Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval of the Study Protocol
2.2. Lentinus edodes: Composition and Dose Selection
2.3. Animal Experiments
2.4. Assays with Gestational Diabetes Mellitus Induced by Strepzotocina (GDM-STZ)
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Blood Collection and Reproductive Performance
2.7. Maternal Hematologic Parameters
2.8. Maternal Biochemical Profile
2.9. Embryofetal Development and Placental Analysis
2.10. Oxidative Stress Biomarkers
2.11. Statistical Analyses
3. Results
3.1. Lentinus edodes
3.2. Gestational Evaluation
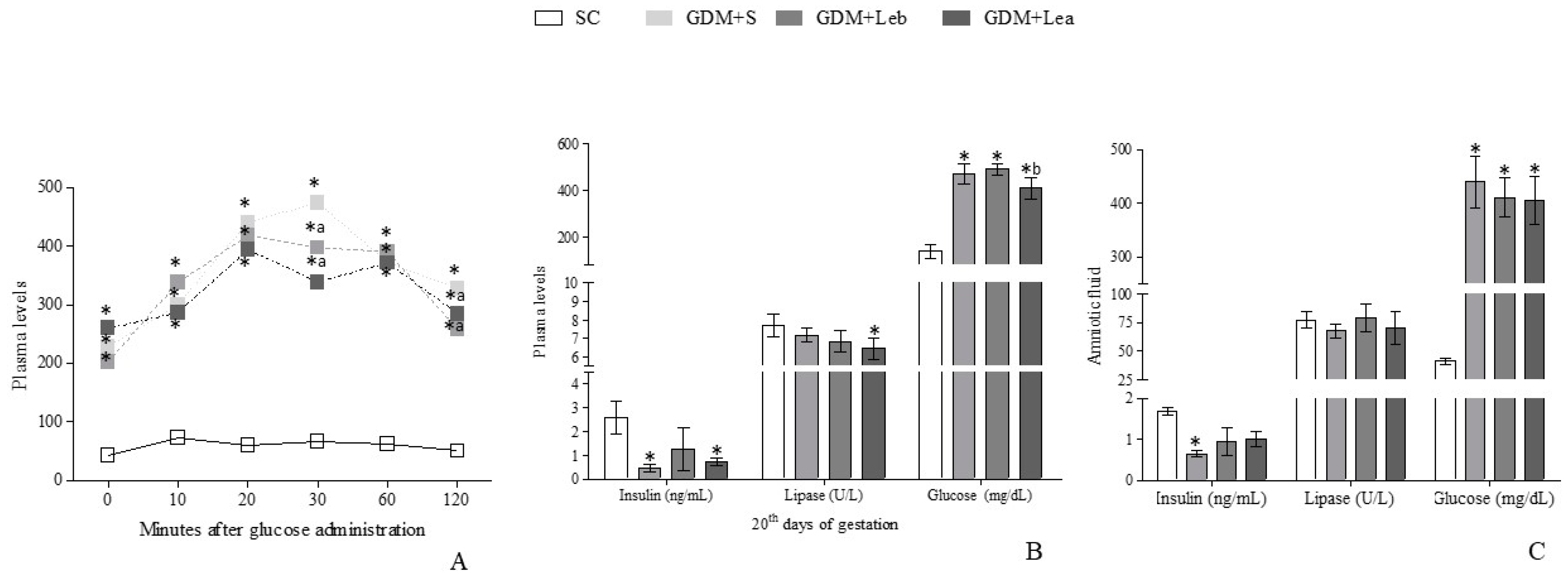
3.3. Glycemic Profile on GMD
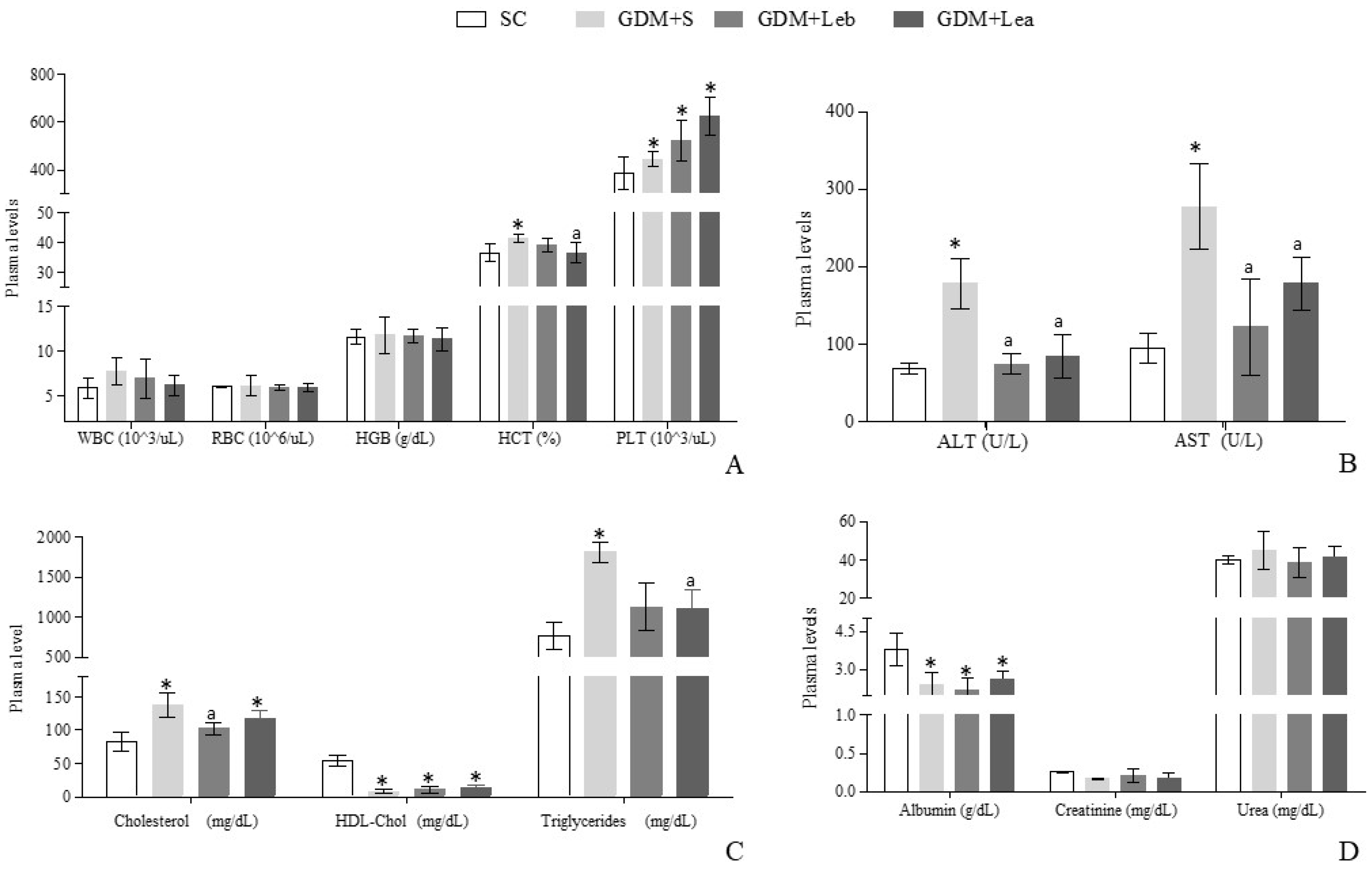
3.4. Maternal Hematologic and Biochemical Evaluation
3.5. Reproductive Performance of Female Rats
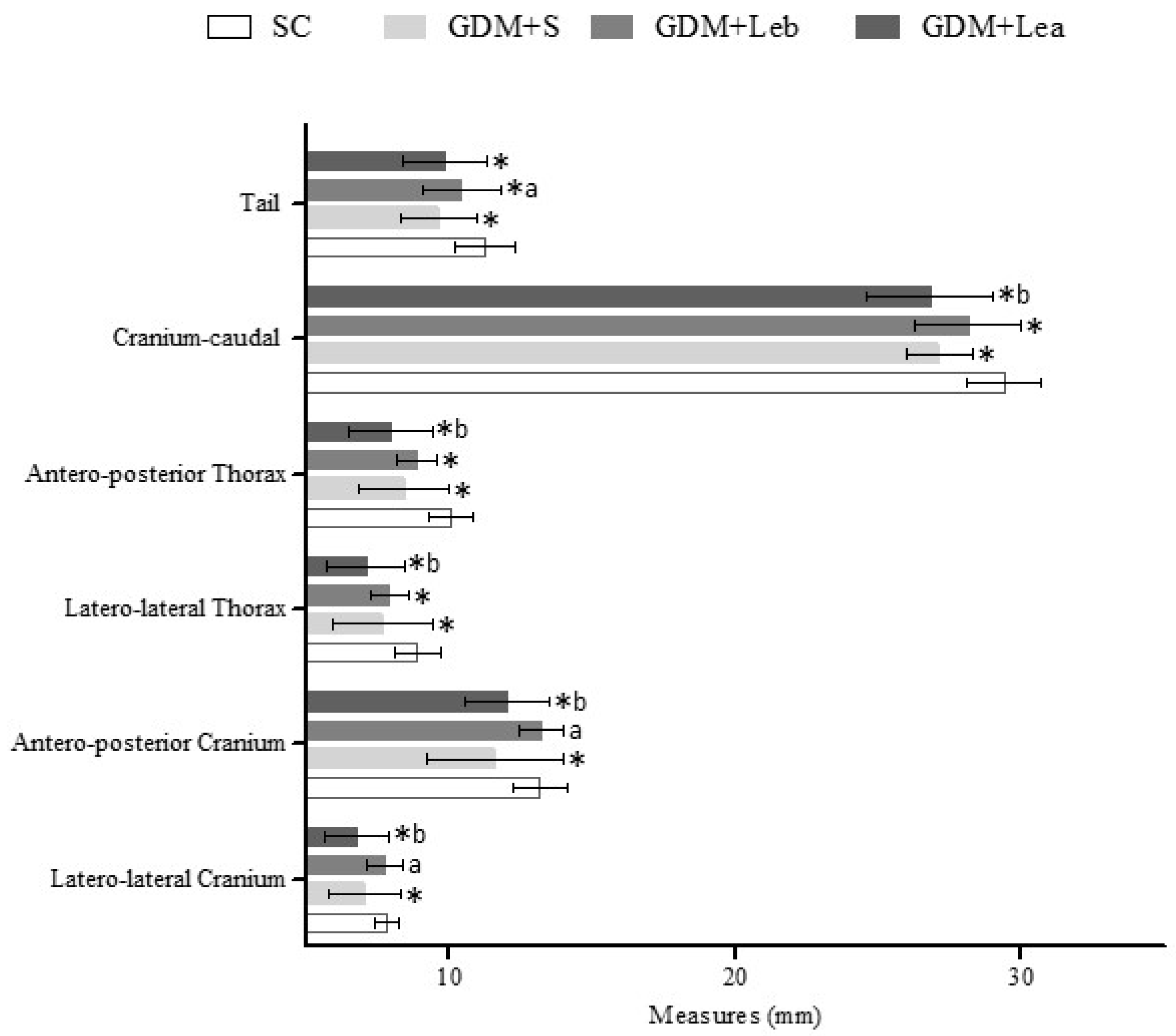
3.6. Embriofetal Development
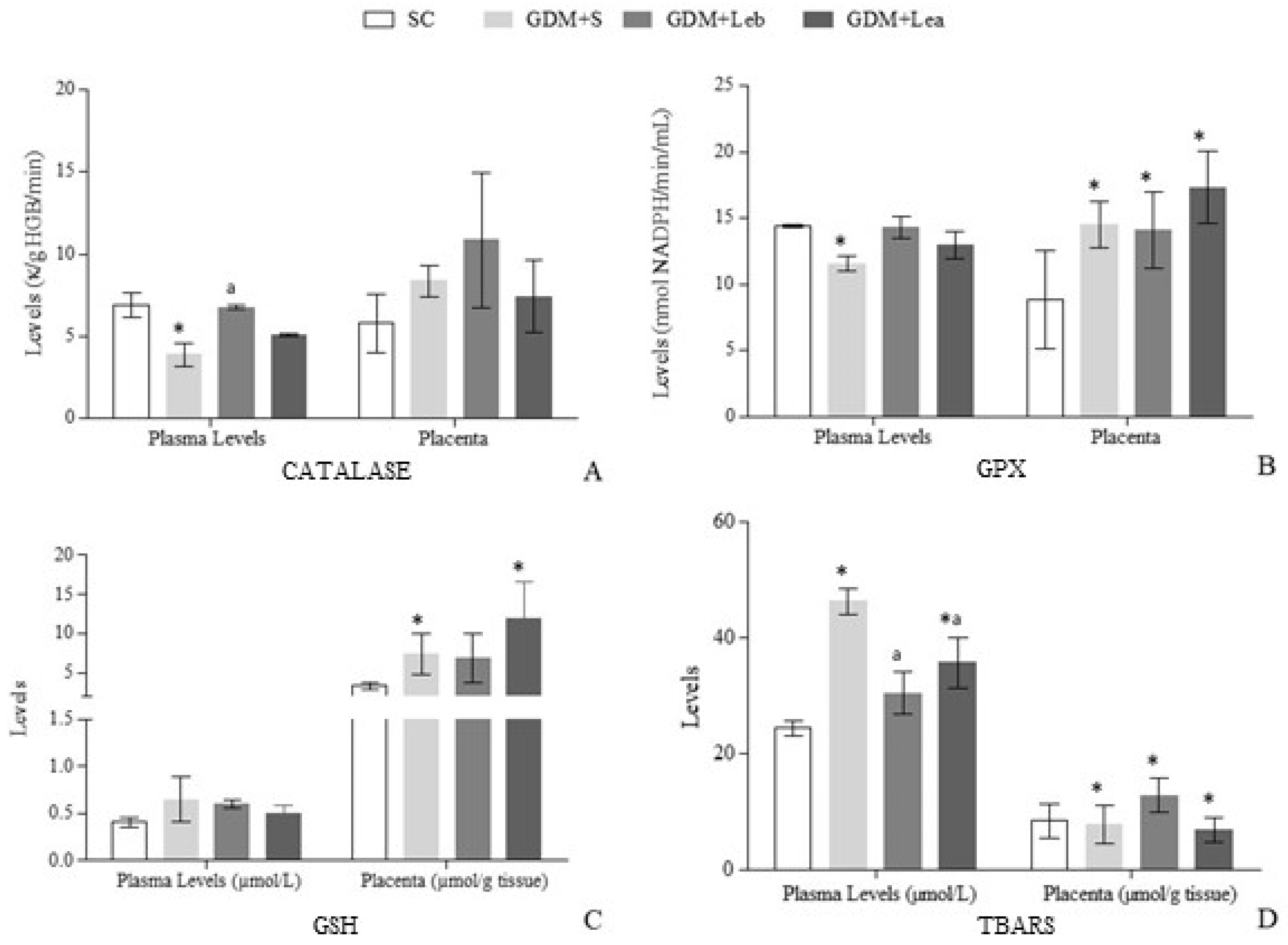
3.7. Oxidative Stress on Maternal Blood and Placenta
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association (ADA). American Diabetes Association standard of medical care in diabetes—2017. Diabetes Care 2017, 40, s4–s128. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-C.; Wasser, S.P. Medicinal mushrooms for glycemic control in diabetes mellitus: History, current status, future perspectives, and unsolved problems (review). Int. J. Med. Mushrooms 2011, 13, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Health Organization Global Report on Diabetes; WHO: Geneva, Switzerland, 2016; 88p. [Google Scholar]
- Coustan, D.R. Gestational diabetes mellitus. Clin. Chem. 2013, 59, 1310–1321. [Google Scholar] [CrossRef]
- Hanafi, M.Y.; Abdelkhalek, T.M.; Saad, M.I.; Saleh, M.M.; Haiba, M.M.; Kamel, M.A. Diabetes-Induced perturbations are subject to intergenerational transmission through maternal line. J. Physiol. Biochem. 2016, 72, 315–326. [Google Scholar] [CrossRef]
- Fetita, L.S.; Sobngwi, E.; Serradas, P.; Calvo, F.; Gautier, J.F. Review: Consequences of fetal exposure to maternal diabetes in offspring. J. Clin. Endocrinol. Metab. 2006, 91, 3718–3724. [Google Scholar] [CrossRef]
