Obesity Affects β2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals
Abstract
:1. Introduction
2. Materials and Methods
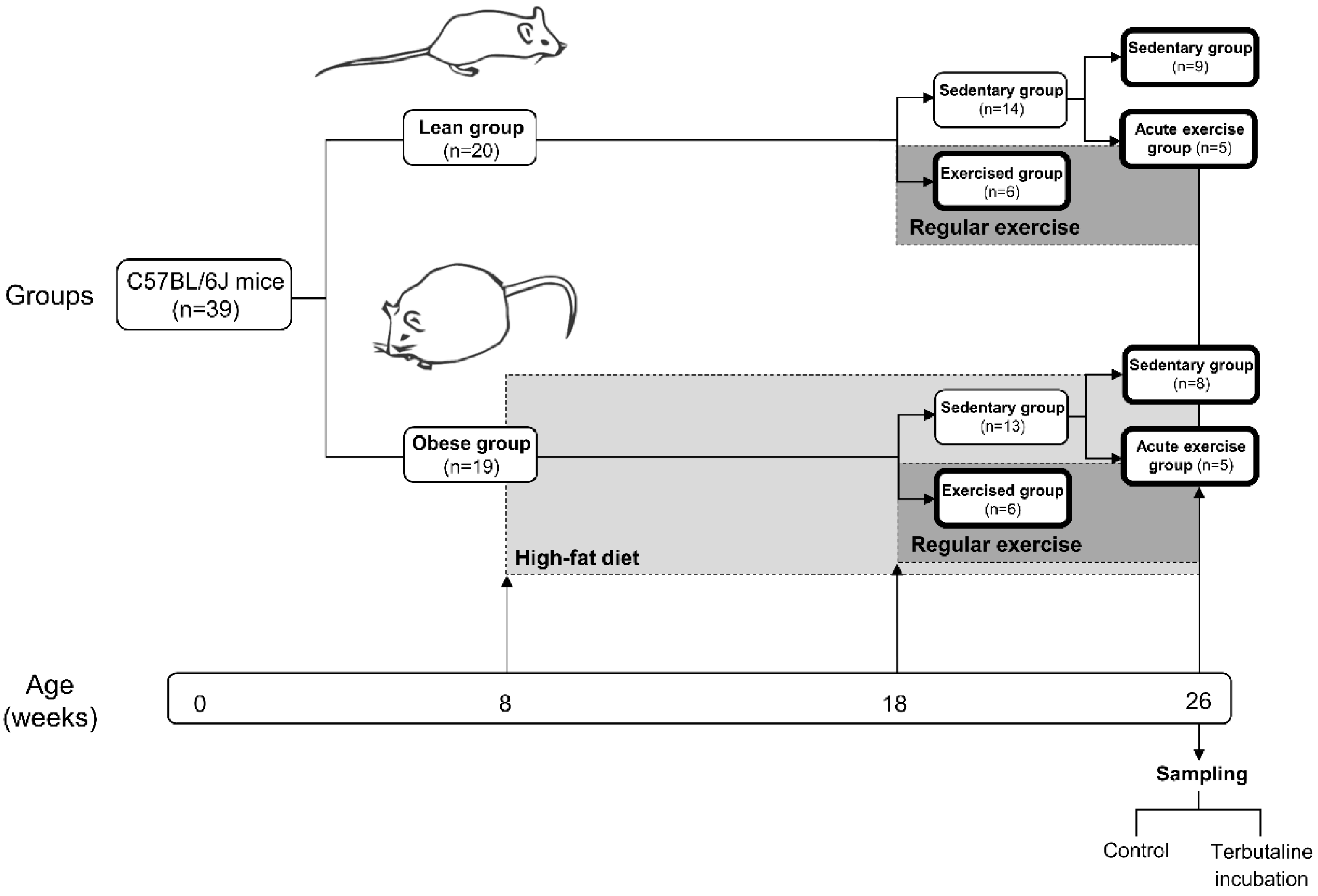
2.1. Animals and Experimental Design
2.2. Exercise Protocol
2.3. Anaesthesia and Whole Blood Collection
2.4. Fasting Glucose Levels and Lipid Profile Determination
2.5. Inflammatory Profile and Phenotype of Circulating Monocytes: Inflammatory Cytokines, Ly6C, Inducible Nitric Oxide Synthase, and Arginase-1 Expression Assays by Flow Cytometry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Weight Measurements, Dietary Intake, Fasting Blood Glucose, and Lipid Profile
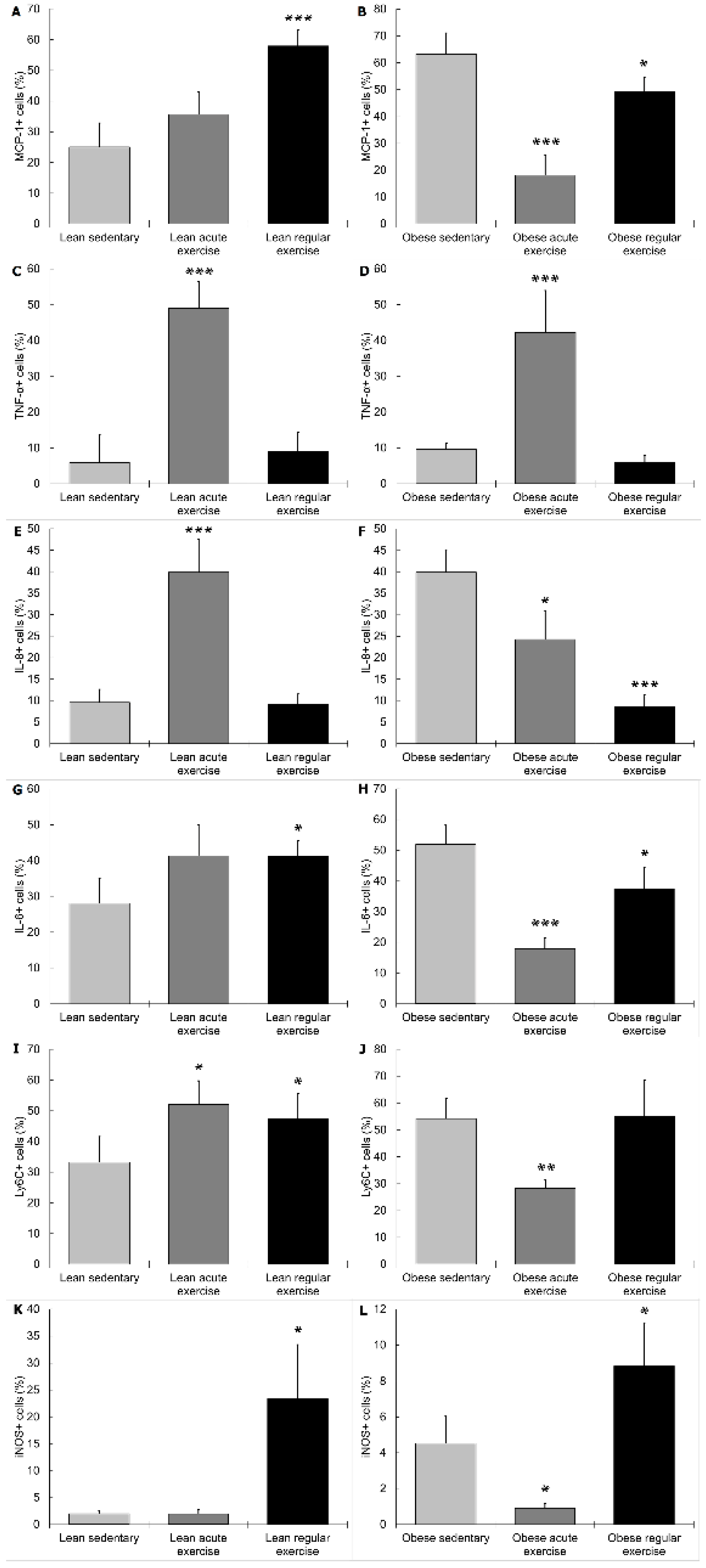
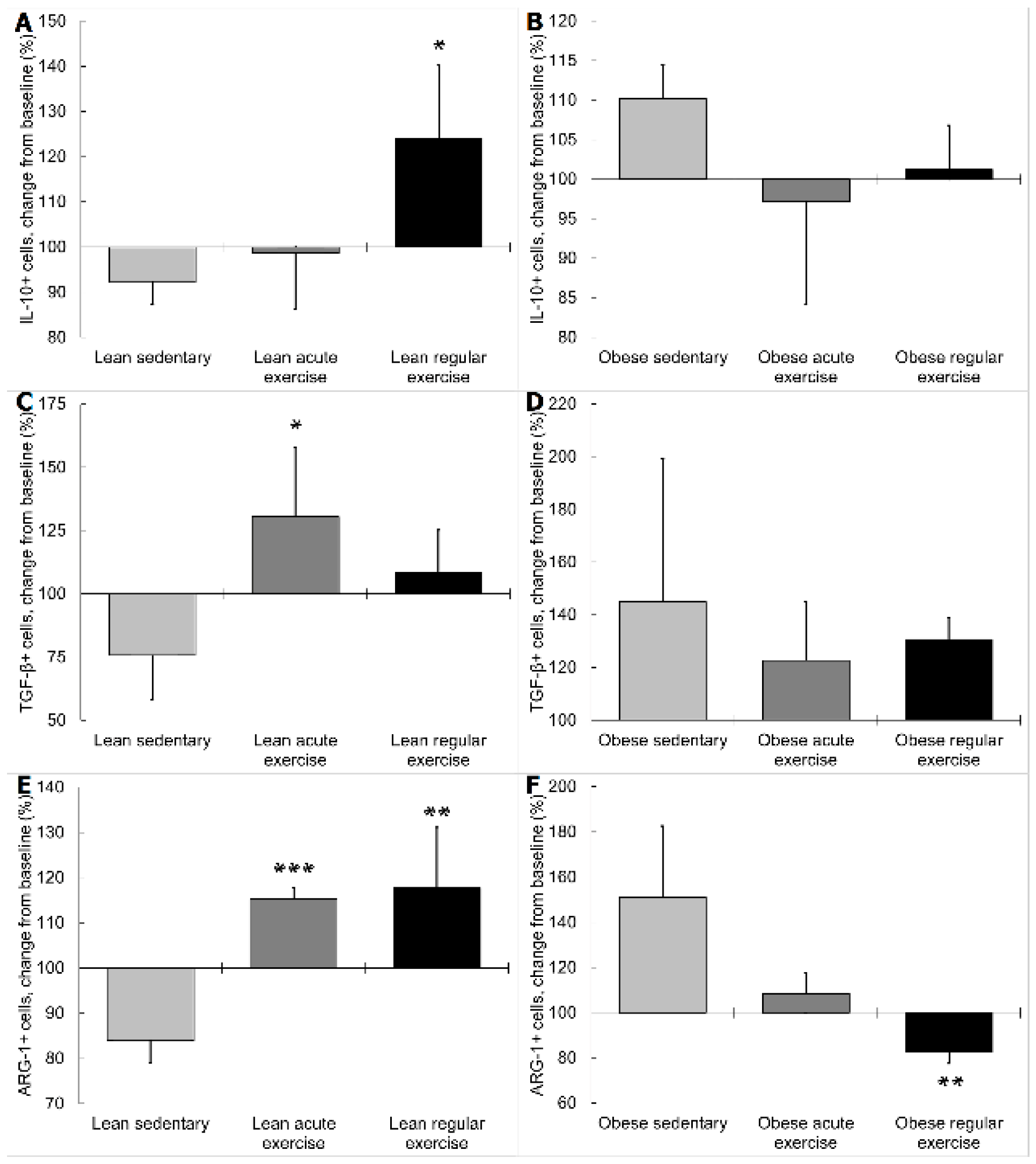
3.2. Influence of Obesity in the Effect of Exercise on the Inflammatory Profile and Phenotype of Circulating Monocytes
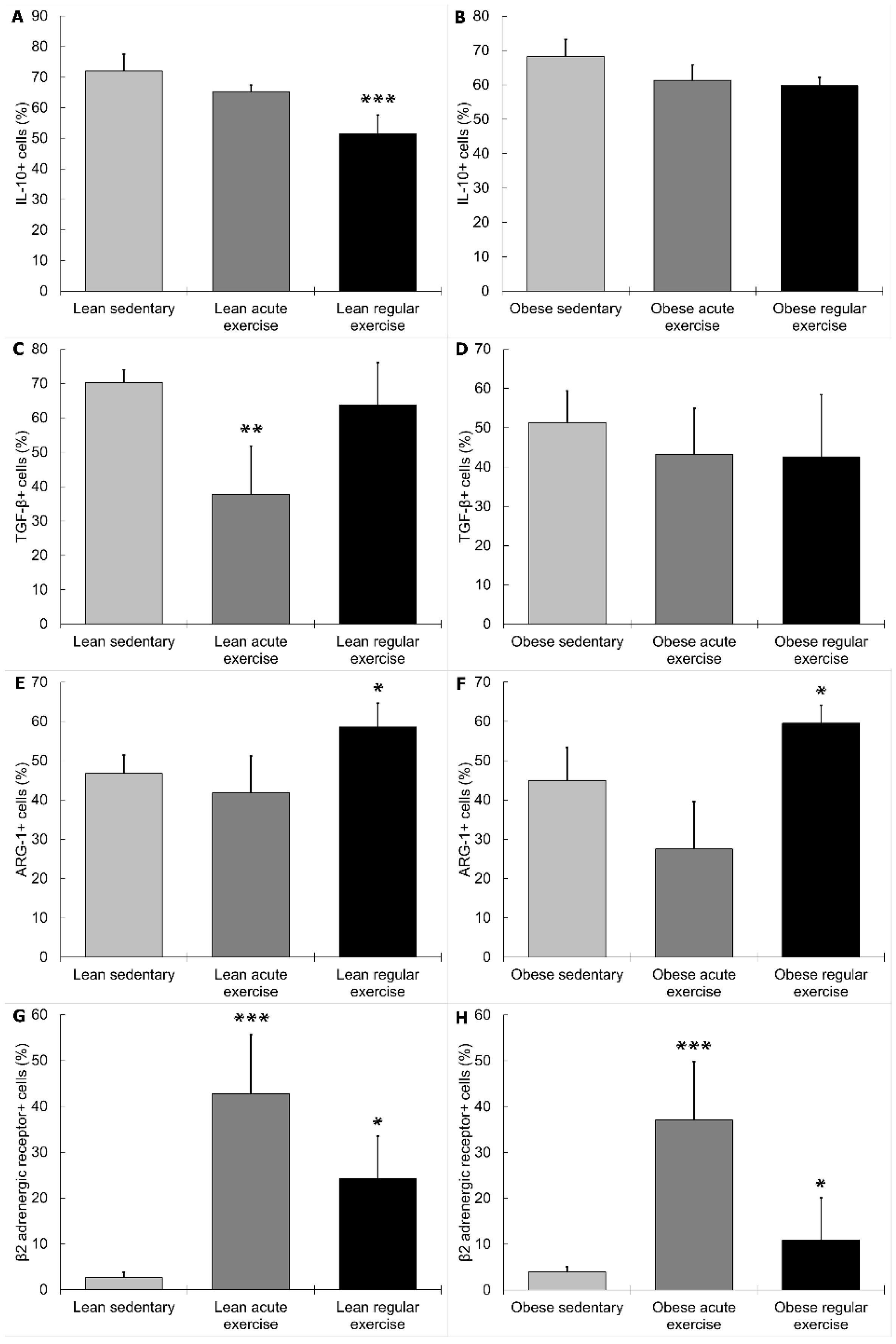
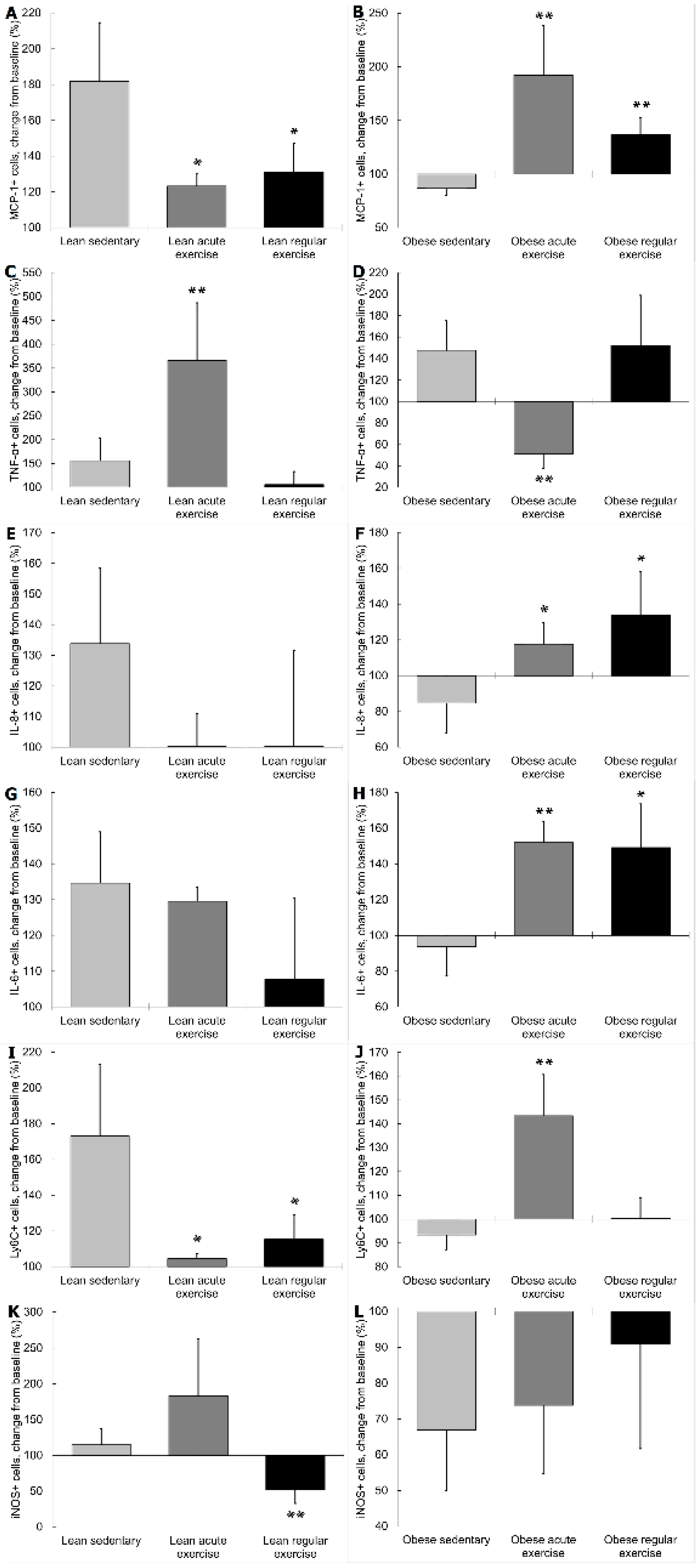
3.3. Influence of Obesity on the β2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vecchié, A.; Dallegri, F.; Carbone, F.; Bonaventura, A.; Liberale, L.; Portincasa, P.; Frühbeck, G.; Montecucco, F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018, 48, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharm. Econ. 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Is metabolic syndrome X an inflammatory condition? Exp. Biol. Med. 2002, 227, 989–997. [Google Scholar] [CrossRef]
