Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients
Abstract
1. Introduction
2. Methods
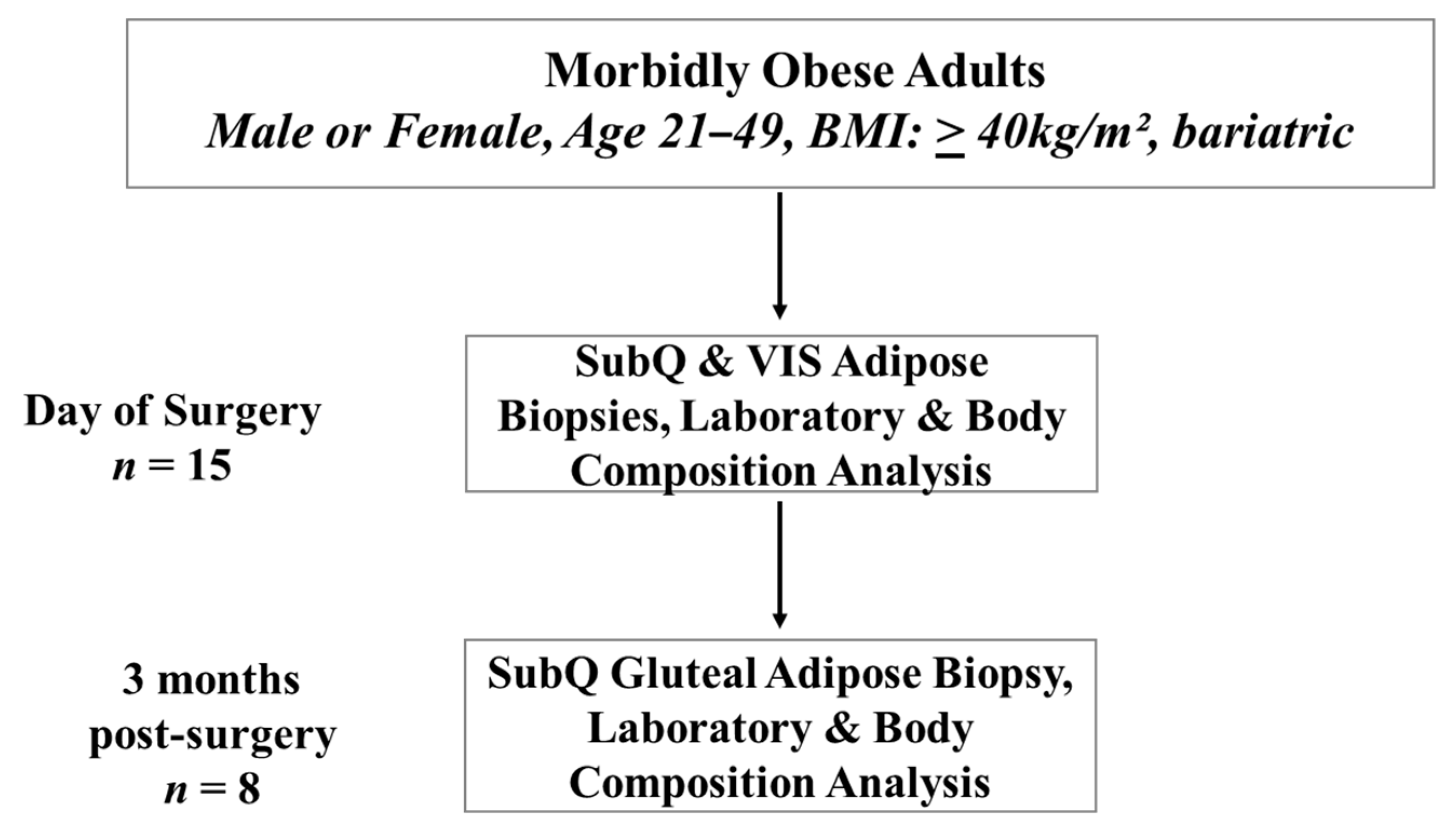
2.1. Human Participants
2.2. Physical and Cardiometabolic Measurements
2.3. Sample Acquisition
2.4. Microvascular Preparation
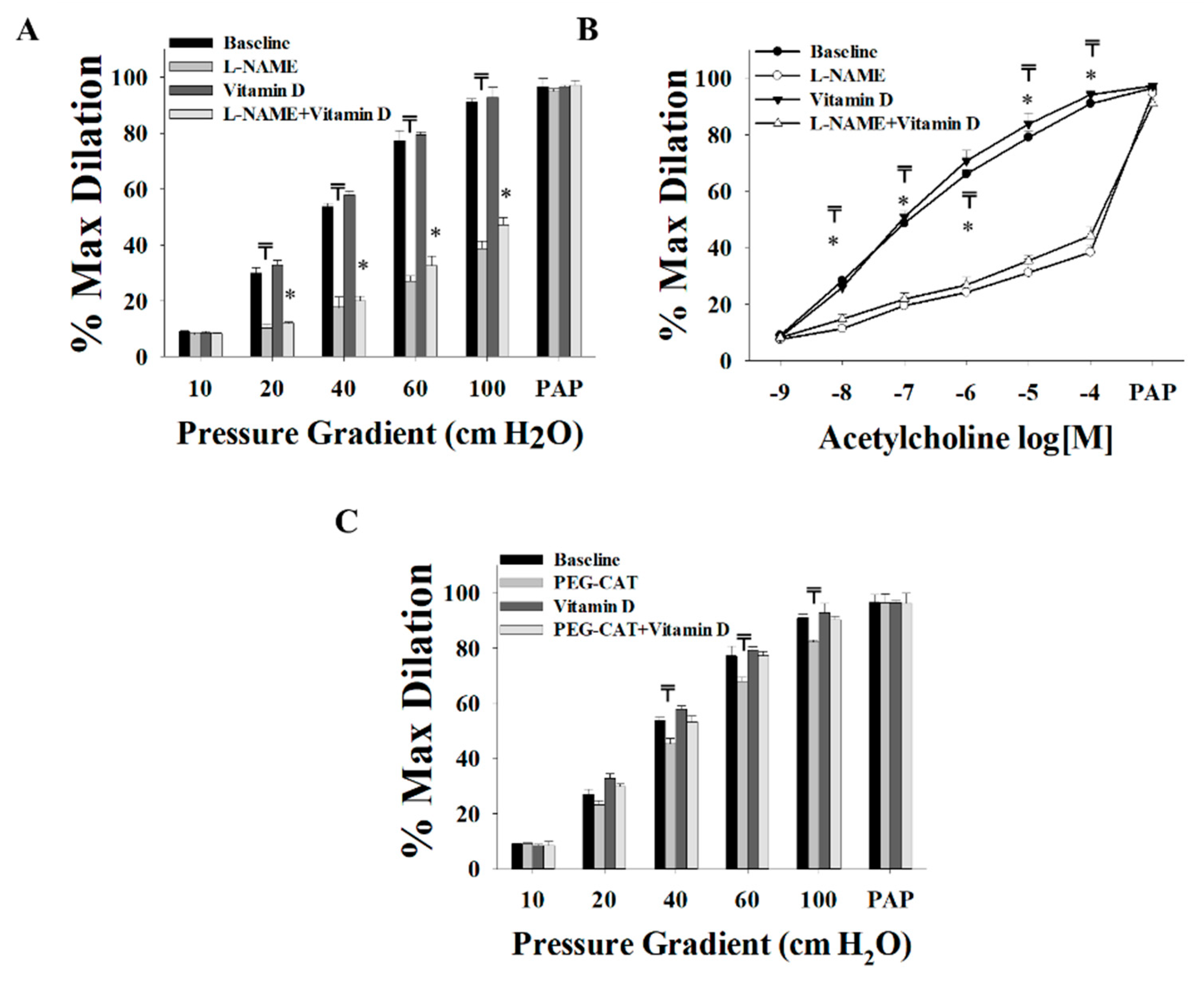
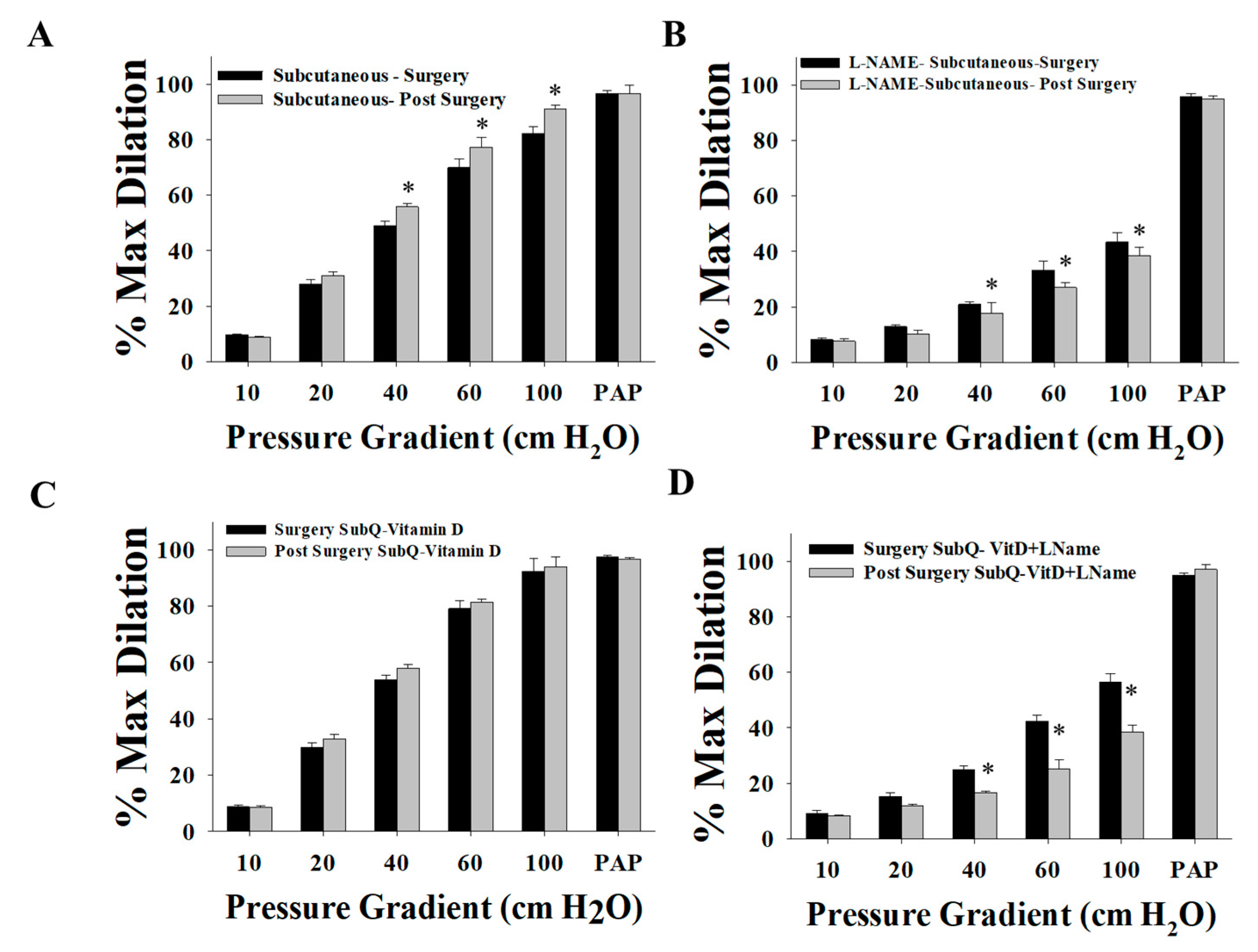
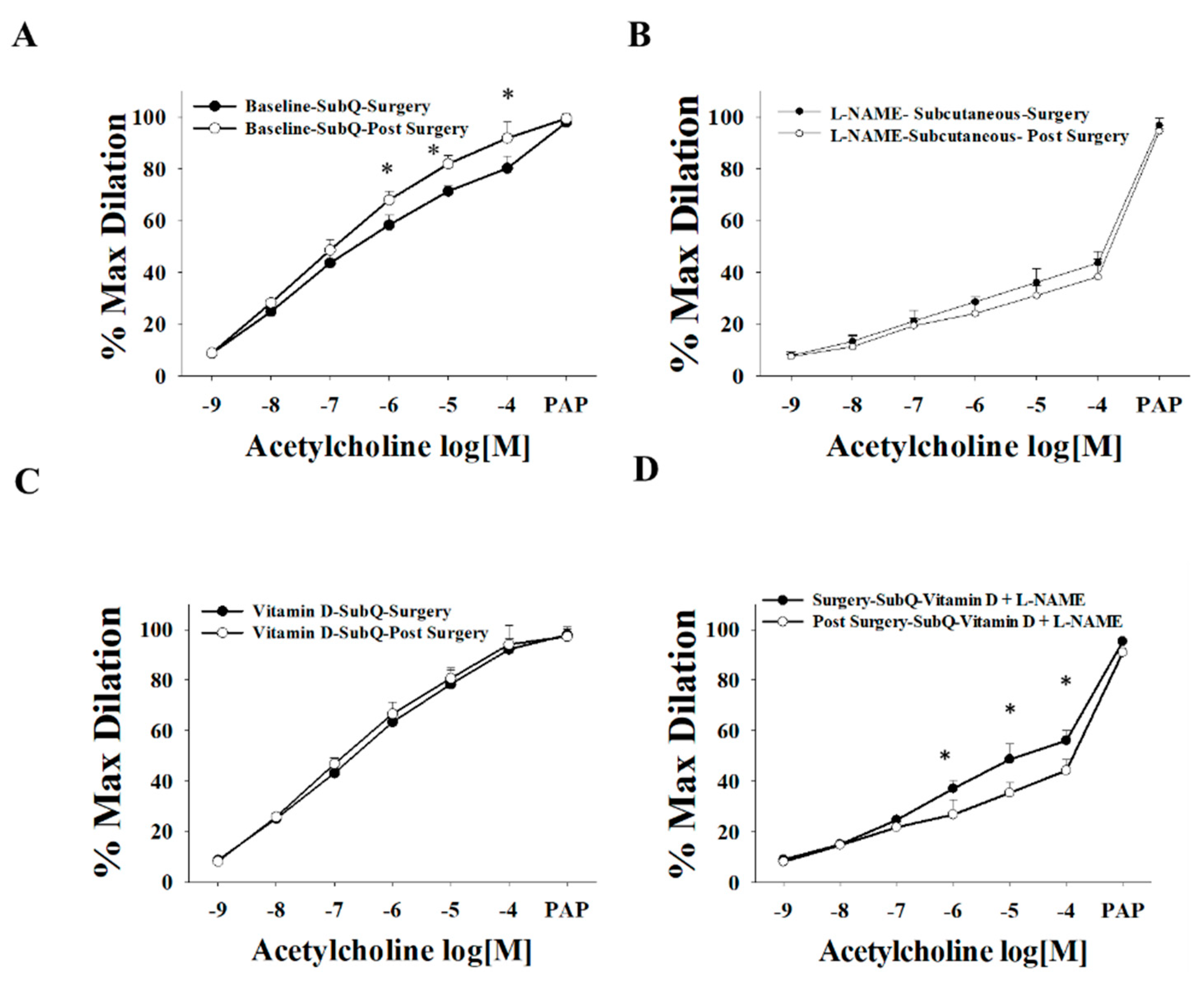
2.5. Measurements of Flow-Induced Dilation
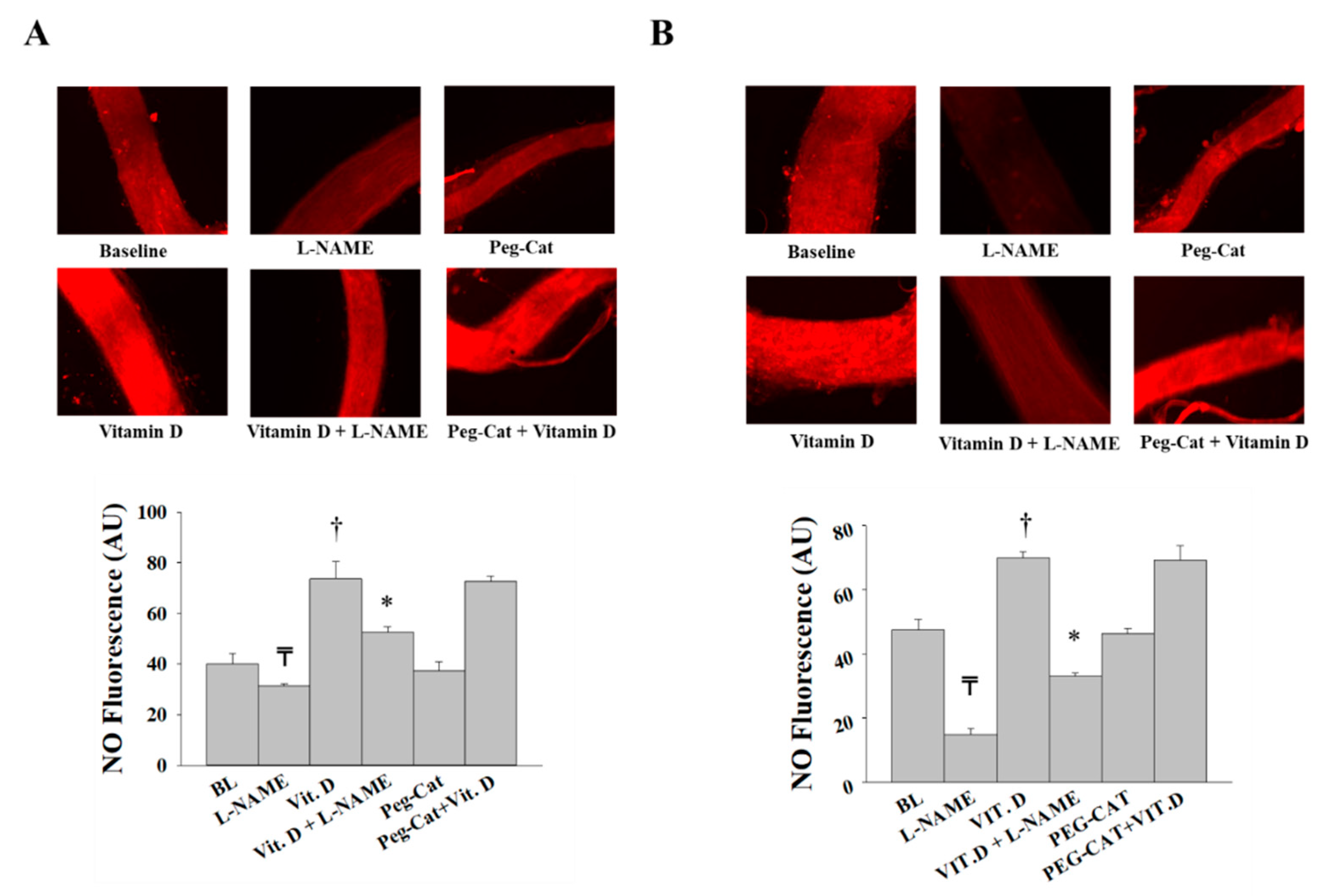
2.6. Measurements of Arteriolar NO
2.7. Statistical Analyses
3. Results
3.1. Physical and Cardiometabolic Parameters
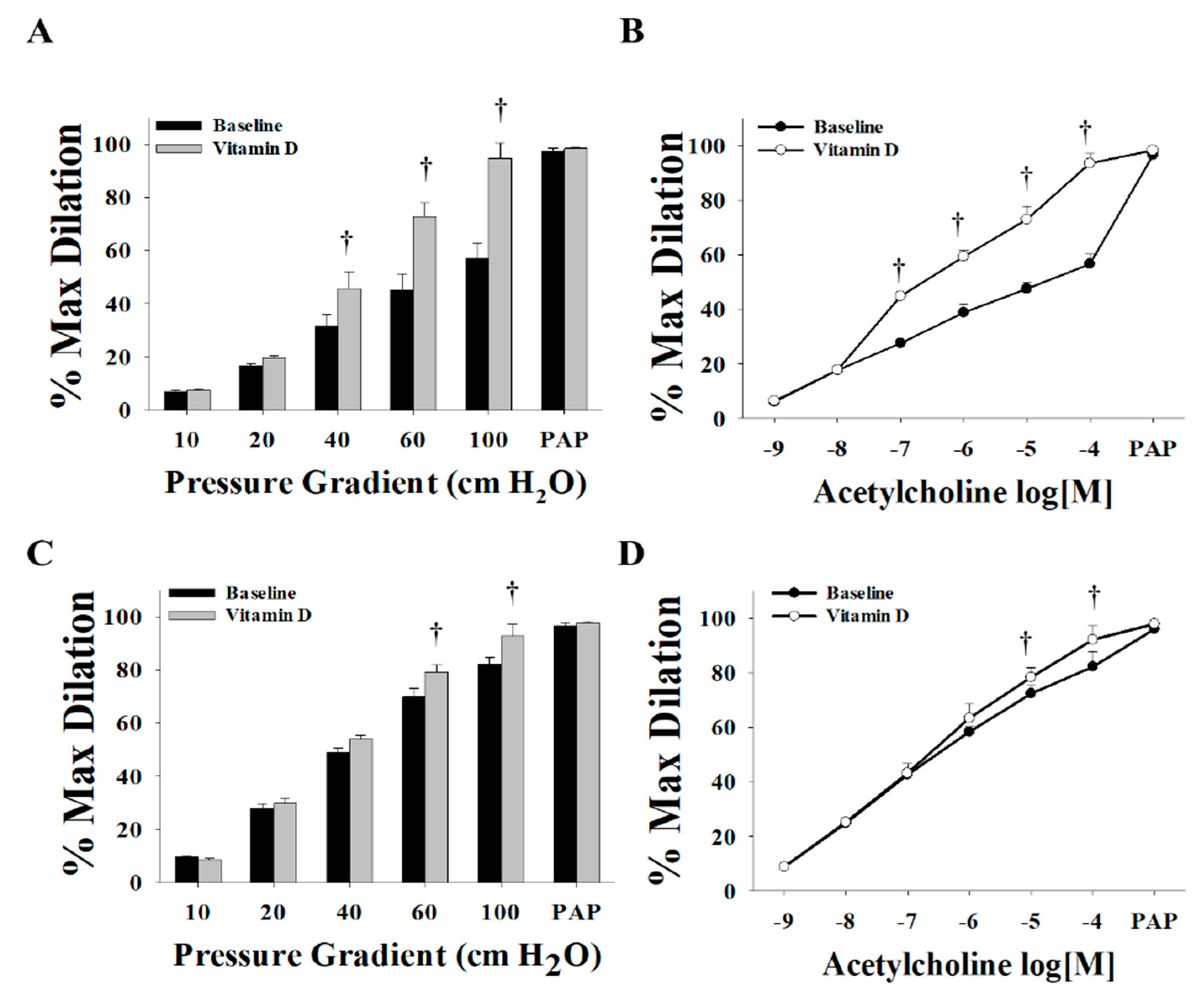
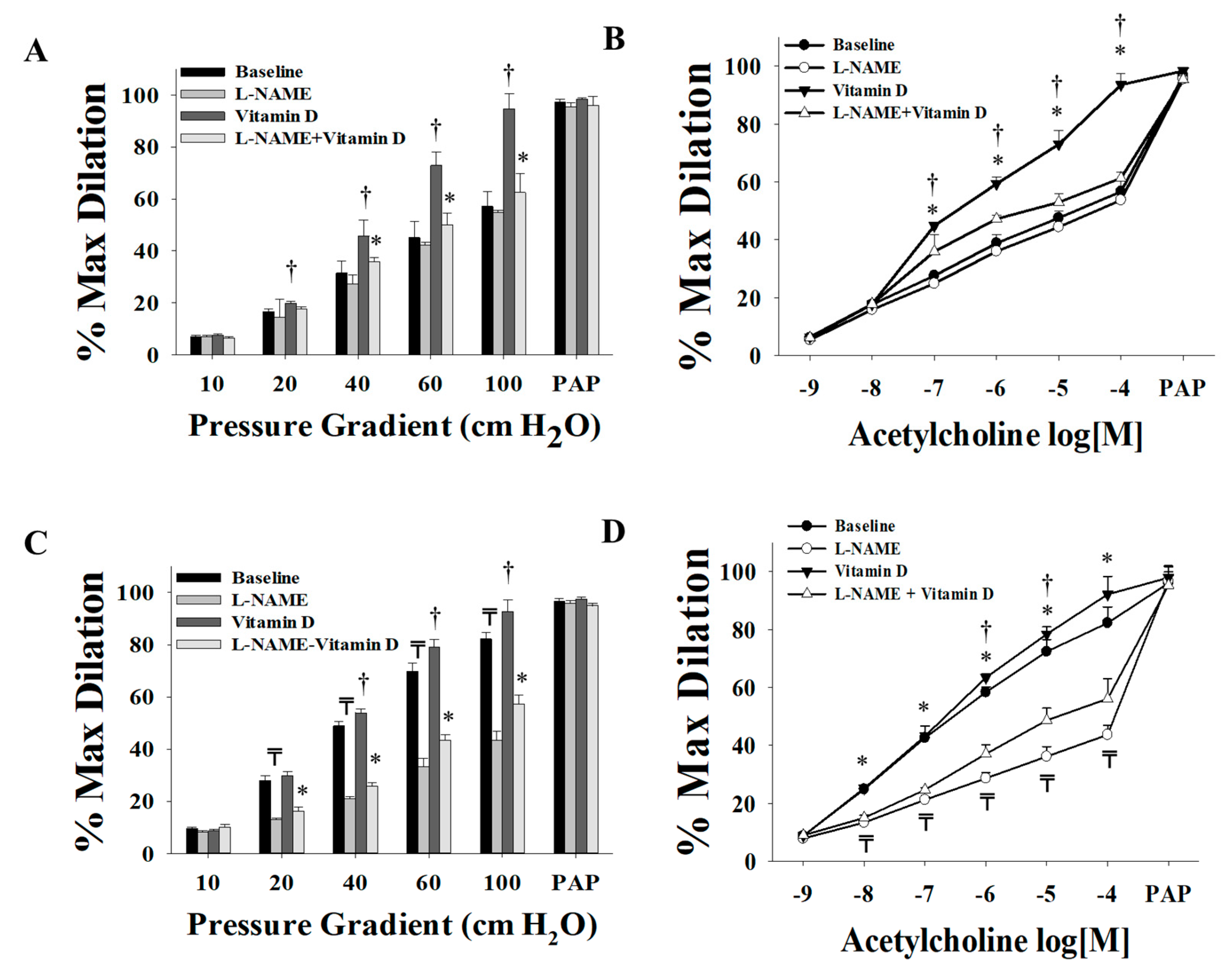
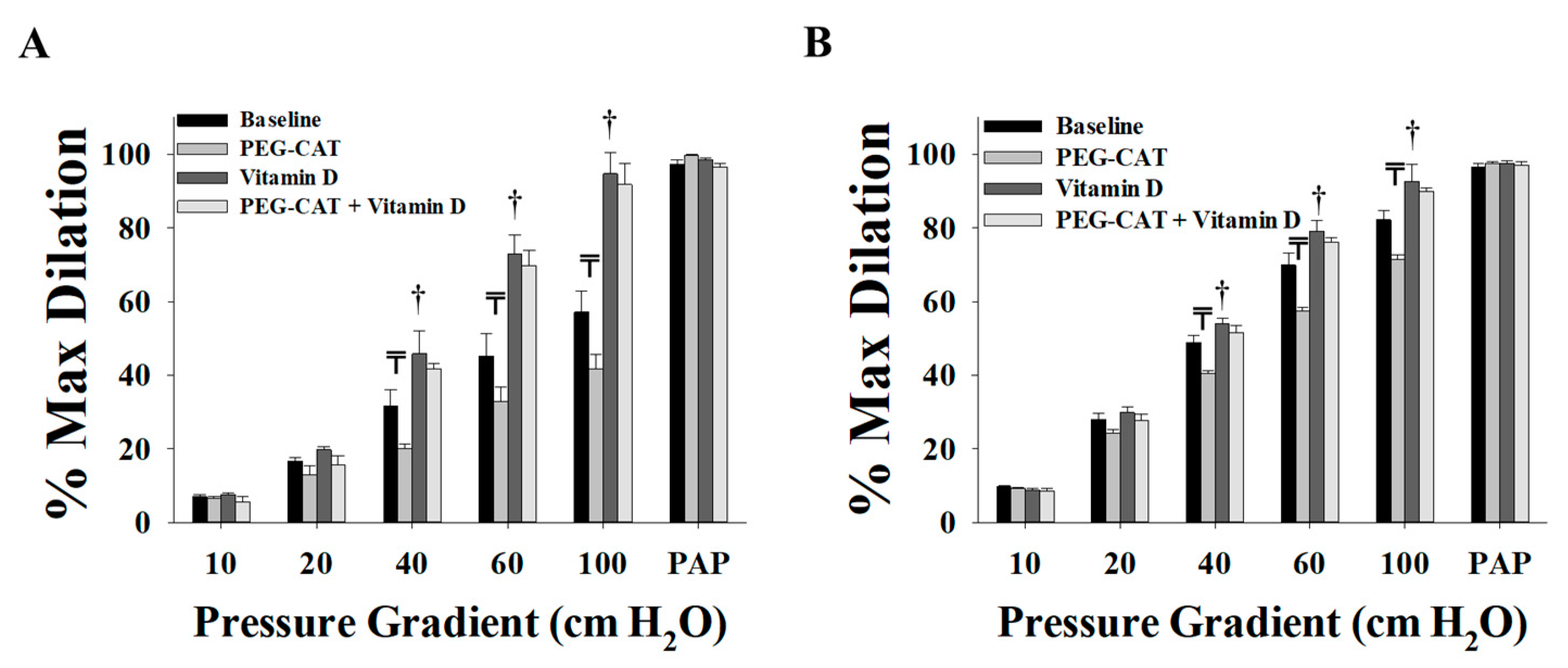
3.2. Effect of Vitamin D on Arteriolar FID, AchID, and NO Production before Weight Loss (at Time of Surgery)
3.3. Effect of Vitamin D on Arteriolar FID, AchID, and NO Production after Weight Loss (Three Months after Surgery)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniel, D.; Hardigan, P.; Bray, N.; Penzell, D.; Savu, C. The incidence of vitamin D deficiency in the obese: A retrospective chart review. J. Community Hosp. Intern. Med. Perspect. 2015, 5, 26069. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.; Vedi, S.; Ledger, J.; Webb, A.; Gazet, J.C.; Pilkington, T.R. Vitamin D status and bone histomorphometry in gross obesity. Am. J. Clin. Nutr. 1981, 34, 2359–2363. [Google Scholar] [CrossRef] [PubMed]