- Bueno, A.; Sinzato, Y.K.; Sudano, M.J.; Alvarenga Fda, C.; Calderon Ide, M.; Rudge, M.V.; Damasceno, D.C. Short and long-term repercussions of the experimental diabetes in embryofetal development. Diabetes. Metab. Res. Rev. 2014, 30, 575–581. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H. Diagnosis, prevention and management of gestational diabetes mellitus. Chronic Dis. Transl. Med. 2016, 6, 2–7. [Google Scholar] [CrossRef]
- Wu, T.; Xu, B. Antidiabetic and antioxidant activities of eight medicinal mushroom species from China. Int. J. Med. Mushrooms 2015, 17, 129–140. [Google Scholar] [CrossRef]
- Chandra, L.C.; Smith, B.J.; Clarke, S.L.; Marlow, D.; D’Offay, J.M.; Kuvibidila, S.R. Differential effects of shiitake- and white button mushroom-supplemented diets on hepatic steatosis in C57BL/6 mice. Food Chem. Toxicol. 2011, 49, 3074–3080. [Google Scholar] [CrossRef]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B.K.S. Lentinus edodes: A Macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.A.J.; Ferreira, I.C.F.R.; Dueñas, M.; Barros, L.; Da Silva, R.; Gomes, E.; Santos-Buelga, C. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chem. 2013, 138, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wu, X.; Ferguson, M.; Simmen, R.C.; Cleves, M.A.; Simmen, F.A.; Fang, N. Diets containing shiitake mushroom reduce serum lipids and serum lipophilic antioxidant capacity in rats. J. Nutr. 2016, 146, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Stanilka, J.M.; Rowe, C.A.; Esteves, E.A.; Nieves, C.; Spaiser, S.J.; Christman, M.C.; Langkamp-Henken, B.; Percival, S.S. Consuming Lentinula edodes (shiitake) mushrooms daily improves human immunity: A randomized dietary intervention in healthy young adults. J. Am. Coll. Nutr. 2015, 34, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Maschio, B.H.; Gentil, B.C.; Caetano, E.L.A.; Rodrigues, L.S.; Laurino, L.F.; Spim, S.R.V.; Jozala, A.F.; Santos, C.A.; Grotto, D.; Gerenutti, M. Effects characterization of shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes), on severe gestational diabetes mellitus in rats. Int. J. Med. Mushroom 2017, 19, 991–1000. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, 6–10. [Google Scholar] [CrossRef]
- National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; ISBN 9780309154000. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in wood: Comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz (IAL). Normas Analíticas; Métodos Químicos e Físicos Para a Análise de Alimentos; IAL: Sao Paolo, Brazil, 2008. [Google Scholar]
- Grotto, D.; Bueno, D.C.R.; Ramos, G.K.; da Costa, S.R.; Spim, S.R.V.; Gerenutti, M. Assessment of the safety of the shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes), in Rats: Biochemical, hematological, and antioxidative parameters. Int. J. Med. Mushrooms 2016, 18, 861–870. [Google Scholar] [CrossRef]
- Gerenutti, M.; Del Fiol, F.; Groppo, F. Reproductive performance of pregnant rats and embryotoxic effects of ciprofloxacin. Pharmazie 2006, 61, 79–80. [Google Scholar]
- Toma, A.; Makonnen, E.; Mekonnen, Y.; Debella, A.; Adisakwattana, S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2015, 15, 242. [Google Scholar] [CrossRef]
- Volpato, G.T.; Damasceno, D.C.; Rudge, M.V.C.; Padovani, C.R.; Calderon, I.M.P. Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2008, 116, 131–137. [Google Scholar] [CrossRef] [PubMed]
- De Mello, M.; de Souza, C.; Braga, L.; dos Santos, J.; Ribeiro, I.; Gobatto, C. Glucose tolerance and insulin action in monosodium glutamate (MSG) obese exercise-trained rats. Physiol. Chem. Phys. Med. NMR 2001, 33, 63–71. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.; Valentine, W. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxide. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Damasceno, D.C.; Netto, A.O.; Iessi, I.L.; Gallego, F.Q.; Corvino, S.B.; Dallaqua, B.; Sinzato, Y.K.; Bueno, A.; Calderon, I.M.P.; Rudge, M.V.C. Streptozotocin-Induced diabetes models: Pathophysiological mechanisms and fetal outcomes. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Caluwaerts, S.; Holemans, K.; Van Bree, R.; Verhaeghe, J.; Van Assche, F.A. Is low-dose streptozotocin in rats an adequate model for gestational diabetes mellitus? J. Soc. Gynecol. Investig. 2003, 10, 216–221. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.; Zhou, T.; Xu, D.P.; Zhang, P.; Li, S.; Li, H. Bin bioactivities and health benefits of mushrooms mainly from China. Molecules 2016, 21, 1–16. [Google Scholar]
- Bequer, L.; Gómez, T.; Molina, J.L.; Álvarez, A.; Chaviano, C.; Clapés, S. Experimental diabetes impairs maternal reproductive performance in pregnant Wistar rats and their offspring. Syst. Biol. Reprod. Med. 2017, 64, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.T.; Damasceno, D.C.; Sinzato, Y.K.; Ribeiro, V.M.; Rudge, M.V.C.; Calderon, I.M.P. Oxidative stress status and placental implications in diabetic rats undergoing swimming exercise after embryonic implantation. Reprod. Sci. 2015, 22, 602–608. [Google Scholar] [CrossRef] [PubMed]
- De Campos, K.E.; Sinzato, Y.K.; Pimenta, W.; Rudge, M.V.C.; Damasceno, D.C. Effect of maternal obesity on diabetes development in adult rat offspring. Life Sci. 2007, 81, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.S.; Rodrigues, L.S.; Neto, L.S.; Moraes-Souza, R.Q.; Soares, T.S.; Américo, M.F.; Campos, K.E.; Damasceno, D.C.; Volpato, G.T. Effect of bauhinia holophylla treatment in streptozotocin-induced diabetic rats. An. Acad. Bras. Cienc. 2017, 89, 263–272. [Google Scholar] [CrossRef]
- López-Soldado, I.; Herrera, E. Different diabetogenic response to moderate doses of streptozotocin in pregnant rats, and its long-term consequences in the offspring. Exp. Diabesity Res. 2003, 4, 107–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.; Shan, W.; Shi, L.; Chang, X.; Zhu, Y.; Chen, F.; Han, X. Lentinan protects pancreatic β cells from STZ-induced damage. J. Cell. Mol. Med. 2016, 20, 1803–1812. [Google Scholar] [CrossRef]
