 36, 2005–2013. [Google Scholar] [CrossRef]
- Shimomura, K.; Okura, T.; Kato, S.; Couraud, P.O.; Schermann, J.M.; Terasaki, T.; Deguchi, Y. Functional expression of a proton-coupled organic cation (H+/OC) antiporter in human brain capillary endothelial cell line hCMEC/D3, a human blood-brain barrier model. Fluids Barriers CNS 2013, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Shimada, A.; Okada, N.; Yamamoto, A. Functional characterization of Na+-independent choline transport in primary cultures of neurons from mouse cerebral cortex. Neurosci. Lett. 2016, 393, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.H.; Yamashita, T.; Higuchi, H.; Tohyama, M. Changes in mRNA for choline transporter-like protein following facial nerve transection. Brain Res. Mol. Brain Res. 2002, 101, 122–125. [Google Scholar] [CrossRef]
| Protein Name | Km for Choline | Sodium-dependency | Sensitivity of HC-3 (Ki) | Tissue Distribution | Substrates |
|---|---|---|---|---|---|
| CHT1 | 0.5–3 µM | Yes | 50–100 nM | Brain, spinal cord | Choline |
| CTL1 | 10–50 µM | No | 10–100 µM | Multiple tissues | Choline, organic cation |
| CTL2 | 50–200 µM | Unknown | Unknown | Placenta, lung | Choline |
| CTL3 | Unknown | Unknown | Unknown | Colon, pancreas | Unknown |
| CTL4 | Unknown | Unknown | Unknown | Prostate, colon | Thiamine pyrophosphate |
| CTL5 | Unknown | Unknown | Unknown | Multiple tissues | Unknown |
| OCT1 | 300–400 µM | No | >250 µM | Liver, kidney | Organic cation |
| OCT2 | 100–500 µM | No | >250 µM | Kidney, brain | Organic cation |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inazu, M. Functional Expression of Choline Transporters in the Blood–Brain Barrier. Nutrients 2019, 11, 2265. https://doi.org/10.3390/nu11102265
Inazu M. Functional Expression of Choline Transporters in the Blood–Brain Barrier. Nutrients. 2019; 11(10):2265. https://doi.org/10.3390/nu11102265
Chicago/Turabian StyleInazu, Masato. 2019. "Functional Expression of Choline Transporters in the Blood–Brain Barrier" Nutrients 11, no. 10: 2265. https://doi.org/10.3390/nu11102265
APA StyleInazu, M. (2019). Functional Expression of Choline Transporters in the Blood–Brain Barrier. Nutrients, 11(10), 2265. https://doi.org/10.3390/nu11102265