Synergistic Cytoprotective Effects of Rutin and Ascorbic Acid on the Proteomic Profile of 3D-Cultured Keratinocytes Exposed to UVA or UVB Radiation
Abstract
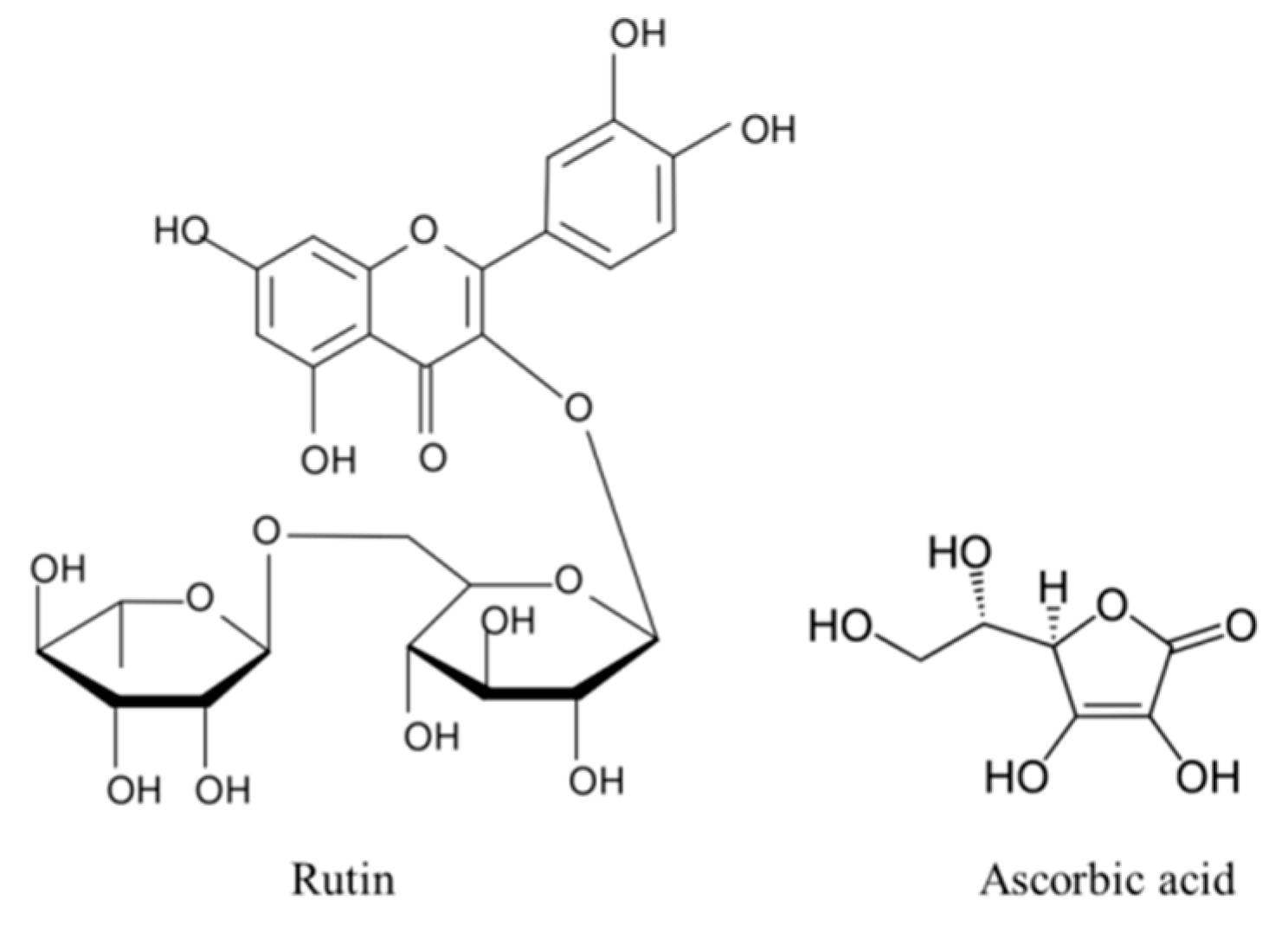
1. Introduction
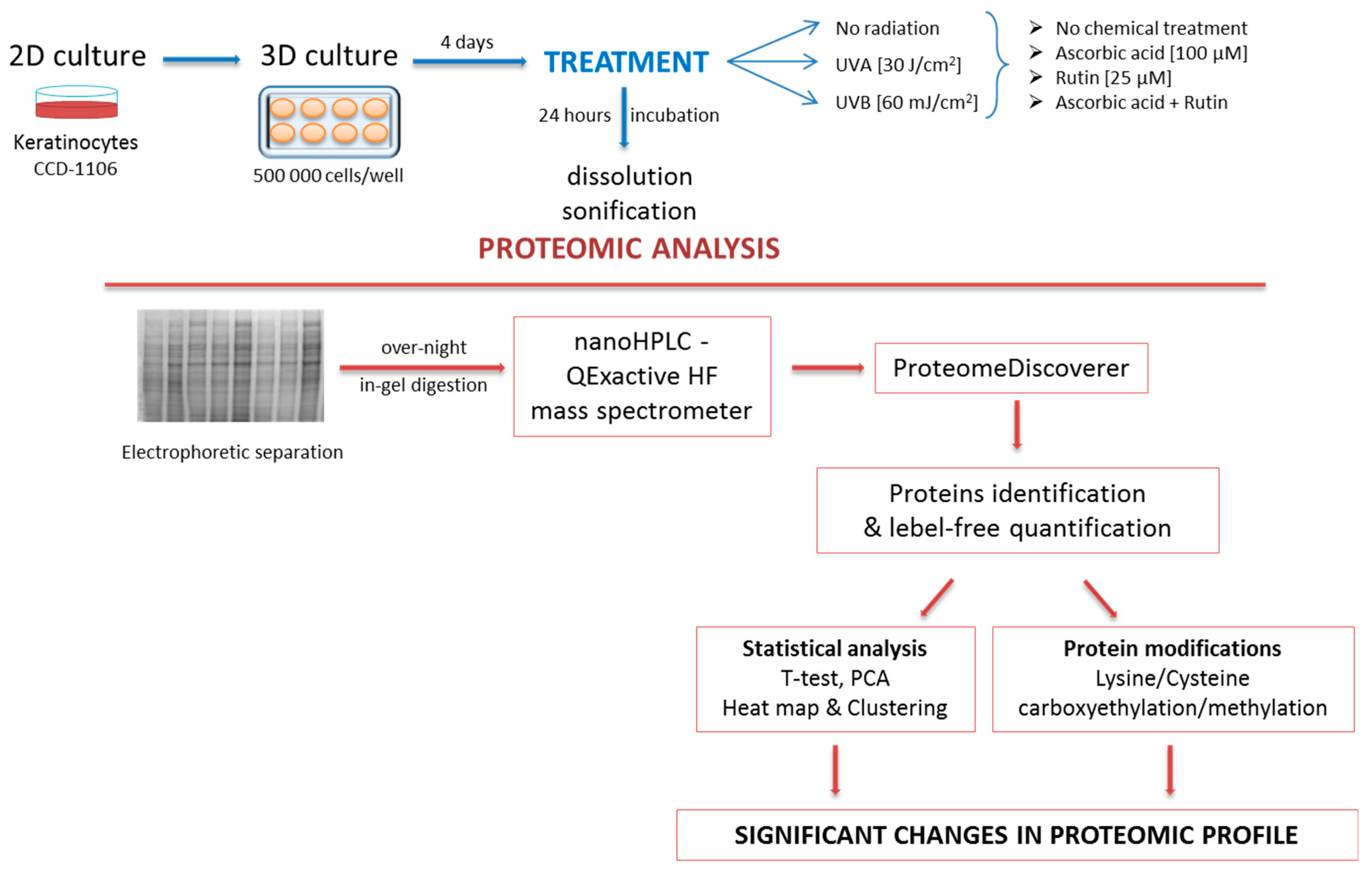
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Proteomic Analysis
2.3. Protein Identification, Grouping, and Label-Free Quantification
2.4. Statistical Analysis
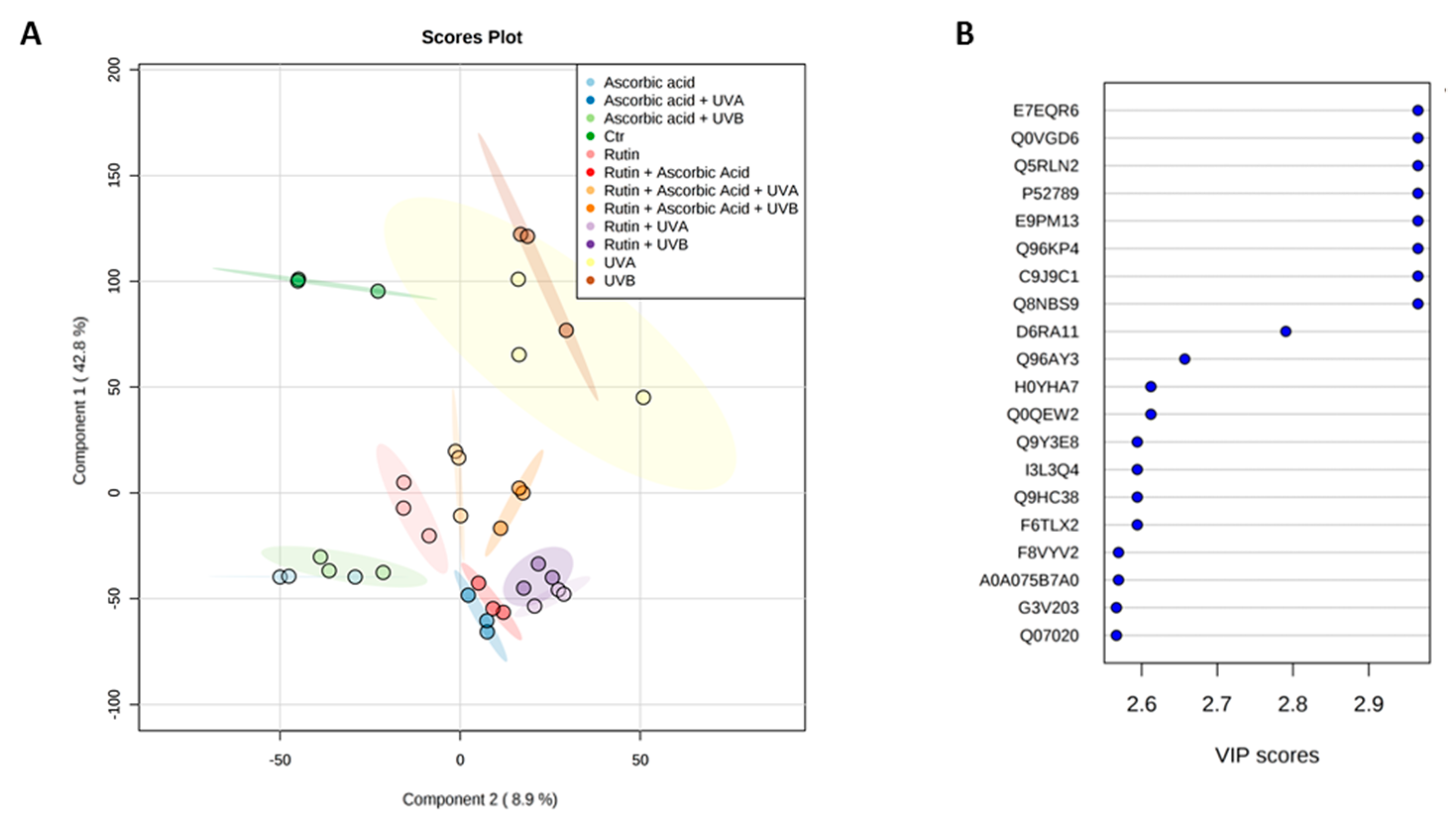
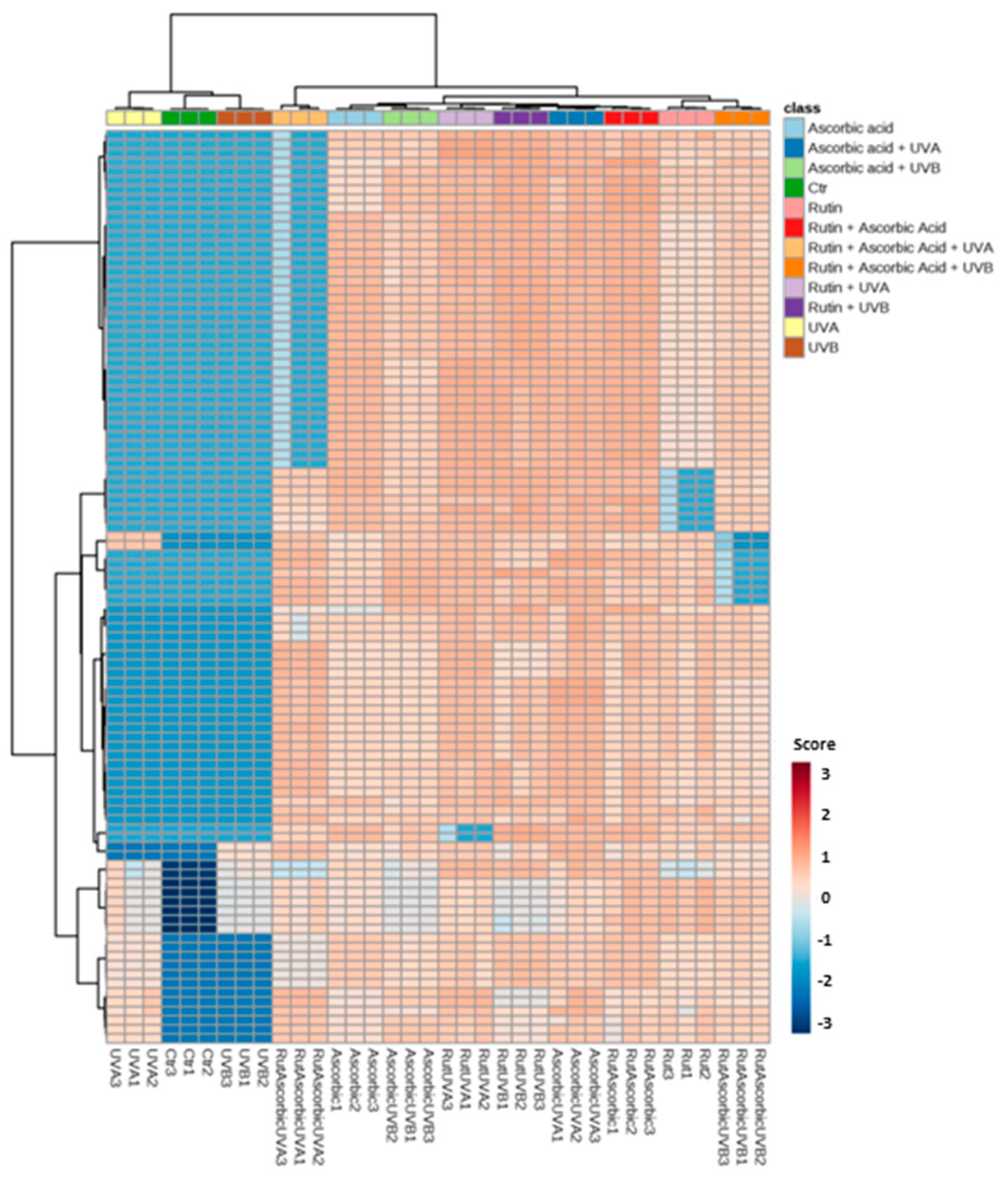
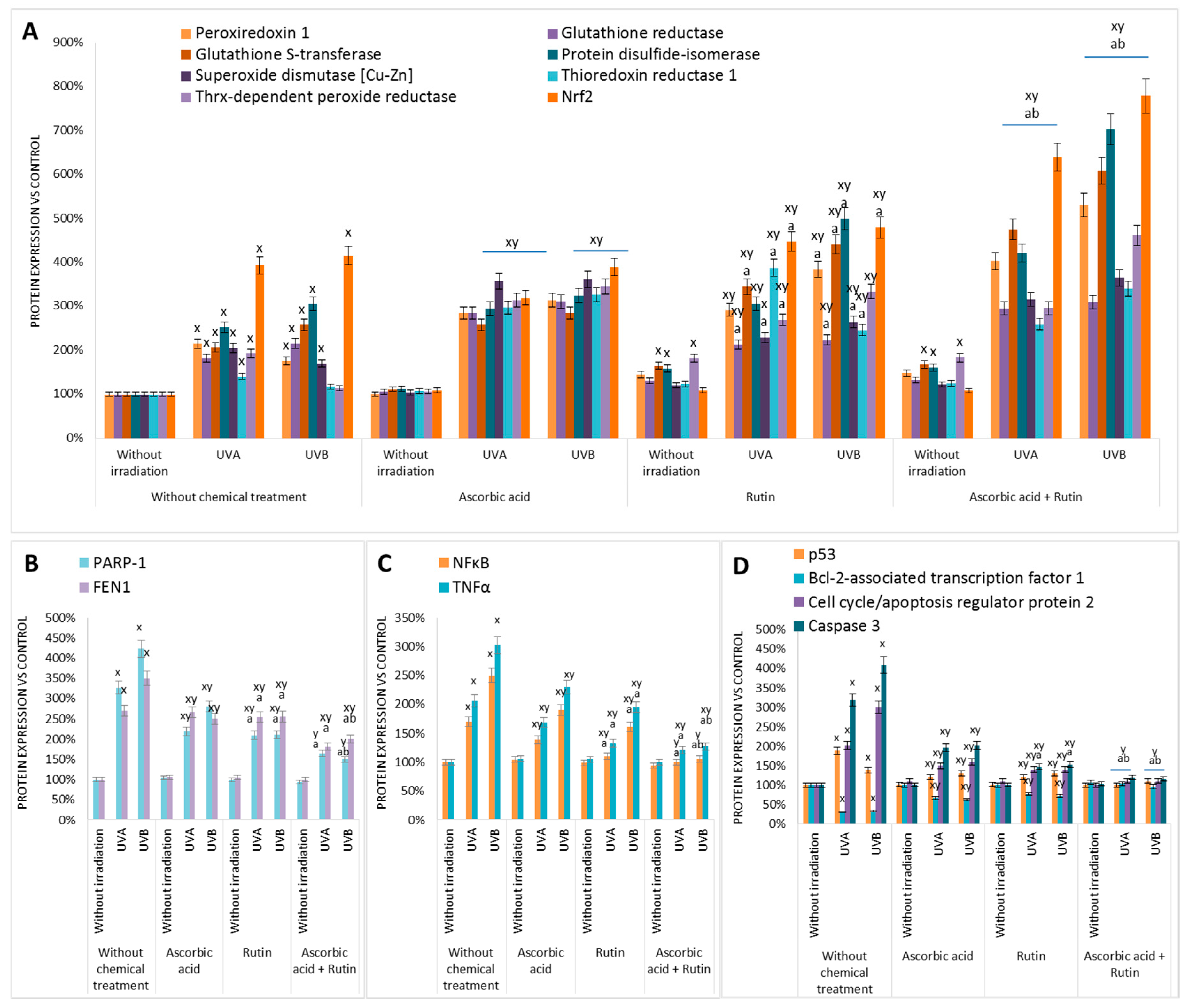
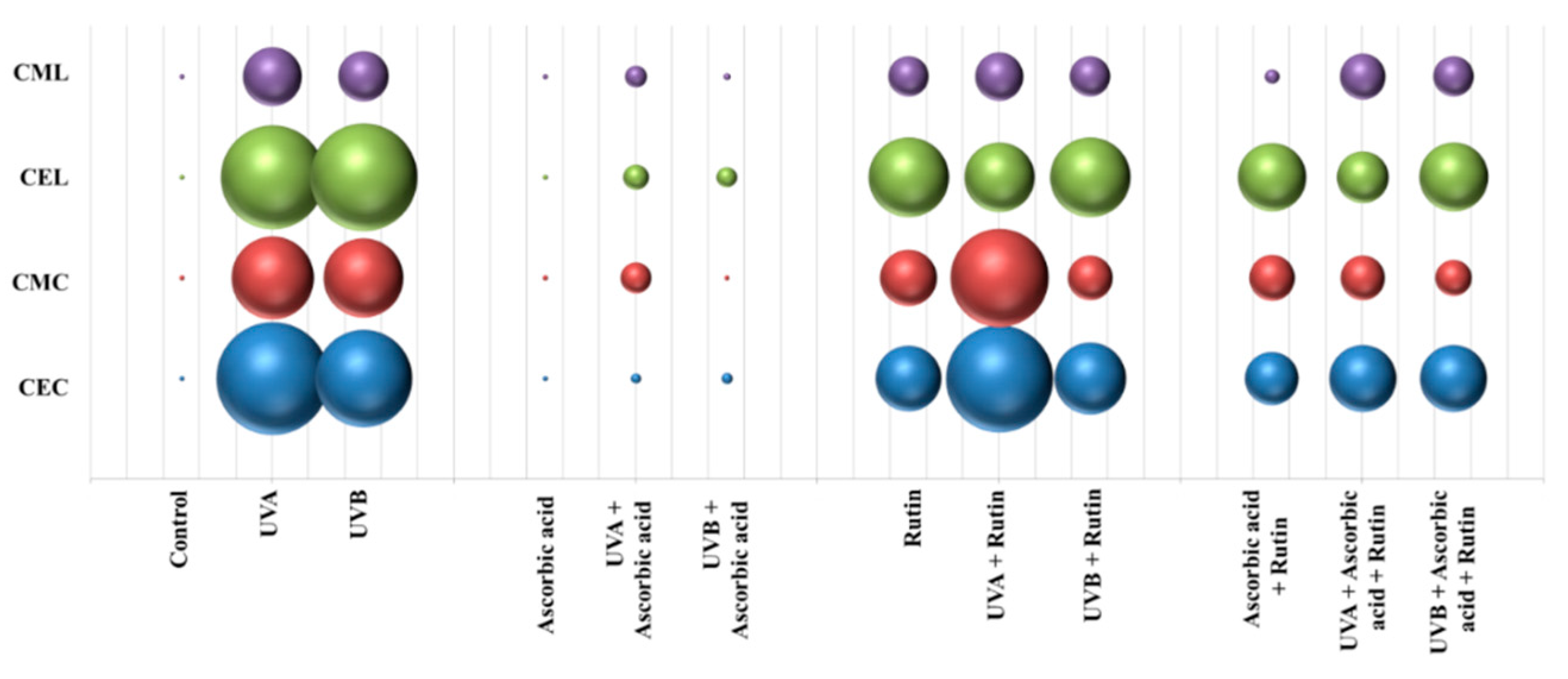
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Invest. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Bernerd, F.; Marionnet, C.; Duval, C. Solar ultraviolet radiation induces biological alterations in human skin in vitro: Relevance of a well-balanced UVA/UVB protection. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 15–23. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Sweetlove, L.J. ROS signaling-specificity is required. Trends Plant Sci. 2010, 15, 370–374. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Aki, K.; Kuge, K.; Tajima, S.; Yamanaka, N.; Kaji, Y.; Yamamoto, N.; Nagai, R.; Yoshii, H.; Fujii, N.; et al. UVB-irradiation enhances the racemization and isomerizaiton of aspartyl residues and production of Nɛ-carboxymethyl lysine (CML) in keratin of skin. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, D.B.; Chappell, P.E.; Eisel, B.; Johnson, S.; Lea, S.M.; Berks, B.C. Mechanism of thiosulfate oxidation in the SoxA family of cysteine-ligated cytochromes. J. Biol. Chem. 2015, 290, 9209–9221. [Google Scholar] [CrossRef] [PubMed]
