Effect of High-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
Statistical analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n–3 Fatty Acids and Cardiovascular Events after Myocardial Infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Barlera, S.; Caimi, V.; Longoni, P.; Marzona, I.; Milani, V.; Silletta, M.G.; Tognoni, G.; et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; Cox, J.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- The ORIGIN Trial Investigators. n-3 Fatty Acids and Cardiovascular Outcomes in Patients with Dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef]
- Bays, H.E.; Ballantyne, C.M.; Doyle, R.T., Jr.; Juliano, R.A.; Philip, S. Icosapent ethyl: Eicosapentaenoic acid concentration and triglyceride-lowering effects across clinical studies. Prostaglandins Other Lipid Mediat. 2016, 125, 57–64. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Beilin, L.J. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 1999, 34, 253–260. [Google Scholar] [CrossRef]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Watts, G.F.; Beilin, L.J. Dietary fish as a major component of a weight-loss diet: Effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am. J. Clin. Nutr 1999, 70, 817–825. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Jiao, L.; Tardif, J.C.; Gregson, J.; Pocock, S.J.; Ballantyne, C.M. Reduction in First and Total Ischemic Events with Icosapent Ethyl Across Baseline Triglyceride Tertiles. J. Am. Coll Cardiol. 2019, 74, 1159–1161. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Marine omega-3 fatty acids and coronary heart disease. Curr. Opin. Cardiol. 2012, 27, 412–419. [Google Scholar] [CrossRef]
- De Caterina, R. n–3 Fatty Acids in Cardiovascular Disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Elajami, T.K.; Ashfaque, H.; Saleh, M.; Bistrian, B.R.; Welty, F.K. Effect of Eicosapentaenoic and Docosahexaenoic Acids Added to Statin Therapy on Coronary Artery Plaque in Patients with Coronary Artery Disease: A Randomized Clinical Trial. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Nishio, R.; Shinke, T.; Otake, H.; Nakagawa, M.; Nagoshi, R.; Inoue, T.; Kozuki, A.; Hariki, H.; Osue, T.; Taniguchi, Y.; et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis 2014, 234, 114–119. [Google Scholar] [CrossRef]
- Niki, T.; Wakatsuki, T.; Yamaguchi, K.; Taketani, Y.; Oeduka, H.; Kusunose, K.; Ise, T.; Iwase, T.; Yamada, H.; Soeki, T.; et al. Effects of the Addition of Eicosapentaenoic Acid to Strong Statin Therapy on Inflammatory Cytokines and Coronary Plaque Components Assessed by Integrated Backscatter Intravascular Ultrasound. Circ. J. 2016, 80, 450–460. [Google Scholar] [CrossRef]
- Watanabe, T.; Ando, K.; Daidoji, H.; Otaki, Y.; Sugawara, S.; Matsui, M.; Ikeno, E.; Hirono, O.; Miyawaki, H.; Yashiro, Y.; et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J. Cardiol. 2017, 70, 537–544. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available online: https://training.cochrane.org/handbook. (accessed on 30 July 2019).