- Vitak, T.Y.; Wasser, S.P.; Nevo, E.; Sybirna, N.O. Structural changes of erythrocyte surface glycoconjugates after treatment with medicinal mushrooms. Int. J. Med. Mushrooms 2015, 17, 867–878. [Google Scholar] [CrossRef]
- Pattanathaiyanon, P.; Phaloprakarn, C.; Tangjitgamol, S. Comparison of gestational diabetes mellitus rates in women with increased and normal white blood cell counts in early pregnancy. J. Obstet. Gynaecol. Res. 2014, 40, 976–982. [Google Scholar] [CrossRef]
- Santilli, F.; Simeone, P.; Liani, R.; Davì, G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015, 120, 28–39. [Google Scholar] [CrossRef]
- Kodner, C. Diagnosis and management of nephrotic syndrome in adults. Am. Fam. Physician 2016, 93, 479–485. [Google Scholar] [PubMed]
- Eneman, B.; Levtchenko, E.; van den Heuvel, B.; Van Geet, C.; Freson, K. Platelet abnormalities in nephrotic syndrome. Pediatr. Nephrol. 2015, 31, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Mooney, M.P.; Cho, D.J. Hemorheological disorders in diabetes mellitus. J. Diabetes Sci. Technol. 2008, 2, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Isakovic, A.; Kocic, G.; Kavaric, N.; Jovanovic, M.; Zvrko, E.; Skerovic, V.; Ninic, A. Relationship between oxidative stress, inflammation and dyslipidemia with fatty liver index in patients with type 2-diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2017, 9, 1–16. [Google Scholar]
- Omodanisi, E.I.; Aboua, Y.G.; Chegou, N.N.; Oguntibeju, O.O. Hepatoprotective, antihyperlipidemic, and anti-inflammatory activity of moringa oleifera in diabetic-induced damage in male wistar rats. Pharmacognosy Res. 2017, 9, 182–187. [Google Scholar] [PubMed]
- Green, R.M.; Flamm, S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002, 123, 1367–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozaki, T.; Minaguchi, J.; Takehana, K.; Ueda, H. Anti-Diabetic activities of traditional Chinese herbal medicine in streptozotocin-induced diabetic rats. Okajimas Folia Anat. Jpn. 2017, 93, 111–118. [Google Scholar] [CrossRef]