- Roduit, R.; Schorderet, D.F. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis 2008, 13, 343–353. [Google Scholar] [CrossRef]
- Gęgotek, A.; Rybałtowska-Kawałko, P.; Skrzydlewska, E. Rutin as a Mediator of Lipid Metabolism and Cellular Signaling Pathways Interactions in Fibroblasts Altered by UVA and UVB Radiation. Oxid. Med. Cell. Longev. 2017, 2017, 4721352. [Google Scholar] [CrossRef]
- Krutmann, J.; Sondenheimer, K.; Grether-Beck, S.; Haarmann-Stemmann, T. Combined, Simultaneous Exposure to Radiation within and Beyond the UV Spectrum: A Novel Approach to Better Understand Skin Damage by Natural Sunlight. In Environment and Skin; Springer: Berlin, Germany, 2018; pp. 11–16. [Google Scholar]
- Ikehata, H.; Yamamoto, M. Roles of the KEAP1-NRF2 system in mammalian skin exposed to UV radiation. Toxicol. Appl. Pharmacol. 2018, 360, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, J.; Pofahl, R.; Haase, I. Epidermal Rac1 regulates the DNA damage response and protects from UV-light-induced keratinocyte apoptosis and skin carcinogenesis. Cell Death Dis. 2017, 8, e2664. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Proteins involved in the antioxidant and inflammatory response in rutin-treated human skin fibroblasts exposed to UVA or UVB irradiation. J. Dermatol. Sci. 2018, 90, 241–252. [Google Scholar] [CrossRef]