- Hengist, A.; Perkin, O.; Gonzalez, J.T.; Betts, J.A.; Hewison, M.; Manolopoulos, K.N.; Jones, K.S.; Koulman, A.; Thompson, D. Mobilising vitamin D from adipose tissue: The potential impact of exercise. Nutr. Bull. 2019, 44, 25–35. [Google Scholar] [CrossRef]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Patel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin D and cardiometabolic outcomes. Ann. Intern. Med. 2010, 152, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Aznaouridis, K.; Rahman, A.; Fike, L.; Kavtaradze, N.; Ahmed, Y.; Uphoff, I.; et al. Vitamin D Status is Associated with Arterial Stiffness and Vascular Dysfunction in Healthy Humans. J. Am. Coll. Cardiol. 2011, 57, E2049. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.S.; Tangpricha, V.; Quyyumi, A.A. Vitamin D and cardiovascular disease: Is the evidence solid? Eur. Hear. J. 2013, 34, 3691–3698. [Google Scholar] [CrossRef]
- Chitalia, N.; Recio-Mayoral, A.; Kaski, J.C.; Banerjee, D. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atheroscler 2012, 220, 265–268. [Google Scholar] [CrossRef]
- Judd, S.; Nanes, M.S.; Ziegler, T.R.; Wilson, P.W.F.; Tangpricha, V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: Results from the third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 2008, 87, 136–141. [Google Scholar] [CrossRef]
- London, G.M.; Guérin, A.P.; Verbeke, F.H.; Pannier, B.; Boutouyrie, P.; Marchais, S.J.; Metivier, F. Mineral Metabolism and Arterial Functions in End-Stage Renal Disease: Potential Role of 25-Hydroxyvitamin D Deficiency. J. Am. Soc. Nephrol. 2007, 18, 613–620. [Google Scholar] [CrossRef]
- Motiwala, S.R.; Wang, T.J. Vitamin D and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 345–353. [Google Scholar] [CrossRef]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, Ethnicity, and Blood Pressure in the Third National Health and Nutrition Examination Survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Yiu, Y.-F.; Chan, Y.-H.; Yiu, K.-H.; Siu, C.-W.; Li, S.-W.; Wong, L.-Y.; Lee, S.W.L.; Tam, S.; Wong, E.W.K.; Cheung, B.M.Y.; et al. Vitamin D Deficiency Is Associated with Depletion of Circulating Endothelial Progenitor Cells and Endothelial Dysfunction in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Simhan, H.N.; Catov, J.M.; Roberts, J.M.; Platt, R.W.; Diesel, J.C.; Klebanoff, M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Vaqueiro, M.; Baré, M.L.; Anton, E. Association of low serum 25-hydroxyvitamin D levels and high arterial blood pressure in the elderly. Nephrol. Dial. Transplant. 