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Mita, T.; Watada, H.; Ogihara, T.; Nomiyama, T.; Ogawa, O.; Kinoshita, J.; Shimizu, T.; Hirose, T.; Tanaka, Y.; Kawamori, R. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis 2007, 191, 162–167. [Google Scholar] [CrossRef]
- Bhatia, L.; Scorletti, E.; Curzen, N.; Clough, G.F.; Calder, P.C.; Byrne, C.D. Improvement in non-alcoholic fatty liver disease severity is associated with a reduction in carotid intima-media thickness progression. Atherosclerosis 2016, 246, 13–20. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Campana, M.C.; De Caterina, R. Omega-3 fatty acids, inflammation and angiogenesis: Basic mechanisms behind the cardioprotective effects of fish and fish oils. Cell. Mol. Biol. 2010, 56, 59–82. [Google Scholar]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012. [Google Scholar] [CrossRef]
- Zampolli, A.; Bysted, A.; Leth, T.; Mortensen, A.; De Caterina, R.; Falk, E. Contrasting effect of fish oil supplementation on the development of atherosclerosis in murine models. Atherosclerosis 2006, 184, 78–85. [Google Scholar] [CrossRef]
- Casos, K.; Saiz, M.P.; Ruiz-Sanz, J.I.; Mitjavila, M.T. Atherosclerosis prevention by a fish oil-rich diet in apoE (−/−) mice is associated with a reduction of endothelial adhesion molecules. Atherosclerosis 2008, 201, 306–317. [Google Scholar] [CrossRef]
- Nakajima, K.; Yamashita, T.; Kita, T.; Takeda, M.; Sasaki, N.; Kasahara, K.; Shinohara, M.; Rikitake, Y.; Ishida, T.; Yokoyama, M.; et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1963–1972. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Matthan, N.R.; Lamon-Fava, S.; Lecker, J.L.; Lichtenstein, A.H. Reduction in dietary omega-6 polyunsaturated fatty acids: Eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse. Atherosclerosis 2009, 204, 147–155. [Google Scholar] [CrossRef]
- Weiner, B.H.; Ockene, I.S.; Levine, P.H.; Cuenoud, H.F.; Fisher, M.; Johnson, B.F.; Daoud, A.S.; Jarmolych, J.; Hosmer, D.; Johnson, M.H.; et al. Inhibition of atherosclerosis by cod-liver oil in a hyperlipidemic swine model. N. Engl. J. Med. 1986, 315, 841–846. [Google Scholar] [CrossRef]
- Davis, H.R.; Bridenstine, R.T.; Vesselinovitch, D.; Wissler, R.W. Fish oil inhibits development of atherosclerosis in rhesus monkeys. Arteriosclerosis 1987, 7, 441–449. [Google Scholar] [CrossRef]