- Bouriche, H.; Miles, E.A.; Selloum, L.; Calder, P.C. Effect of Cleome arabica leaf extract, rutin and quercetin on soybean lipoxygenase activity and on generation of inflammatory eicosanoids by human neutrophils. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 195–201. [Google Scholar] [CrossRef]
- Sunada, S.; Fujisawa, H.; Cartwright, I.M.; Maeda, J.; Brents, C.A.; Mizuno, K.; Aizawa, Y.; Kato, T.A.; Uesaka, M. Monoglucosyl rutin as a potential radioprotector in mammalian cells. Mol. Med. Rep. 2014, 10, 10–14. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domínguez, F.; García-Carrancá, A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch. Med. Res. 2013, 44, 346–351. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Si, Y.X.; Yin, S.J.; Oh, S.; Wang, Z.J.; Ye, S.; Yan, L.; Yang, J.M.; Park, Y.D.; Lee, J.; Qian, G.Y. An integrated study of tyrosinase inhibition by rutin: Progress using a computational simulation. J. Biomol. Struct. Dyn. 2012, 29, 999–1012. [Google Scholar] [CrossRef]
- Guo, R.; Wei, P.; Liu, W. Combined antioxidant effects of rutin and vitamin C in Triton X-100 micelles. J. Pharm. Biomed. Anal. 2007, 43, 1580–1586. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Sagun, K.C.; Cárcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar] [PubMed]
- Seo, A.; Kitagawa, N.; Matsuura, T.; Sato, H.; Inai, T. Formation of keratinocyte multilayers on filters under airlifted or submerged culture conditions in medium containing calcium, ascorbic acid, and keratinocyte growth factor. Histochem. Cell Biol. 2016, 146, 585–597. [Google Scholar] [CrossRef]