- Akamatsu, S.; Watanabe, A.; Tamesada, M.; Nakamura, R.; Hayashi, S.; Kodama, D.; Kawase, M.; Yagi, K. Hepatoprotective effect of extracts from lentinus edodes mycelia on dimethylnitrosamine-induced liver injury. Pharm. Soc. Japan 2004, 27, 1957–1960. [Google Scholar] [CrossRef]
- Rief, P.; Pichler, M.; Raggam, R.; Hafner, F.; Gerger, A.; Eller, P.; Brodmann, M.; Gary, T. The AST/ALT (De-Ritis) ratio A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine 2016, 95, 1–4. [Google Scholar] [CrossRef]
- Afiune, L.A.F.; Leal-Silva, T.; Sinzato, Y.K.; Moraes-Souza, R.Q.; Soares, T.S.; Campos, K.E.; Fujiwara, R.T.; Herrera, E.; Damasceno, D.C.; Volpato, G.T. Beneficial effects of Hibiscus rosa-sinensis L. flower aqueous extract in pregnant rats with diabetes. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef]
- El-Sayyad, H.I.H.; Al-Haggar, M.M.S.; El-Ghawet, H.A.; Bakr, I.H.M. Effect of maternal diabetes and hypercholesterolemia on fetal liver of albino Wistar rats. Nutrition 2014, 30, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Spim, S.R.V.; de Oliveira, B.G.C.C.; Leite, F.G.; Gerenutti, M.; Grotto, D. Effects of Lentinula edodes consumption on biochemical, hematologic and oxidative stress parameters in rats receiving high-fat diet. Eur. J. Nutr. 2016, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; HaiYan, Z.; JianHong, Z.; Jing, G. The pharmacological effect of polysaccharides from Lentinus edodes on the oxidative status and expression of VCAM-1mRNA of thoracic aorta endothelial cell in high-fat-diet rats. Carbohydr. Polym. 2008, 74, 445–450. [Google Scholar] [CrossRef]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Calderon, I.D.M.P.; Rudge, M.V.C.; Ramos, M.D.; Peraçoli, J.C. Estudo longitudinal, bioquímico e histoquímico, de placentas de ratas diabéticas: Relação com a macrossomia e o retardo de crescimento intra-uterino. Rev. Bras. Ginecol. Obs. 1999, 21, 91–98. [Google Scholar] [CrossRef]