- Ma, J.; Folsom, A.R.; Lewis, L.; Eckfeldt, J.H. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 1997, 65, 551–559. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Mayer-Davis, E.; Jenny, N.S.; Jiang, R.; Ouyang, P.; Steffen, L.M.; Siscovick, D.; Wu, C.; et al. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am. J. Clin. Nutr. 2008, 88, 1111–1118. [Google Scholar] [CrossRef]
- Heine-Broring, R.C.; Brouwer, I.A.; Proenca, R.V.; van Rooij, F.J.; Hofman, A.; Oudkerk, M.; Witteman, J.C.; Geleijnse, J.M. Intake of fish and marine n-3 fatty acids in relation to coronary calcification: The Rotterdam Study. Am. J. Clin. Nutr. 2010, 91, 1317–1323. [Google Scholar] [CrossRef]
- Greenland, P.; Bonow, R.O.; Brundage, B.H.; Budoff, M.J.; Eisenberg, M.J.; Grundy, S.M.; Lauer, M.S.; Post, W.S.; Raggi, P.; Redberg, R.F.; et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation 2007, 115, 402–426. [Google Scholar] [CrossRef]
- Hino, A.; Adachi, H.; Toyomasu, K.; Yoshida, N.; Enomoto, M.; Hiratsuka, A.; Hirai, Y.; Satoh, A.; Imaizumi, T. Very long chain N-3 fatty acids intake and carotid atherosclerosis: An epidemiological study evaluated by ultrasonography. Atherosclerosis 2004, 176, 145–149. [Google Scholar] [CrossRef]
- Sekikawa, A.; Mahajan, H.; Kadowaki, S.; Hisamatsu, T.; Miyagawa, N.; Fujiyoshi, A.; Kadota, A.; Maegawa, H.; Murata, K.; Miura, K.; et al. Association of blood levels of marine omega-3 fatty acids with coronary calcification and calcium density in Japanese men. Eur. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Sekikawa, A.; Curb, J.D.; Ueshima, H.; El-Saed, A.; Kadowaki, T.; Abbott, R.D.; Evans, R.W.; Rodriguez, B.L.; Okamura, T.; Sutton-Tyrrell, K.; et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: A cross-sectional study. J. Am. Coll. Cardiol. 2008, 52, 417–424. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Ahn, J.; Park, S.K.; Park, T.S.; Kim, J.H.; Yun, E.; Kim, S.P.; Lee, H.W.; Oh, J.H.; Choi, J.H.; Cha, K.S.; et al. Effect of n-3 Polyunsaturated Fatty Acids on Regression of Coronary Atherosclerosis in Statin Treated Patients Undergoing Percutaneous Coronary Intervention. Korean Circ. J. 2016, 46, 481–489. [Google Scholar] [CrossRef]