- Savini, I.; Rossi, A.; Duranti, G.; Avigliano, L.; Catani, M.V.; Melino, G. Characterization of keratinocyte differentiation induced by ascorbic acid: Protein kinase C involvement and vitamin C homeostasis. J. Invest. Dermatol. 2002, 118, 372–379. [Google Scholar] [CrossRef]
- Milde, J.; Elstner, E.F.; Grassmann, J. Synergistic inhibition of low-density lipoprotein oxidation by rutin, γ-terpinene, and ascorbic acid. Phytomedicine 2004, 11, 105–113. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef]
- Bissell, M.J. Architecture Is the Message: The role of extracellular matrix and 3-D structure in tissue-specific gene expression and breast cancer. Pezcoller Found. J. 2007, 16, 2–17. [Google Scholar]
- Ishii, K.; Furuta, T.; Kasuya, Y. Determination of rutin in human plasma by high-performance liquid chromatography utilizing solid-phase extraction and ultraviolet detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 161–168. [Google Scholar] [CrossRef]
- Serrano, G.; Almudéver, P.; Serrano, J.M.; Milara, J.; Torrens, A.; Expósito, I.; Cortijo, J. Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders. Clin. Cosmet. Invest. Dermatol. 2015, 8, 591–599. [Google Scholar]
- Kang, J.S.; Kim, H.N.; Kim, J.E.; Mun, G.H.; Kim, Y.S.; Cho, D.; Shin, D.H.; Hwang, Y.I.; Lee, W.J. Regulation of UVB-induced IL-8 and MCP-1 production in skin keratinocytes by increasing vitamin C uptake via the redistribution of SVCT-1 from the cytosol to the membrane. J. Invest. Dermatol. 2007, 127, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Njälsson, R.; Norgren, S. Physiological and pathological aspects of GSH metabolism. Acta Paediatr. 