- Saito, F.H.; Damasceno, D.C.; Kempinas, W.G.; Morceli, G.; Sinzato, Y.K.; Taylor, K.N.; Rudge, M.V. Repercussions of mild diabetes on pregnancy in Wistar rats and on the fetal development. Diabetol. Metab. Syndr. 2010, 2, 26. [Google Scholar] [CrossRef]
- Kc, K.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66, 14–20. [Google Scholar] [CrossRef]
- Heshmat, S.H. Intrauterine growth restriction. Anat. Physiol. Biochem. Int. J. 2017, 1, 5. [Google Scholar]
- Matkovics, B.; Kotorman, M.; Varga, I.; Hai, D.; Varga, C. Oxidative stress in experimental diabetes induced by streptozotocin. Acta Physiol. Hung. 1997, 85, 29–38. [Google Scholar]
- Myatt, L. Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, 66–69. [Google Scholar] [CrossRef]
- Damasceno, D.C.; Sinzato, Y.K.; Bueno, A.; Netto, A.O.; Dallaqua, B.; Gallego, F.Q.; Iessi, I.L.; Corvino, S.B.; Serrano, R.G.; Marini, G.; et al. Mild diabetes models and their maternal-fetal repercussions. J. Diabetes Res. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Mouzon, S.H.; Jawerbaum, A.; Editors, R.; Eriksson, U.; Jain, S.; Levy, E.; et al. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Chen, J.; Das, K.C.; Kavdia, M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc. Diabetol. 2013, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Santhakumari, P.; Prakasam, A.; Pugalendi, K. Others modulation of oxidative stress parameters by treatment with Piper betle leaf in streptozotocin induced diabetic rats. Indian J. Pharmacol. 2003, 35, 373–378. [Google Scholar]
- Damasceno, D.C.; Volpato, G.T.; Paranhos Calderon, I.; Cunha Rudge, M.V. Oxidative stress and diabetes in pregnant rats. Anim. Reprod. Sci. 2002, 72, 235–244. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Khan, I. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar]