- Angerer, P.; Kothny, W.; Stork, S.; von Schacky, C. Effect of dietary supplementation with omega-3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovasc. Res. 2002, 54, 183–190. [Google Scholar] [CrossRef]
- Baldassarre, D.; Amato, M.; Eligini, S.; Barbieri, S.S.; Mussoni, L.; Frigerio, B.; Kozakova, M.; Tremoli, E.; Sirtori, C.R.; Colli, S. Effect of n-3 fatty acids on carotid atherosclerosis and haemostasis in patients with combined hyperlipoproteinemia: A double-blind pilot study in primary prevention. Ann. Med. 2006, 38, 367–375. [Google Scholar] [CrossRef]
- Lonn, E.M.; Bosch, J.; Diaz, R.; Lopez-Jaramillo, P.; Ramachandran, A.; Hancu, N.; Hanefeld, M.; Krum, H.; Ryden, L.; Smith, S.; et al. Effect of insulin glargine and n-3FA on carotid intima-media thickness in people with dysglycemia at high risk for cardiovascular events: The glucose reduction and atherosclerosis continuing evaluation study (ORIGIN-GRACE). Diabetes Care 2013, 36, 2466–2474. [Google Scholar] [CrossRef]
- Von Schacky, C.; Angerer, P.; Kothny, W.; Theisen, K.; Mudra, H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1999, 130, 554–562. [Google Scholar] [CrossRef]
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Chang, C.H.; Tseng, P.T.; Chen, N.Y.; Lin, P.C.; Lin, P.Y.; Chang, J.P.; Kuo, F.Y.; Lin, J.; Wu, M.C.; Su, K.P. Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot. Essent. Fatty Acids 2018, 129, 1–12. [Google Scholar] [CrossRef]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; Mozaffarian, D.; et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin. Cardiol. 2018, 41, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; O’Neal, D.N.; Best, J.D.; Beilin, L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000, 71, 1085–1094. [Google Scholar] [CrossRef]
- Grimsgaard, S.; Bonaa, K.H.; Hansen, J.B.; Nordoy, A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am. J. Clin. Nutr. 1997, 66, 649–659. [Google Scholar] [CrossRef]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lepine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009, 139, 861–868. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef]
- Lahoute, C.; Herbin, O.; Mallat, Z.; Tedgui, A. Adaptive immunity in atherosclerosis: Mechanisms and future therapeutic targets. Nat. Rev. 2011, 8, 348–358. [Google Scholar] [CrossRef]