2005, 94, 132–137. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Ono, R.; Masaki, T.; Dien, S.; Yu, X.; Fukunaga, A.; Yodoi, J.; Nishigori, C. Suppressive effect of recombinant human thioredoxin on ultraviolet light-induced inflammation and apoptosis in murine skin. J. Dermatol. 2012, 39, 843–851. [Google Scholar] [CrossRef]
- Telorack, M.; Meyer, M.; Ingold, I.; Conrad, M.; Bloch, W.; Werner, S. A glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair. PLoS Genet. 2016, 12, e1005800. [Google Scholar] [CrossRef]
- Thongrakard, V.; Ruangrungsi, N.; Ekkapongpisit, M.; Isidoro, C.; Tencomnao, T. Protection from UVB toxicity in human keratinocytes by Thailand native herbs extracts. Photochem. Photobiol. 2014, 90, 214–224. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Spentzos, D.; Fountzilas, E.; Francoeur, N.; Sanisetty, S.; Grammatikos, A.P.; Hecht, J.L.; Cannistra, S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011, 71, 5081–5089. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Tarumoto, T.; Nagai, T.; Ohmine, K.; Miyoshi, T.; Nakamura, M.; Kondo, T.; Mitsugi, K.; Nakano, S.; Muroi, K.; Komatsu, N.; et al. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp. Hematol. 2004, 32, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Boeing, S.; Williamson, L.; Encheva, V.; Gori, I.; Saunders, R.E.; Instrell, R.; Aygün, O.; Rodriguez-Martinez, M.; Weems, J.C.; Kelly, G.P.; et al. Multiomic analysis of the UV-induced DNA damage response. Cell Rep. 2016, 15, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Purohit, N.K.; Robu, M.; Shah, R.G.; Geacintov, N.E.; Shah, G.M. Characterization of the interactions of PARP-1 with UV-damaged DNA in vivo and in vitro. Sci. Rep. 2016, 6, 19020. [Google Scholar] [CrossRef]
