Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Lifestyle Factors
2.3. Lifestyle Factors
2.4. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Associations Between Green Tea Intake and Risk of CRC by Anatomical Site
3.3. Associations Between Lifestyle Factors and Risk of CRC by Anatomical Site
3.4. Protective Effect of Green Tea Consumption and Health-Related Factors on CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxley, R.R.; Ansary-Moghaddam, A.; Clifton, P.; Czernichow, S.; Parr, C.L.; Woodward, M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: A quantitative overview of the epidemiological evidence. Int. J. Cancer 2009, 125, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Kim, K.Z.; Jung, K.W.; Park, S.; Won, Y.J.; Kim, J.; Kim, D.Y.; Oh, J.H. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res. Treat. 2012, 44, 219–226. [Google Scholar] [CrossRef]
- Shin, H.R.; Jung, K.W.; Won, Y.J.; Kong, H.J.; Yim, S.H.; Sung, J.; Seo, S.W.; Kim, K.Y.; Lee, S.Y.; Kong, I.S.; et al. National cancer incidence for the year 2002 in Korea. Cancer Res. Treat. 2007, 39, 139–149. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef]
- Lee, H.S.; Duffey, K.J.; Popkin, B.M. South Koreas entry to the global food economy: Shifts in consumption of food between 1998 and 2009. Asia Pac. J. Clin. Nutr. 2012, 21, 618–629. [Google Scholar]
- Hao, X.; Xiao, H.; Ju, J.; Lee, M.J.; Lambert, J.D.; Yang, C.S. Green tea polyphenols inhibit colorectal tumorigenesis in azoxymethane-treated F344 rats. Nutr. Cancer 2017, 69, 623–631. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y. Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Somashekar, R.; Meng, X.Q.; Gopalsamy, G.; Bambaca, L.; McKinnon, R.A.; Young, G.P. Supplementation with Brazil nuts and green tea extract regulates targeted biomarkers related to colorectal cancer risk in humans. Br. J. Nutr. 2016, 116, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Du, M.; Chu, H.; Zhu, L.; Tong, N.; Zhang, Z.; Wang, M.; Gu, D.; Chen, J. An inverse association between tea consumption and colorectal cancer risk. Oncotarget 2017, 8, 37367. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Shu, X.-O.; Li, H.-L.; Yang, G.; Ji, B.-T.; Xiang, Y.-B.; Cai, H.; Chow, W.-H.; Gao, Y.-T.; Zheng, W. Prospective cohort study of tea consumption and risk of digestive system cancers: Results from the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2012, 96, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; De Dauwe, P.; Boyle, T.; Tabatabaei, S.M.; Fritschi, L.; Heyworth, J.S. Tea, coffee, and milk consumption and colorectal cancer risk. J. Epidemiol. 2014, 24, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition, Physical Activity and Colorectal Cancer. Available online: https://www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf (accessed on 3 September 2019).