- Calder, P.C.; Albers, R.; Antoine, J.M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabba, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. TEM 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A. Could Ceramides Become the New Cholesterol? Cell. Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.M.; Leventhal, A.R.; Kuriakose, G.; Schuchman, E.H.; Williams, K.J.; Tabas, I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1723–1730. [Google Scholar] [CrossRef]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvanne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E45–E52. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferre, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schottker, B.; Laaperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma Ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Volzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Midtbo, L.K.; Borkowska, A.G.; Bernhard, A.; Ronnevik, A.K.; Lock, E.J.; Fitzgerald, M.L.; Torstensen, B.E.; Liaset, B.; Brattelid, T.; Pedersen, T.L.; et al. Intake of farmed Atlantic salmon fed soybean oil increases hepatic levels of arachidonic acid-derived oxylipins and ceramides in mice. J. Nutr. Biochem. 2015, 26, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Taltavull, N.; Ras, R.; Marine, S.; Romeu, M.; Giralt, M.; Mendez, L.; Medina, I.; Ramos-Romero, S.; Torres, J.L.; Nogues, M.R. Protective effects of fish oil on pre-diabetes: A lipidomic analysis of liver ceramides in rats. Food Funct. 2016, 7, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Skorve, J.; Hilvo, M.; Vihervaara, T.; Burri, L.; Bohov, P.; Tillander, V.; Bjorndal, B.; Suoniemi, M.; Laaksonen, R.; Ekroos, K.; et al. Fish oil and krill oil differentially modify the liver and brain lipidome when fed to mice. Lipids Health Dis. 2015, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Libby, P.; Hansson, G.K. Adaptive immunity and atherosclerosis. Clin. Immunol. 2010, 134, 33–46. [Google Scholar] [CrossRef]
- Tracy, R.P.; Doyle, M.F.; Olson, N.C.; Huber, S.A.; Jenny, N.S.; Sallam, R.; Psaty, B.M.; Kronmal, R.A. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: The multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000117. [Google Scholar] [CrossRef]