- Yoon, J.H.; Swiderski, P.M.; Kaplan, B.E.; Takao, M.; Yasui, A.; Shen, B.; Pfeifer, G.P. Processing of UV damage in vitro by FEN-1 proteins as part of an alternative DNA excision repair pathway. Biochemistry 1999, 38, 4809–4817. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Assefa, Z.; Van Laethem, A.; Garmyn, M.; Agostinis, P. Ultraviolet radiation-induced apoptosis in keratinocytes: On the role of cytosolic factors. Biochim. Biophys. Acta 2005, 1755, 90–106. [Google Scholar] [CrossRef]
- Shinozaki, K.; Hosokawa, Y.; Hazawa, M.; Kashiwakura, I.; Okumura, K.; Kaku, T.; Nakayama, E. Ascorbic acid enhances radiation-induced apoptosis in an HL60 human leukemia cell line. J. Radiat. Res. 2011, 52, 229–237. [Google Scholar] [CrossRef]
- Singh, S.; Rana, S.V.S. Ascorbic acid improves mitochondrial function in liver of arsenic-treated rat. Toxicol. Ind. Health 2010, 26, 265–272. [Google Scholar] [CrossRef]
- Catani, M.V.; Savini, I.; Rossi, A.; Melino, G.; Avigliano, L. Biological role of vitamin C in keratinocytes. Nutr. Rev. 2005, 63, 81–90. [Google Scholar] [CrossRef]
- Perk, A.A.; Shatynska-Mytsyk, I.; Gerçek, Y.C.; Boztaş, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 124–129. [Google Scholar] [CrossRef]