- Das, U.N. Metabolic syndrome X: An inflammatory condition? Curr. Hypertens. Rep. 2004, 6, 66–73. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- Lumeng, C.N. Innate Immune Activation in Obesity. Mol. Asp. Med. 2013, 34, 12–29. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Neels, J.G.; Olefsky, J.M. Inflamed fat: What starts the fire? J. Clin. Investig. 2006, 116, 33–35. [Google Scholar] [CrossRef]
- Pasquali, R.; Vicennati, V.; Cacciari, M.; Pagotto, U. The Hypothalamic-Pituitary-Adrenal Axis Activity in Obesity and the Metabolic Syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 111–128. [Google Scholar] [CrossRef]
- Lambert, G.W.; Straznicky, N.E.; Lambert, E.A.; Dixon, J.B.; Schlaich, M.P. Sympathetic nervous activation in obesity and the metabolic syndrome—Causes, consequences and therapeutic implications. Pharm. Ther. 2010, 126, 159–172. [Google Scholar] [CrossRef]
- Ortega, E.; Martín-Cordero, L.; García-Roves, P.M.; Chicco, A.J.; González-Franquesa, A.; Marado, D. Diabetes Mellitus and Metabolic Syndrome. In Biomarkers of Cardiometabolic Risk, Inflammation and Disease; Palavra, F., Reis, F., Marado, D., Sena, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 55–80. [Google Scholar]