- Zhang, P.; Kim, W.; Zhou, L.; Wang, N.; Ly, L.H.; McMurray, D.N.; Chapkin, R.S. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J. Nutr. 2006, 136, 2391–2398. [Google Scholar] [CrossRef]
- Serhan, C.; Arita, M.; Hong, S.; Gotlinger, K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 2004, 39, 1125–1132. [Google Scholar] [CrossRef]
- Back, M.; Yurdagul, A., Jr.; Tabas, I.; Oorni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Aspects Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Itakura, H.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Kita, T.; et al. Relationships between plasma fatty acid composition and coronary artery disease. J. Atheroscler. Thromb. 2011, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Manku, M.S.; Bays, H.E.; Philip, S.; Granowitz, C.; Doyle, R.T., Jr.; Juliano, R.A. Icosapent Ethyl Effects on Fatty Acid Profiles in Statin-Treated Patients with High Triglycerides: The Randomized, Placebo-controlled ANCHOR Study. Cardiol. Ther. 2019, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Hsu, A.; Wolski, K.; Hu, B.; Bayturan, O.; Lavoie, A.; Uno, K.; Tuzcu, E.M.; Nissen, S.E. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J. Am. Coll. Cardiol. 2010, 55, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Agostoni, P.; Abbate, A.; Castagno, D.; Lipinski, M.J.; Vetrovec, G.W.; Frati, G.; Presutti, D.G.; Quadri, G.; Moretti, C.; et al. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: A meta-regression of randomized clinical trials. Atherosclerosis 2013, 226, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R., 3rd; Raichlen, J.S.; Riley, W.A.; Evans, G.W.; Palmer, M.K.; O’Leary, D.H.; Grobbee, D.E.; Bots, M.L. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The METEOR Trial. JAMA 2007, 297, 1344–1353. [Google Scholar] [CrossRef]
- Taylor, A.J.; Villines, T.C.; Stanek, E.J.; Devine, P.J.; Griffen, L.; Miller, M.; Weissman, N.J.; Turco, M. Extended-Release Niacin or Ezetimibe and Carotid Intima-Media Thickness. N. Engl. J. Med. 2009, 361, 2113–2122. [Google Scholar] [CrossRef]
- Costanzo, P.; Perrone-Filardi, P.; Vassallo, E.; Paolillo, S.; Cesarano, P.; Brevetti, G.; Chiariello, M. Does Carotid Intima-Media Thickness Regression Predict Reduction of Cardiovascular Events? A Meta-Analysis of 41 Randomized Trials. J. Am. Coll. Cardiol. 2010, 56, 2006–2020. [Google Scholar] [CrossRef]