- Liang, P.S.; Chen, T.Y.; Giovannucci, E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2406–2415. [Google Scholar] [CrossRef]
- Romieu, I.; Straif, K.; Jenab, M.; Fedirko, V.; Islami, F.; Negri, E.; Tramacere, I.; La Vecchia, C.; Scotti, L.; Bagnardi, V.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar]
- Cho, Y.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Genetic Risk Score, Combined Lifestyle Factors and Risk of Colorectal Cancer. Cancer Res. Treat. 2019, 51, 1033. [Google Scholar] [CrossRef]
- Yang, G.; Shu, X.-O.; Li, H.; Chow, W.-H.; Ji, B.-T.; Zhang, X.; Gao, Y.-T.; Zheng, W. Prospective cohort study of green tea consumption and colorectal cancer risk in women. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1219–1223. [Google Scholar] [CrossRef]
- Gunathilake, M.N.; Lee, J.; Cho, Y.A.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Interaction between physical activity, PITX1 rs647161 genetic polymorphism and colorectal cancer risk in a Korean population: A case-control study. Oncotarget 2018, 9, 7590–7603. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.; Oh, J.; Shin, A.; Kim, J. Dietary inflammatory index and risk of colorectal cancer: A case-control study in Korea. Nutrients 2016, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.T.; Chow, W.H.; Hsing, A.W.; McLaughlin, J.K.; Dai, Q.; Gao, Y.T.; Blot, W.J.; Fraumeni J.F., Jr. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer 1997, 70, 255–258. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef]
- Morris, J.; Moseley, V.R.; Cabang, A.B.; Coleman, K.; Wei, W.; Garrett-Mayer, E.; Wargovich, M.J. Reduction in promotor methylation utilizing EGCG (epigallocatechin-3-gallate) restores RXRα expression in human colon cancer cells. Oncotarget 2016, 7, 35313. [Google Scholar] [CrossRef]