- Ma, J.Q.; Liu, C.M.; Yang, W. Protective effect of rutin against carbon tetrachloride-induced oxidative stress, inflammation and apoptosis in mouse kidney associated with the ceramide, MAPKs, p53 and calpain activities. Chem. Biol. Interact. 2018, 286, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, E.; Moloudizargari, M.; Asghari, M.H.; Abdollahi, M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin. Drug Metab. Toxicol. 2017, 13, 525–536. [Google Scholar] [CrossRef]
- Golden, T.; Swingle, M.; Honkanen, R.E. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008, 27, 169–178. [Google Scholar] [CrossRef] [PubMed]
- País, S.M.; Téllez-Iñón, M.T.; Capiati, D.A. Serine/threonine protein phosphatases type 2A and their roles in stress signaling. Plant. Signal. Behav. 2009, 4, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, H.G.; Yan, Q.; Deng, M.; Liu, J.; Doerge, S.; Ma, W.; Dong, Z.; Li, D.W. Protein phosphatase-2A is a target of epigallocatechin-3-gallate and modulates p53-Bak apoptotic pathway. Cancer Res. 2008, 68, 4150–4162. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sharma, S.; Hasan, M.; Pareek, A.; Singla-Pareek, S.; Sopory, S. Characteristic variations and similarities in biochemical, molecular, and functional properties of glyoxalases across prokaryotes and eukaryotes. Int. J. Mol. Sci. 2017, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, T.M.; Kettner, K.; Rödel, G.; Sträter, N. Regulatory function of hexokinase 2 in glucose signaling in Saccharomyces cerevisiae. J. Biol. Chem. 2016, 291, 16477. [Google Scholar] [CrossRef]
- Boesten, D.M.; von Ungern-Sternberg, S.N.; den Hartog, G.J.; Bast, A. Protective Pleiotropic Effect of Flavonoids on NAD. Oxid. Med. Cell Longev. 2015, 2015, 894597. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef]