- Martín-Cordero, L.; García, J.J.; Hinchado, M.D.; Ortega, E. The interleukin-6 and noradrenaline mediated inflammation-stress feedback mechanism is dysregulated in metabolic syndrome: Effect of exercise. Cardiovasc. Diabetol. 2011, 10, 42. [Google Scholar] [CrossRef]
- Martín-Cordero, L.; García, J.J.; Ortega, E. Noradrenaline-mediated inhibition of inflammatory cytokines is altered in macrophages from obese Zucker rats: Effect of habitual exercise. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 234–239. [Google Scholar] [CrossRef]
- Ortega, E.; García, J.J.; De la Fuente, M. Modulation of adherence and chemotaxis of macrophages by norepinephrine. Influence of ageing. Mol. Cell. Biochem. 2000, 203, 113–117. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef]
- García, J.J.; Sáez, M.D.C.; De La Fuente, M.; Ortega, E. Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol. Cell. Biochem. 2003, 254, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; Del Rey, A. Physiology of psychoneuroimmunology: A personal view. Brain Behav. Immun. 2007, 21, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014, 182, 15–41. [Google Scholar] [CrossRef]
- Ortega, E.; Galvez, I.; Martin-Cordero, L. Adrenergic Regulation of Macrophage-Mediated Innate/Inflammatory Responses in Obesity and Exercise in this Condition: Role of B2 Adrenergic Receptors. Endocr. Metab. Immune Disord. Drug Targets 2019, 19. [Google Scholar] [CrossRef]