- Zhang, X.; Albanes, D.; Beeson, W.L.; Van Den Brandt, P.A.; Buring, J.E.; Flood, A.; Freudenheim, J.L.; Giovannucci, E.L.; Goldbohm, R.A.; Jaceldo-Siegl, K. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: Pooled analysis of prospective cohort studies. J. Natl. Cancer Inst. 2010, 102, 771–783. [Google Scholar] [CrossRef]
- Shin, A.; Joo, J.; Yang, H.-R.; Bak, J.; Park, Y.; Kim, J.; Oh, J.H.; Nam, B.-H. Risk prediction model for colorectal cancer: National Health Insurance Corporation study, Korea. PLoS ONE 2014, 9, e88079. [Google Scholar] [CrossRef]
- Driver, J.A.; Gaziano, J.M.; Gelber, R.P.; Lee, I.-M.; Buring, J.E.; Kurth, T. Development of a risk score for colorectal cancer in men. Am. J. Med. 2007, 120, 257–263. [Google Scholar] [CrossRef]
- Wei, E.K.; Colditz, G.A.; Giovannucci, E.L.; Fuchs, C.S.; Rosner, B.A. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses’ Health Study. Am. J. Epidemiol. 2009, 170, 863–872. [Google Scholar] [CrossRef]
- Serafini, M.; Villano, D.; Spera, G.; Pellegrini, N. Redox molecules and cancer prevention: The importance of understanding the role of the antioxidant network. Nutr. Cancer 2006, 56, 232–240. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Daniel, C.R.; Graubard, B.I.; Wu, J.W.; Hollenbeck, A.R.; Gunter, M.J.; Park, Y.; Freedman, N.D. Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. Am. J. Clin. Nutr. 2012, 96, 374–381. [Google Scholar] [CrossRef] [PubMed]
- van den Brandt, P.A. Coffee or Tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women. Eur. J. Epidemiol. 2018, 33, 183–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; He, B.C.; Yan, L.J.; Liu, F.P.; Huang, J.F.; Hu, Z.J.; Lin, Z.; Zheng, X.Y.; Lin, L.S.; Zhang, Z.F.; et al. Tea consumption and its interactions with tobacco smoking and alcohol drinking on oral cancer in southeast China. Eur. J. Clin. Nutr. 2017, 71, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette smoking and variations in systemic immune and inflammation markers. J. Natl. Cancer Inst. 2014, 106, dju294. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X. Green tea liquid consumption alters the human intestinal and oral microbiome. Mol. Nutr. Food Res. 2018, 62, 1800178. [Google Scholar] [CrossRef]
- Ward, R.E.; Benninghoff, A.D.; Healy, B.J.; Li, M.; Vagu, B.; Hintze, K.J. Consumption of the total Western diet differentially affects the response to green tea in rodent models of chronic disease compared to the AIN93G diet. Mol. Nutr. Food Res. 2017, 61, 1600720. [Google Scholar] [CrossRef]
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Inflammatory Dietary Pattern, IL-17F Genetic Variant, and the Risk of Colorectal Cancer. Nutrients 2018, 10, 724. [Google Scholar] [CrossRef]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Shin, A.; Jung, H.S.; Oh, J.H.; Kim, J. Effects of interactions between common genetic variants and smoking on colorectal cancer. BMC Cancer 2017, 17, 869. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Rennert, G.; Cuadras, D.; Salazar, R.; Cordero, D.; Rennert, H.S.; Lejbkowicz, F.; Kopelovich, L.; Lipkin, S.M.; Gruber, S.B. Polymorphisms in alcohol metabolism genes ADH1B and ALDH2, alcohol consumption and colorectal cancer. PLoS ONE 2013, 8, e80158. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B. Are there two sides to colorectal cancer. Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmstrom, M.; Koskensalo, S.; Bohling, T.; Rautelin, H.; et al. Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms. Dig. Dis. Sci. 2018, 63, 2950–2958. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Controls (n = 1820) | Cases (n = 922) | p-Value |