- Yurkiv, B.; Wasser, S.P.; Nevo, E.; Sybirna, N.O. Antioxidant effects of medicinal mushrooms Agaricus brasiliensis and Ganoderma lucidum (Higher Basidiomycetes): Evidence from animal studies. Int. J. Med. Mushrooms 2015, 17, 943–955. [Google Scholar] [CrossRef]
| Groups | Uterus Weight (g) | Ovary Weight (g) | Placenta Weight (g) | Number of Alive Fetus Per Mother | Post-Implantation Loss (%) |
|---|---|---|---|---|---|
| SC | 44.48 ± 13.83 | 0.142 ± 0.015 | 0.415 ± 0.09 | 9.75 ± 1.83 | 4.6 |
| GDM + S | 35.40 ± 12.49 * | 0.112 ± 0.019 * | 0.494 ± 0.143 * | 8.16 ± 1.53 | 16 * |
| GDM + Leb | 41.64 ± 8.36 | 0.105 ± 0.020 * | 0.485 ± 0.102 * | 10.83 ± 2.03 | 6.9 a |
| GDM + Lea | 42.79 ± 11.79 | 0.123 ± 0.019 | 0.443 ± 0.081 | 11.33 ± 2.92 | 13.8 *b |
| (F = 0.5187, p = 0.6774) | (F = 3.336, p = 0.0287) | (F = 10.66, p < 0.0001) | (F = 0.8614, p = 0.4790) | (p = 0.0287) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurino, L.F.; Viroel, F.J.M.; Caetano, E.; Spim, S.; Pickler, T.B.; Rosa-Castro, R.M.; Vasconcelos, E.A.; Jozala, A.F.; Hataka, A.; Grotto, D.; et al. Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus. Nutrients 2019, 11, 2720. https://doi.org/10.3390/nu11112720
Laurino LF, Viroel FJM, Caetano E, Spim S, Pickler TB, Rosa-Castro RM, Vasconcelos EA, Jozala AF, Hataka A, Grotto D, et al. Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus. Nutrients. 2019; 11(11):2720. https://doi.org/10.3390/nu11112720
Chicago/Turabian StyleLaurino, Leticia F., Fabia J. M. Viroel, Erika Caetano, Sara Spim, Thaisa B. Pickler, Raquel M. Rosa-Castro, Edilma Albuquerque Vasconcelos, Angela F. Jozala, Alessandre Hataka, Denise Grotto, and et al. 2019. "Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus" Nutrients 11, no. 11: 2720. https://doi.org/10.3390/nu11112720
APA StyleLaurino, L. F., Viroel, F. J. M., Caetano, E., Spim, S., Pickler, T. B., Rosa-Castro, R. M., Vasconcelos, E. A., Jozala, A. F., Hataka, A., Grotto, D., & Gerenutti, M. (2019). Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus. Nutrients, 11(11), 2720. https://doi.org/10.3390/nu11112720