2010, 25, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Sun, B.; Larson, C.; Forman, J.P.; Williams, J.S. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: An interventional study. J. Clin. Endocrinol. Metab. 2012, 97, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, E.A.S.; Munir, N.; Iniesta, R.R. Effect of vitamin D supplementation on cardiovascular disease risk factors and exercise performance in healthy participants: A randomized placebo-controlled preliminary study. Ther. Adv. Endocrinol. Metab. 2016, 7, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; DeMay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.-C.; Slater, S.; Jurutka, P.W. Vitamin D receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008, 66, 98–112. [Google Scholar] [CrossRef]
- Elliott, P.; McKenna, W. Hypertrophic cardiomyopathy: A 50th anniversary. Heart 2008, 94, 1247–1248. [Google Scholar] [CrossRef]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Bukhari, I.; Yakout, S.M.; Sabico, S.; Khattak, M.N.K.; Aziz, I.; Alokail, M.S. Associations of Serum Nitric Oxide with Vitamin D and Other Metabolic Factors in Apparently Healthy Adolescents. Biomed. Res. Int. 2018, 2018, 1489132. [Google Scholar] [CrossRef] [PubMed]
- Arfian, N.; Kusuma, M.H.; Anggorowati, N.; Nugroho, D.B.; Jeffilano, A.; Suzuki, Y.; Ikeda, K.; Emoto, N. Vitamin D upregulates endothelin-1, ETBR, eNOS mRNA expression and attenuates vascular remodelling and ischemia in kidney fibrosis model in mice. Physiol. Res. 2018, 67, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1alpha,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol. Biochem. 2011, 27, 661–668. [Google Scholar] [CrossRef]
- Robinson, A.T.; Szczurek, M.; Bian, J.T.; Cavka, A.; Grizelj, I.; Phillips, S. Mitochondrial reactive oxygen species contribute to impaired flow-induced dilation in visceral but not subcutaneous adipose tissue resistance arteries in human obesity. FASEB J. 2013, 27, 687. [Google Scholar]
- Phillips, S.A.; Hatoum, O.A.; Gutterman, D.D. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am. J. Physiol. Circ. Physiol. 2007, 292, 93–100. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J. Pharmacol. Sci. 2003, 92, 1–6. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Szczurek, M.R.; Blackburn, B.K.; Mey, J.T.; Chen, Z.; Robinson, A.T.; Bian, J.-T.; Unterman, T.G.; Minshall, R.D.; Brown, M.D.; et al. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol. Rep. 2016, 4, e12895. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hwang, C.-L.; Szczurek, M.R.; Bian, J.