|---|---|---|---|
| Age (years) | 56.1 ± 9.1 | 56.6 ± 9.7 | 0.18 1 |
| Sex (men) | 1235 (67.9) | 624 (67.7) | 0.93 2 |
| BMI (kg/m2) | |||
| <25 | 1210 (66.5) | 639 (69.3) | 0.14 2 |
| ≥25 | 610 (33.5) | 283 (30.7) | |
| Prior BMI (kg/m2) 3 | |||
| <25 | 1239 (68.1) | 593 (64.3) | 0.048 2 |
| ≥25 | 581 (31.9) | 329 (35.7) | |
| Family history of CRC (yes) 4 | 96 (5.3) | 86 (9.3) | <0.001 2 |
| Education (years) | |||
| <12 | 852 (47.9) | 690 (74.8) | <0.001 2 |
| ≥12 | 926 (52.1) | 232 (25.2) | |
| Regular exercise (yes) | 1030 (58.1) | 310 (33.6) | <0.001 2 |
| Alcohol consumption | |||
| Never-drinker | 552 (30.3) | 279 (30.3) | 0.97 2 |
| Ever-drinker | 1268 (69.7) | 643 (69.7) | |
| Smoking status | |||
| Never-smoker | 801 (44.0) | 408 (44.3) | 0.91 2 |
| Ever-smoker | 1019 (56.0) | 514 (55.7) | |
| Total energy intake (kcal/d) | 1701.5 ± 554.6 | 2027.6 ± 533.0 | <0.001 2 |
| DII 5 | 2.7 ± 1.2 | 2.9 ± 1.3 | 0.001 1 |
| Green tea consumption (g/d) 5 | 65.8 ± 169.1 | 22.6 ± 75.7 | <0.001 1 |
| Green Tea Intake (g/d) | No. of Controls | No. of Cases | Crude OR (95% CI) | Multivariate OR (95% CI) 1 |
|---|---|---|---|---|
| CRC | ||||
| T1 (≤0.01) | 605 | 368 | 1.0 (ref) | 1.0 (ref) |
| T2 (0.02–25.49) | 607 | 378 | 1.02 (0.85–1.23) | 1.29 (1.02–1.63) |
| T3 (≥25.50) | 608 | 176 | 0.48 (0.39–0.59) | 0.59 (0.46–0.76) |
| p for trend | <0.001 | <0.001 | ||
| Colon cancer | ||||
| T1 (≤0.01) | 605 | 179 | 1.0 (ref) | 1.0 (ref) |
| T2 (0.02–21.28) | 607 | 178 | 0.99 (0.78–1.25) | 1.09 (0.81–1.47) |
| T3 (≥21.29) | 608 | 105 | 0.51 (0.39–0.66) | 0.56 (0.41–0.78) |
| p for trend | <0.001 | <0.001 | ||
| Rectal cancer | ||||
| T1 (≤0.01) | 605 | 174 | 1.0 (ref) | 1.0 (ref) |
| T2 (0.02–21.51) | 607 | 176 | 1.01 (0.79–1.28) | 1.35 (1.00–1.82) |