- Landmann, R. Beta-adrenergic receptors in human leukocyte subpopulations. Eur. J. Clin. Investig. 1992, 22, 30–36. [Google Scholar]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharm. Rev. 2000, 52, 595–638. [Google Scholar]
- Sanders, V.M.; Kavelaars, A. Adrenergic Regulation of Immunity. In Psychoneuroimmunology; Ader, R., Ed.; Academic Press: Amsterdam, The Netherlands, 2007; pp. 63–84. [Google Scholar]
- Kohm, A.P.; Sanders, V.M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharm. Rev. 2001, 53, 487–525. [Google Scholar]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Padro, C.J.; Sanders, V.M. Neuroendocrine regulation of inflammation. Semin. Immunol. 2014, 26, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharm. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Iezzoni, D.G.; Daly, A.; Harris, A.G.; Chrousos, G.P. Cytokine Dysregulation, Inflammation and Well-Being. Neuroimmunomodulation 2005, 12, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Álvarez-Barrientos, A.; Ortega, E. Anti-inflammatory effect of β2 adrenergic stimulation on circulating monocytes with a pro-inflammatory state in high-fat diet-induced obesity. Brain Behav. Immun. 2019, 80, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Dimitrov, S.; Pruitt, C.; Shaikh, F.; Beg, N. Benefit of physical fitness against inflammation in obesity: Role of beta adrenergic receptors. Brain Behav. Immun. 2014, 39, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Hulteng, E.; Hong, S. Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via β (2)-adrenergic activation. Brain Behav. Immun. 2017, 61, 60–68. [Google Scholar] [CrossRef]