- Goldberger, Z.D.; Valle, J.A.; Dandekar, V.K.; Chan, P.S.; Ko, D.T.; Nallamothu, B.K. Are changes in carotid intima-media thickness related to risk of nonfatal myocardial infarction? A critical review and meta-regression analysis. Am. Heart J. 2010, 160, 701–714. [Google Scholar] [CrossRef]
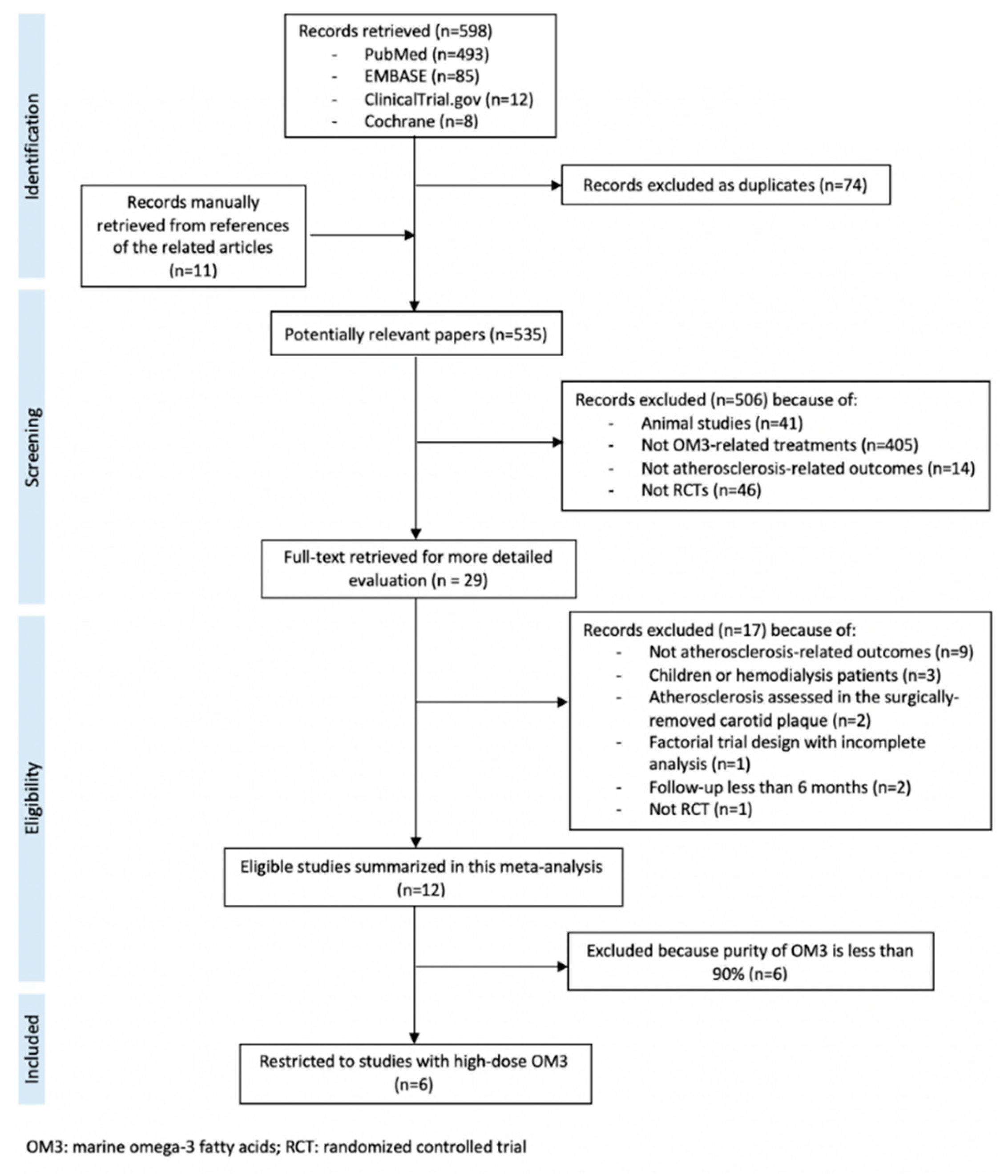
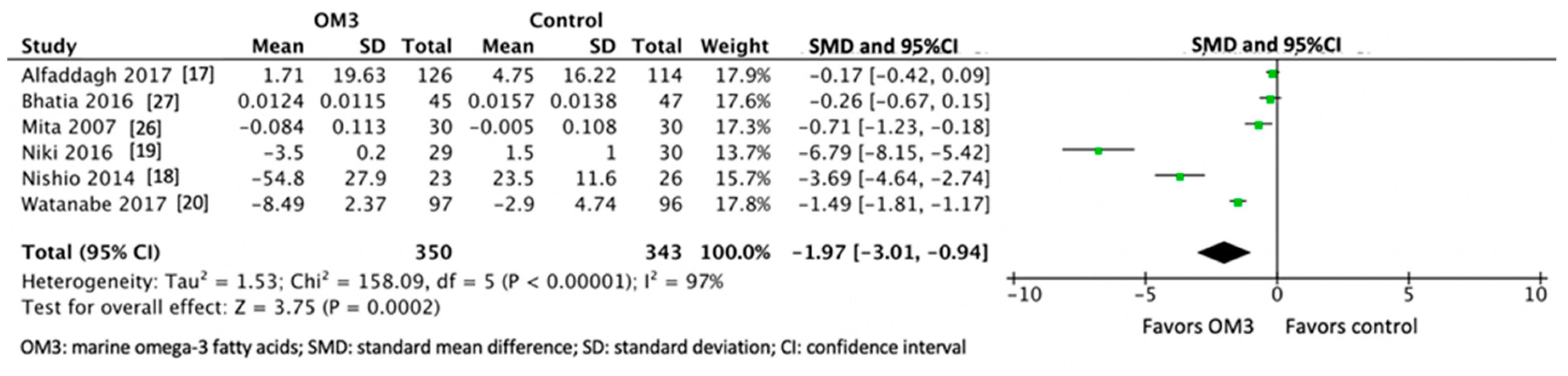
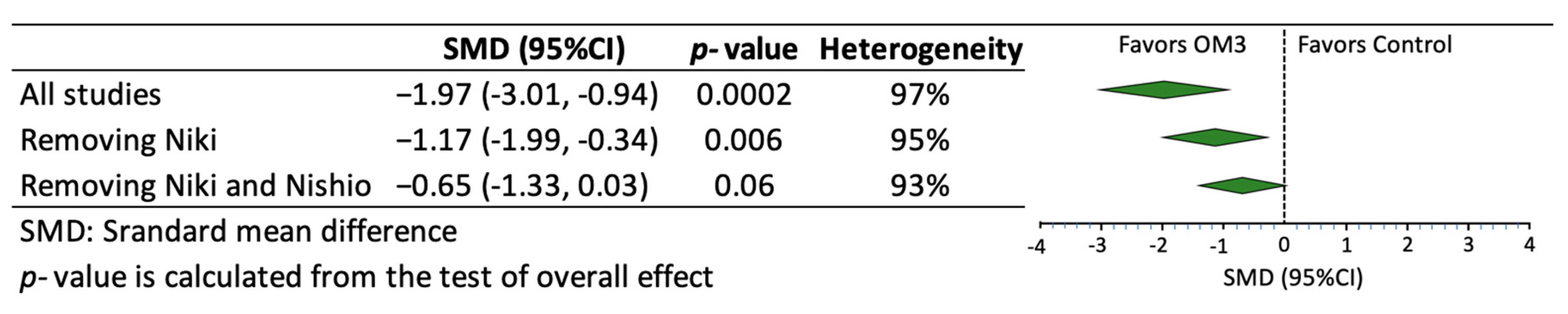
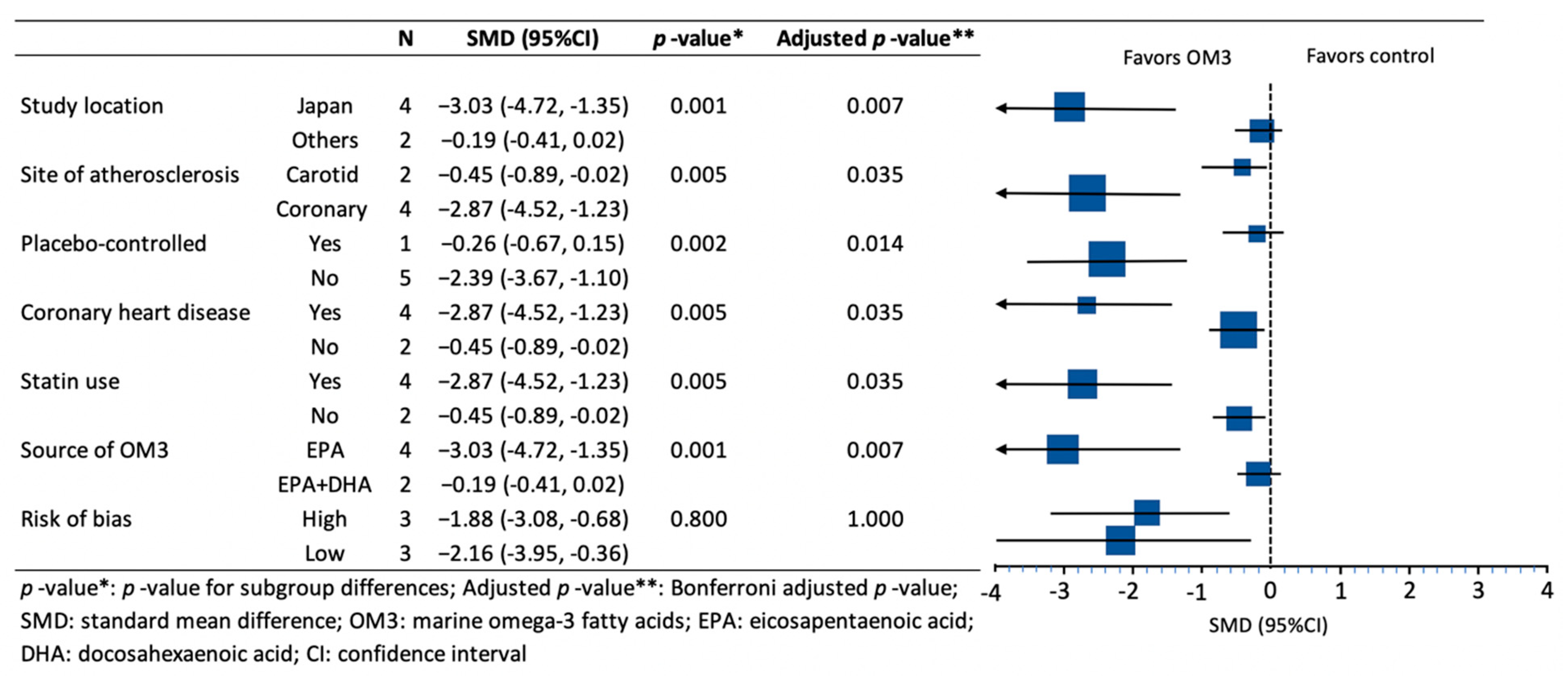
| Author, Year, Location, Reference | Number of Participants Treatment/Control | Age (years) Treatment/Control | Characteristics of the Participants | Use of Statin | Use of Placebo | Dose and Type of OM3 (g/day) | Purity of OM3 (%) | Duration of Intervention (months) |
|---|---|---|---|---|---|---|---|---|
| Alfaddagh, 2017, US [17] | 143/142 | 63 ± 8 vs. 64 ± 8 | CHD or CV risk factor | Yes | No | 1.86 EPA + 1.50 DHA | 90% | 30 |
| Bhatia, 2016, UK [27] | 51/52 | 49 ± 11 vs. 54 ± 9 | NAFLD | No | Yes | 1.368 EPA + 1.656 DHA | 90% | 15 to 18 |
| Mita, 2006, Japan [26] | 40/41 | 59 ± 11 vs. 61 ± 8 | T2DM | No | No | 1.8 EPA | >98% | 25 ± 2 |
| Niki, 2016, Japan [19] | 48/47 | 68 ± 10 vs. 69 ± 11 | CHD and DL | Yes | No | 1.8 EPA | >98% | 6 |
| Nishio, 2014, Japan [18] | 25/27 | 61 ± 13 vs. 64 ± 10 | CHD and DL | Yes | No | 1.8 EPA | >98% | 9 |
| Watanabe, 2017, Japan [20] | 122/119 | 67 ± 10 vs. 68 ± 10 | CHD | Yes | No | 1.8 EPA | >98% | 6 to 8 |
| First Author, Year, Country, Reference | Imaging Techniques | Primary Outcome | Baseline Measurement Treatment vs. Control Groups | Difference in Primary Outcome between the End of Intervention and Baseline in Each of Treatment and Control Groups Treatment vs. Control Groups | Net Difference between Intervention and Control Groups | p-Value for Net Difference |
|---|---|---|---|---|---|---|
| Alfaddagh, 2017, US [17] | cCTA | Percent change in non-calcified plaque volume (%) | 26.4 (14.3, 39.7) vs. 23.7 (14.3, 36.8) | 1.71 ± 19.9 vs. 4.75 ± 16.44 | −3.04 | 0.14 |
| Bhatia, 2016, UK [27] | B-Mode ultrasound | Change in mean carotid IMT (mm) | 0.649 ± 0.095 vs. 0.674 ± 0.098 | 0.0124 ± 0.0115 vs. 0.0157 ± 0.0138 | −0.003 | 0.17 |