| T3 (≥21.52) | 608 | 94 | 0.47 (0.36–0.62) | 0.60 (0.43–0.84) |
| p for trend | <0.001 | <0.001 | ||
| Categories of Lifestyle Factors | No. of Controls | CRC | Colon Cancer | Rectal Cancer | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Crude OR (95% CI) | p-Value | Multivariate OR (95% CI) 1 | p-Value | No. of Cases | Crude OR (95% CI) | p-Value | Multivariate OR (95% CI) 1 | p-Value | No. of Cases | Crude OR (95% CI) | p-Value | Multivariate OR (95% CI) 1 | p-Value | ||
| Prior BMI | ||||||||||||||||
| ≥25 kg/m2 | 581 | 329 | 1.0 (ref) | 0.048 | 1.0 (ref) | 0.02 | 190 | 1.0 (ref) | <0.001 | 1.0 (ref) | <0.001 | 137 | 1.0 (ref) | 0.66 | 1.0 (ref) | 0.84 |
| <25 kg/m2 | 1239 | 593 | 0.85 (0.72–1.00) | 0.78 (0.63–0.97) | 272 | 0.67 (0.54–0.83) | 0.60 (0.46–0.78) | 307 | 1.05 (0.84–1.32) | 0.97 (0.74–1.29) | ||||||
| Physical activity | ||||||||||||||||
| No | 790 | 612 | 1.0 (ref) | <0.001 | 1.0 (ref) | <0.001 | 299 | 1.0 (ref) | <0.001 | 1.0 (ref) | <0.001 | 301 | 1.0 (ref) | <0.001 | 1.0 (ref) | <0.001 |
| Yes | 1030 | 310 | 0.39 (0.33–0.46) | 0.47 (0.38–0.57) | 163 | 0.42 (0.34–0.52) | 0.51 (0.39–0.66) | 143 | 0.36 (0.29–0.45) | 0.43 (0.33–0.56) | ||||||
| Smoking | ||||||||||||||||
| Ever | 1019 | 514 | 1.0 (ref) | 0.9 | 1.0 (ref) | 0.56 | 238 | 1.0 (ref) | 0.08 | 1.0 (ref) | 0.07 | 266 | 1.0 (ref) | 0.14 | 1.0 (ref) | 0.41 |
| Never | 801 | 408 | 1.01 (0.86–1.18) | 1.07 (0.86–1.33) | 224 | 1.20 (0.98–1.47) | 1.30 (0.98–1.71) | 178 | 0.85 (0.67–1.05) | 0.89 (0.67–1.18) | ||||||
| Alcohol drinking | ||||||||||||||||
| Ever | 1268 | 643 | 1.0 (ref) | 0.97 | 1.0 (ref) | 0.08 | 318 | 1.0 (ref) | 0.73 | 1.0 (ref) | 0.16 | 315 | 1.0 (ref) | 0.6 | 1.0 (ref) | 0.29 |
| Never | 552 | 279 | 1.00 (0.84–1.18) | 0.81 (0.64–1.03) | 144 | 1.04 (0.83–1.30) | 0.81 (0.60–1.09) | 129 | 0.94 (0.75–1.18) | 0.85 (0.62–1.15) | ||||||