- Yu, J.; Wei, W.; Danner, E.; Ashley, R.K.; Israelachvili, J.N.; Waite, J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011, 7, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Y.; Zhang, L. Chemical modification and antitumor activities of two polysaccharide-protein complexes from Pleurotus tuber-regium. Int. J. Biol. Macromol. 2009, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Synergistic Cytoprotective Effects of Rutin and Ascorbic Acid on the Proteomic Profile of 3D-Cultured Keratinocytes Exposed to UVA or UVB Radiation. Nutrients 2019, 11, 2672. https://doi.org/10.3390/nu11112672
Gęgotek A, Jarocka-Karpowicz I, Skrzydlewska E. Synergistic Cytoprotective Effects of Rutin and Ascorbic Acid on the Proteomic Profile of 3D-Cultured Keratinocytes Exposed to UVA or UVB Radiation. Nutrients. 2019; 11(11):2672. https://doi.org/10.3390/nu11112672
Chicago/Turabian StyleGęgotek, Agnieszka, Iwona Jarocka-Karpowicz, and Elżbieta Skrzydlewska. 2019. "Synergistic Cytoprotective Effects of Rutin and Ascorbic Acid on the Proteomic Profile of 3D-Cultured Keratinocytes Exposed to UVA or UVB Radiation" Nutrients 11, no. 11: 2672. https://doi.org/10.3390/nu11112672
APA StyleGęgotek, A., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Synergistic Cytoprotective Effects of Rutin and Ascorbic Acid on the Proteomic Profile of 3D-Cultured Keratinocytes Exposed to UVA or UVB Radiation. Nutrients, 11(11), 2672. https://doi.org/10.3390/nu11112672