-T.; Ranieri, C.; Gutterman, D.D.; Phillips, S.A. Low-Fat Diet Designed for Weight Loss but not Weight Maintenance Improves Nitric Oxide-Dependent Arteriolar Vasodilation in Obese Adults. Nutrition 2019, 11, 1339. [Google Scholar] [CrossRef]
- Miura, H.; Wachtel, R.E.; Liu, Y.; Loberiza, F.R.; Saito, T.; Miura, M.; Gutterman, D.D. Flow-induced dilation of human coronary arterioles: Important role of Ca (2+)-activated K (+) channels. Circulation 2001, 103, 1992–1998. [Google Scholar] [CrossRef]
- Robinson, A.T.; Franklin, N.C.; Norkeviciute, E.; Bian, J.T.; Babana, J.C.; Szczurek, M.R.; Phillips, S.A. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J. Hypertens. 2016, 34, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Grizelj, I.; Cavka, A.; Bian, J.-T.; Szczurek, M.; Robinson, A.; Shinde, S.; Nguyen, V.; Braunschweig, C.; Wang, E.; Drenjancevic, I.; et al. Reduced flow- and acetylcholine-induced dilations in visceral compared to subcutaneous adipose arterioles in human morbid obesity. Microcirculation 2015, 22, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Thengchaisri, N.; Kuo, L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: Role of cyclooxygenase and potassium channels. Am. J. Physiol. Circ. Physiol. 