| Mita, 2006, Japan [26] | B-mode ultrasound | Annual change in maximum carotid IMT (mm/year) | 1.505 ± 0.412 vs. 1.706 ± 0.423 | −0.084 ± 0.113 vs. −0.005 ± 0.108 | −0.079 | <0.01 |
| Niki, 2016, Japan [19] | IVUS | Change in lipid plaque volume (mm3) | 18.5 ± 1.3 vs. 17.8 ± 1.3 | −3.5 ± 0.2 vs. 1.5 ± 1.0 | −5.0 | <0.01 |
| Nishio, 2014, Japan [18] | OCT | Change in fibrous-cap thickness (um) | 47.5 ± 7.4 vs. 46.5 ± 10.9 | −54.8 ± 27.9 vs. −23.5 ± 11.6 | −31.3 | <0.01 |
| Watanabe, 2017, Japan [20] | IVUS | Change in normalized total atheroma volume (mm3) | 74.2 (55.9, 99.2) vs. 74.2 (57.5, 96.8) | −8.49 ± 2.37 vs. −2.90 ± 4.74 | −5.59 | <0.01 |
| Study | Selection Bias | Performance bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | Total | |
|---|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Source of Bias | Low on Risk of Bias | |
| Alfaddagh, 2017, US [17] | low | high | high | low | low | low | low | 5/7 |
| Bhatia, 2016, UK [27] | low | unclear | low | low | low | low | low | 6/7 |
| Mita, 2006, Japan [26] | unclear | unclear | high | low | low | unclear | low | 3/7 |
| Niki, 2016, Japan [19] | low | low | high | low | low | low | low | 6/7 |
| Nishio, 2014, Japan [18] | unclear | unclear | high | low | low | unclear | low | 3/7 |
| Watanabe, 2017, Japan [20] | low | high | high | unclear | low | low | low | 4/7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekikawa, A.; Cui, C.; Sugiyama, D.; Fabio, A.; Harris, W.S.; Zhang, X. Effect of High-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 2599. https://doi.org/10.3390/nu11112599
Sekikawa A, Cui C, Sugiyama D, Fabio A, Harris WS, Zhang X. Effect of High-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients. 2019; 11(11):2599. https://doi.org/10.3390/nu11112599
Chicago/Turabian StyleSekikawa, Akira, Chendi Cui, Daisuke Sugiyama, Anthony Fabio, William S. Harris, and Xiao Zhang. 2019. "Effect of High-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Nutrients 11, no. 11: 2599. https://doi.org/10.3390/nu11112599
APA StyleSekikawa, A., Cui, C., Sugiyama, D., Fabio, A., Harris, W. S., & Zhang, X. (2019). Effect of High-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients, 11(11), 2599. https://doi.org/10.3390/nu11112599