| DII | ||||||||||||||||
| High | 682 | 390 | 1.0 (ref) | 0.012 | 1.0 (ref) | 0.14 | 189 | 1.0 (ref) | 0.2 | 1.0 (ref) | 0.49 | 193 | 1.0 (ref) | 0.015 | 1.0 (ref) | 0.20 |
| Low | 681 | 308 | 0.79 (0.66–0.95) | 0.86 (0.70–1.05) | 162 | 0.86 (0.68–1.09) | 0.91 (0.71–1.18) | 143 | 0.74 (0.58–0.94) | 0.84 (0.65–1.10) | ||||||
| Anatomical Sites | Lifestyle Factors | Categories | Green Tea Intake (g/day) | p-Value 1 | ||
|---|---|---|---|---|---|---|
| T1 (≤0.01) | T2 (0.02–25.49) | T3 (≥25.50) | ||||
| CRC | Prior BMI | ≥25 kg/m2 | 1.0 (ref) | 1.16 (0.77–1.74) | 0.45 (0.28–0.71) | 0.36 |
| <25 kg/m2 | 0.67 (0.48–0.93) | 1.17 (0.72–1.92) | 1.49 (0.86–2.58) | |||
| Physical activity | No | 1.0 (ref) | 1.34 (0.99–1.81) | 0.59 (0.43–0.82) | 0.92 | |
| Yes | 0.48 (0.35–0.67) | 0.91 (0.56–1.45) | 0.97 (0.58–1.63) | |||
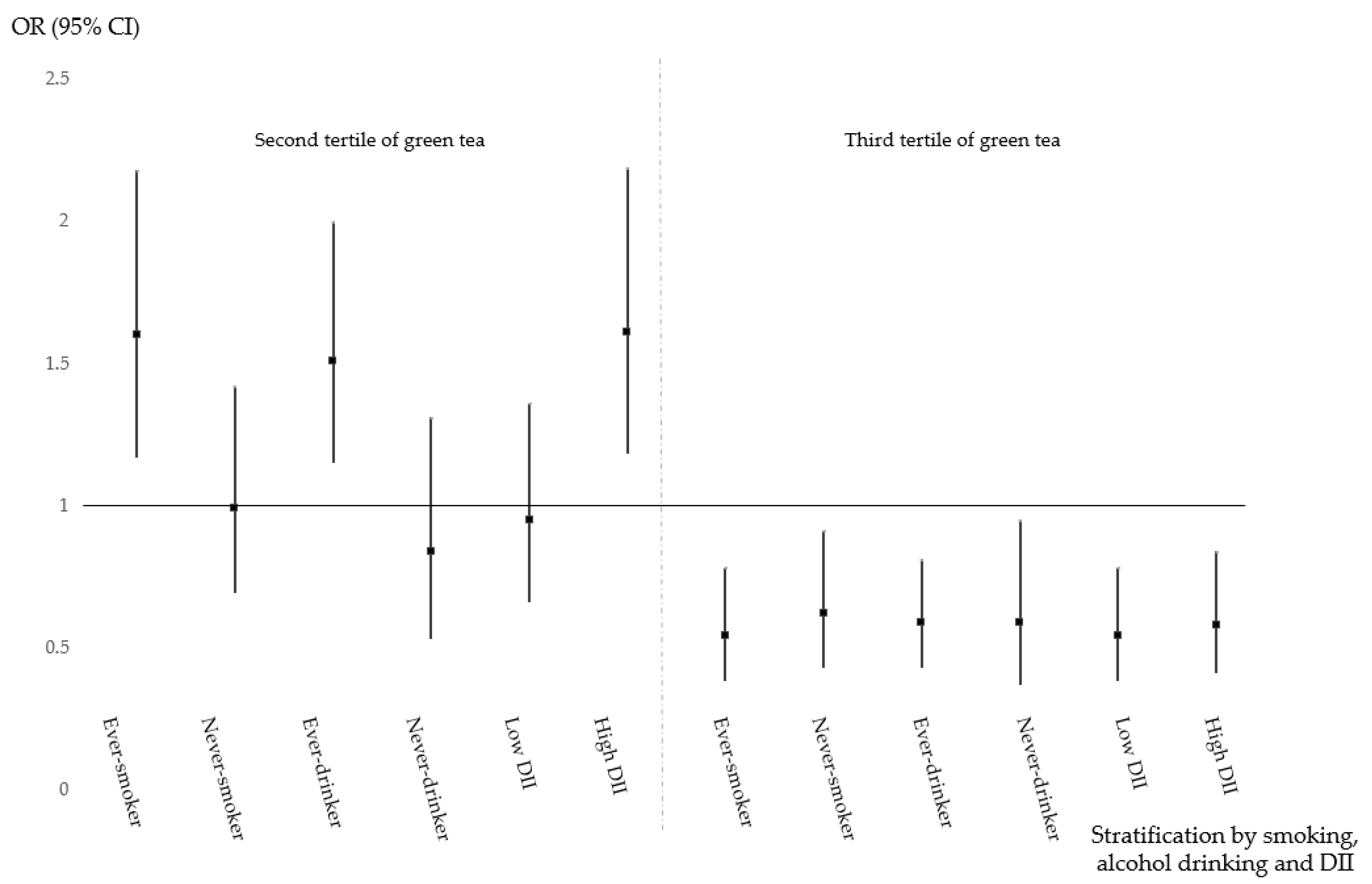
| Smoking | Ever | 1.0 (ref) | 1.59 (1.17–2.17) | 0.55 (0.38–0.78) | 0.041 | |
| Never | 1.23 (0.88–1.72) | 0.61 (0.38–0.78) | 1.12 (0.67–1.87) | |||
| Alcohol drinking | Ever | 1.0 (ref) | 1.58 (1.19–2.08) | 0.61 (0.45–0.84) | 0.024 | |
| Never | 1.07 (0.75–1.52) | 0.51 (0.31–0.84) | 0.89 (0.52–1.53) | |||
| DII | High (≥2.73) | 1.0 (ref) | 1.65 (1.21–2.25) | 0.60 (0.41–0.85) | 0.06 | |
| Low (<2.73) | 1.05 (0.76–1.46) | 0.58 (0.36–0.92) | 0.90 (0.54–1.51) | |||
| Colon cancer | Prior BMI | ≥25 kg/m2 | 1.0 (ref) | 1.07 (0.66–1.73) | 0.48 (0.28–0.81) | 0.76 |
| <25 kg/m2 | 0.53 (0.35–0.79) | 1.21 (0.66–2.20) | 1.24 (0.63–2.42) | |||
| Physical activity | No | 1.0 (ref) | 1.34 (0.93–1.95) | 0.58 (0.38–0.88) | 0.63 | |
| Yes | 0.58 (0.39–0.88) | 0.75 (0.41–1.35) | 0.85 (0.43–1.65) | |||
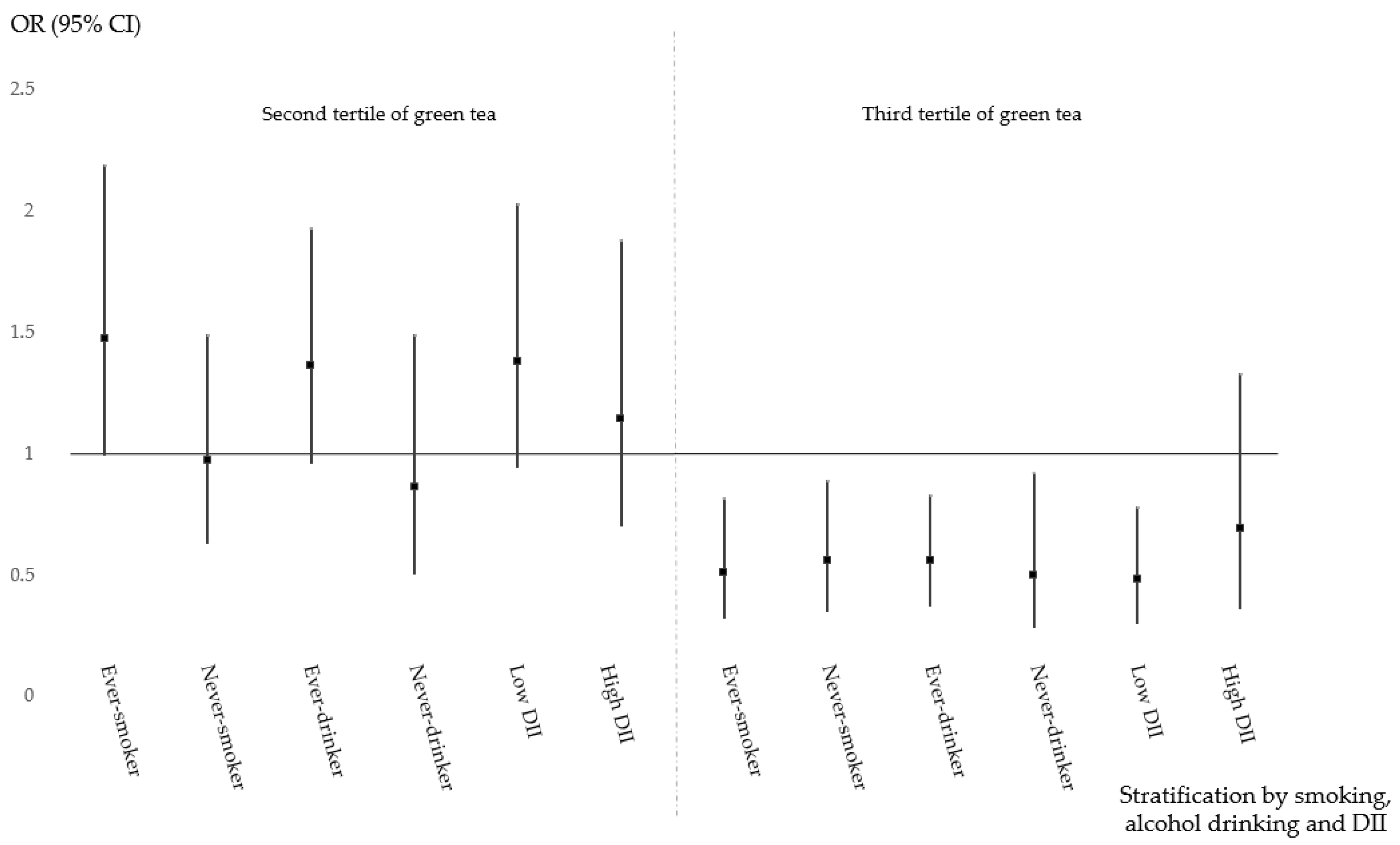
| Smoking | Ever | 1.0 (ref) | 1.49 (1.00–2.21) | 0.52 (0.32–0.84) | 0.2 | |