2003, 285, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Matoba, T.; Shimokawa, H.; Kubota, H.; Morikawa, K.; Fujiki, T.; Kunihiro, I.; Mukai, Y.; Hirakawa, Y.; Takeshita, A. Hydrogen Peroxide is an Endothelium-Derived Hyperpolarizing Factor in Human Mesenteric Arteries. Biochem. Biophys. Res. Commun. 2002, 290, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.K.; Delansorne, R.; Man, R.Y.K.; Vanhoutte, P.M. Vitamin D derivatives acutely reduce endothelium—Dependent contractions in the aorta of the spontaneously hypertensive rat. Am. J. Physiol. Circ. Physiol. 2008, 295, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, S.; Sambuceti, G.; Giusti, M.; Morbelli, S.; Murialdo, G.; Garibotto, G.; Lara, V.; Pietro, A.; Barbara, R.; Mehrdad, N.; et al. 1,25-Dihydroxy vitamin D and coronary microvascular function. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Merke, J.; Milde, P.; Lewicka, S.; Hügel, U.; Klaus, G.; Mangelsdorf, D.J.; Haussler, M.R.; Rauterberg, E.W.; Ritz, E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J. Clin. Investig. 1989, 83, 1903–1915. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin D3-1 α-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.-F.; Liu, S.Q.; Cao, L.