- Ortega, E. The “bioregulatory effect of exercise” on the innate/inflammatory responses. J. Physiol. Biochem. 2016, 72, 361–369. [Google Scholar] [CrossRef]
- Kim, T.-W.; Choi, H.-H.; Chung, Y.-R. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J. Exerc. Rehabil. 2016, 12, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, J.M.; Heiss, V.J.; Maurya, S.K.; Kalyanasundaram, A.; Periasamy, M.; LaFountain, R.A.; Wilson, J.M.; Simonetti, O.P.; Ziouzenkova, O. Graded Maximal Exercise Testing to Assess Mouse Cardio-Metabolic Phenotypes. PLoS ONE 2016, 11, e0148010. [Google Scholar] [CrossRef]
- Ortega, E. Physiology and biochemistry: Influence of exercise on phagocytosis. Int. J. Sports Med. 1994, 3, 172–178. [Google Scholar]
- Ortega, E. Neuroendocrine mediators in the modulation of phagocytosis by exercise: Physiological implications. Exerc. Immunol. Rev. 2003, 9, 70–93. [Google Scholar]
- Martin-Cordero, L.; Garcia, J.J.; Giraldo, E.; De la Fuente, M.; Manso, R.; Ortega, E. Influence of exercise on the circulating levels and macrophage production of IL-1beta and IFNgamma affected by metabolic syndrome: An obese Zucker rat experimental animal model. Eur. J. Appl. Physiol. 