| Never | 1.50 (0.99–2.27) | 0.63 (0.35–1.12) | 1.06 (0.55–2.03) | |||
| Alcohol drinking | Ever | 1.0 (ref) | 1.44 (1.01–2.04) | 0.59 (0.39–0.88) | 0.19 | |
| Never | 1.06 (0.69–1.64) | 0.56 (0.30–1.04) | 0.80 (0.40–1.60) | |||
| DII | High (≥2.73) | 1.0 (ref) | 1.45 (0.98–2.14) | 0.51 (0.32–0.83) | 0.29 | |
| Low (<2.73) | 1.02 (0.68–1.55) | 0.67 (0.37–1.20) | 1.05 (0.54–2.04) | |||
| Rectal cancer | Prior BMI | ≥25 kg/m2 | 1.0 (ref) | 1.35 (0.80–2.30) | 0.38 (0.19–0.74) | 0.16 |
| <25 kg/m2 | 0.80 (0.52–1.25) | 1.09 (0.58–2.06) | 2.08 (0.96–4.51) | |||
| Physical activity | No | 1.0 (ref) | 1.41 (0.98–2.03) | 0.61 (0.40–0.93) | 0.91 | |
| Yes | 0.39 (0.25–0.62) | 1.07 (0.57–1.99) | 1.16 (0.58–2.34) | |||
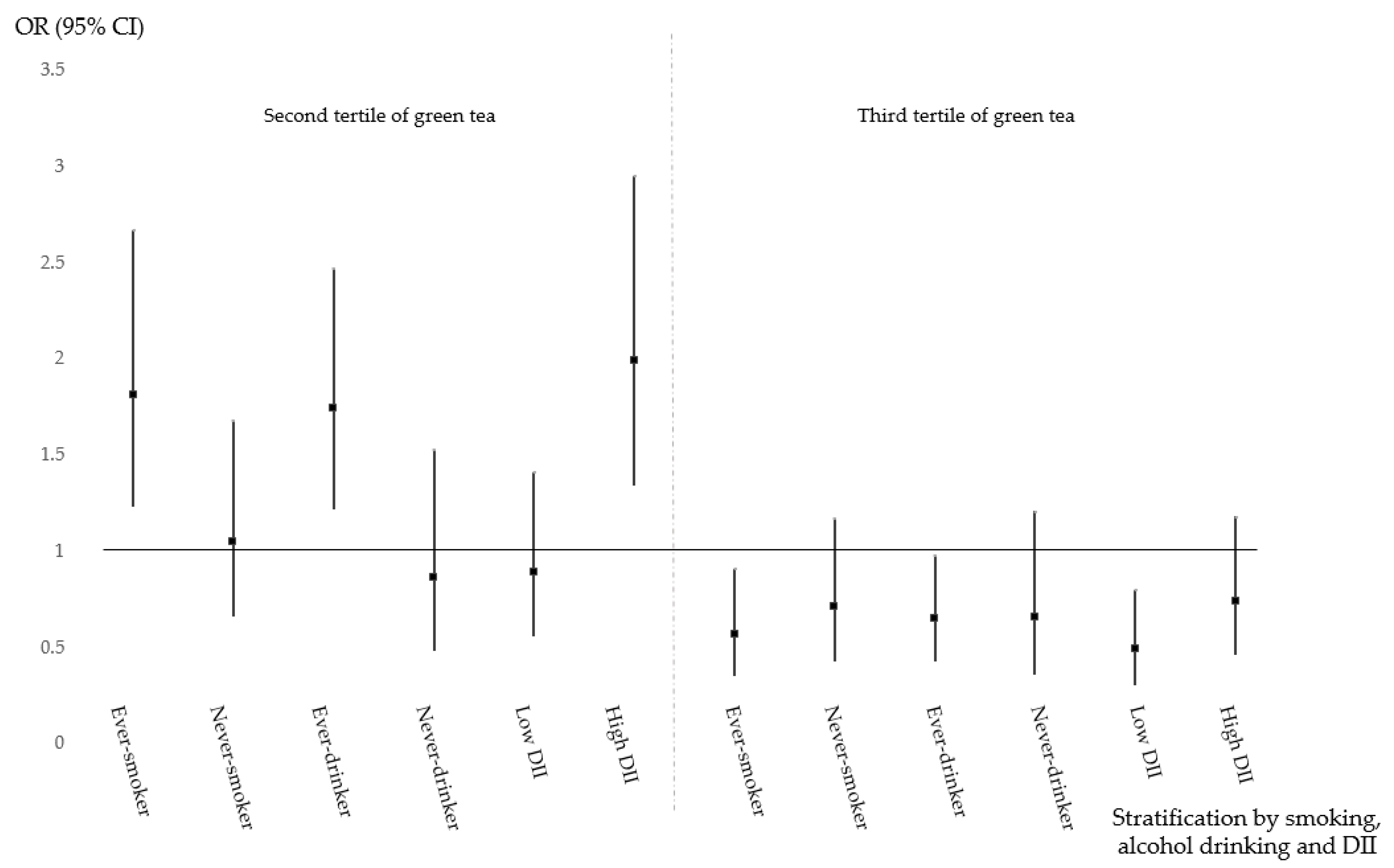
| Smoking | Ever | 1.0 (ref) | 1.77 (1.21–2.59) | 0.57 (0.36–0.90) | 0.08 | |
| Never | 1.00 (0.65–1.54) | 0.60 (0.33–1.10) | 1.25 (0.64–2.44) | |||
| Alcohol drinking | Ever | 1.0 (ref) | 1.78 (1.25–2.54) | 0.67 (0.44–1.00) | 0.06 | |
| Never | 1.14 (0.73–1.80) | 0.47 (0.25–0.91) | 0.90 (0.44–1.83) | |||
| DII | High (≥2.73) | 1.0 (ref) | 1.99 (1.35–2.94) | 0.73 (0.46–1.17) | 0.04 | |
| Low (<2.73) | 1.20 (0.79–1.83) | 0.46 (0.25–0.84) | 0.69 (0.35–1.35) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors. Nutrients 2019, 11, 2612. https://doi.org/10.3390/nu11112612
Kim H, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, Kim J. Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors. Nutrients. 2019; 11(11):2612. https://doi.org/10.3390/nu11112612
Chicago/Turabian StyleKim, Hyejin, Jeonghee Lee, Jae Hwan Oh, Hee Jin Chang, Dae Kyung Sohn, Aesun Shin, and Jeongseon Kim. 2019. "Protective Effect of Green Tea Consumption on Colorectal Cancer Varies by Lifestyle Factors" Nutrients 11, no. 11: 2612. https://doi.org/10.3390/nu11112612