2009, 107, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Kraemer, W.J.; Mastro, A.M.; Denegar, C.R.; Volek, J.S.; Häkkinen, K.; Anderson, J.M.; Lee, E.C.; Maresh, C.M. Leukocyte β2-Adrenergic Receptor Expression in Response to Resistance Exercise. Med. Sci. Sports Exerc. 2011, 43, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Burman, K.D.; Ferguson, E.W.; Djuh, Y.-Y.; Wartofsky, L.; Latham, K. Beta receptors in peripheral mononuclear cells increase acutely during exercise. Eur. J. Endocrinol. 1985, 109, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Landmann, R.; Portenier, M.; Staehelin, M.; Wesp, M.; Box, R. Changes in beta-adrenoceptors and leucocyte subpopulations after physical exercise in normal subjects. Naunyn Schmiedeberg’s Arch. Pharm. 1988, 337, 261–266. [Google Scholar] [CrossRef]
- Ratge, D.; Wiedemann, A.; Kohse, K.P.; Wisser, H. Alterations of beta-adrenoceptors on human leukocyte subsets induced by dynamic exercise: Effect of prednisone. Clin. Exp. Pharm. Physiol. 1988, 15, 43–53. [Google Scholar] [CrossRef]
- Ortega, E.; Giraldo, E.; Hinchado, M.D.; Martín, L.; García, J.J.; De La Fuente, M. Neuroimmunomodulation during Exercise: Role of Catecholamines as ‘Stress Mediator’ and/or ‘Danger Signal’ for the Innate Immune Response. Neuroimmunomodulation 2007, 14, 206–212. [Google Scholar] [CrossRef]
- Johansson, L.H. Factors behind the functional beta 2-adrenoceptor selectivity of terbutaline. Pharm. Toxicol. 1995, 77, 21–24. [Google Scholar] [CrossRef]
- Keränen, T.; Hömmö, T.; Hämäläinen, M.; Moilanen, E.; Korhonen, R. Anti-Inflammatory Effects of β2-Receptor Agonists Salbutamol and Terbutaline Are Mediated by MKP. PLoS ONE 2016, 11, e0148144. [Google Scholar] [CrossRef]
- Ortega, E.; Bote, M.E.; Giraldo, E.; García, J.J. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand. J. Med. Sci. Sports 2012, 22, 104–112. [Google Scholar] [CrossRef]
- De La Fuente, M.; Cruces, J.; Hernandez, O.; Ortega, E. Strategies to improve the functions and redox state of the immune system in aged subjects. Curr. Pharm. Des. 2011, 17, 3966–3993. [Google Scholar] [CrossRef]
- Noh, H.; Yu, M.R.; Kim, H.J.; Lee, J.H.; Park, B.-W.; Wu, I.-H.; Matsumoto, M.; King, G.L. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 2017, 92, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lean | Obese | |||||
|---|---|---|---|---|---|---|
| Sedentary | Acute Exercise | Regular Exercise | Sedentary | Acute Exercise | Regular Exercise | |
| Body weight (g) | 29.28 ± 1.17 | 30.15 ± 2.49 | 25.7 ± 1.27 | 42.28 ± 1.15 *** | 40.37 ± 2.93 | 36.14 ± 2.98 ● |
| Dietary intake (g/day) | 3.96 ± 0.22 | 4.16 ± 0.09 | 4.05 ± 0.06 | 2.68 ± 0.11 *** | 2.62 ± 0.08 | 2.49 ± 0.03 ●● |
| Energy intake (kJ/day) | 55.32 ± 3.15 | 58.16 ± 1.38 | 56.63 ± 0.86 | 62.01 ± 2.67 *** | 60.50 ± 1.90 | 57.63 ± 0.71 ●● |
| Glucose (mg/dL) | 218.90 ± 13.26 | 174.45 ± 32.06 ● | 196.37 ± 25.53 | 311.50 ± 30.93 ** | 222.75 ± 23.12 ● | 282.5 ± 27.85 |
| Cholesterol (mg/dL) | ||||||
| Total cholesterol HDL-C cLDL-C | 103.69 ± 2.22 42.15 ± 2.93 50.75 ± 3.49 | <99 † | 106.75 ± 2.90 51.75 ± 3.91 ● 39.4 ± 1.74 ● | 172.70 ± 19.28 *** 59.70 ± 5.69 ** 88.83 ± 16.05 * | 175 ± 41.82 55.5 ± 2.72 78 ± 12 | 178.12 ± 24.68 75.25 ± 4.39 ● 38.5 ± 1.5 ● |
| Triglycerides (mg/dL) | 86.80 ± 1.86 | 88 ± 0.01 | 76.62 ± 0.73 ● | 91.55 ± 1.99 * | 98.75 ± 7 | 80 ± 1.43 ●●● |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Álvarez-Barrientos, A.; Ortega, E. Obesity Affects β2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals. Nutrients 2019, 11, 2630. https://doi.org/10.3390/nu11112630
Gálvez I, Martín-Cordero L, Hinchado MD, Álvarez-Barrientos A, Ortega E. Obesity Affects β2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals. Nutrients. 2019; 11(11):2630. https://doi.org/10.3390/nu11112630
Chicago/Turabian StyleGálvez, Isabel, Leticia Martín-Cordero, María Dolores Hinchado, Alberto Álvarez-Barrientos, and Eduardo Ortega. 2019. "Obesity Affects β2 Adrenergic Regulation of the Inflammatory Profile and Phenotype of Circulating Monocytes from Exercised Animals" Nutrients 11, no. 11: 2630. https://doi.org/10.3390/nu11112630