-P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Martínez-Miguel, P.; Valdivielso, J.M.; Medrano-Andrés, D.; Román-García, P.; Cano-Peñalver, J.L.; Rodríguez-Puyol, M.; Rodríguez-Puyol, D.; López-Ongil, S. The active form of vitamin D, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. Am. J. Physiol. Metab. 2014, 307, 1085–1096. [Google Scholar] [CrossRef]
- Khan, A.; Dawoud, H.; Malinski, T. Nanomedical studies of the restoration of nitric oxide/peroxynitrite balance in dysfunctional endothelium by 1,25-dihydroxy vitamin D3—Clinical implications for cardiovascular diseases. Int. J. Nanomed. 2018, 13, 455–466. [Google Scholar] [CrossRef]
- Jia, X.; Xu, J.; Gu, Y.; Gu, X.; Li, W.; Wang, Y. Vitamin D suppresses oxidative stress-induced microparticle release by human umbilical vein endothelial cells. Boil. Reprod. 2017, 96, 199–210. [Google Scholar] [CrossRef]
- Xu, J.; Jia, X.; Gu, Y.; Lewis, D.F.; Gu, X.; Wang, Y. Vitamin D Reduces Oxidative Stress—Induced Procaspase-3/ROCK1 Activation and MP Release by Placental Trophoblasts. J. Clin. Endocrinol. Metab. 2017, 102, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Hoe, E.; Nathanielsz, J.; Toh, Z.Q.; Spry, L.; Marimla, R.; Balloch, A.; Mulholland, K.; Licciardi, P.V. Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection. Nutrition 2016, 8, 806. [Google Scholar] [CrossRef]
- Naeini, A.E.; Moeinzadeh, F.; Vahdat, S.; Ahmadi, A.; Hedayati, Z.P.; Shahzeidi, S. The Effect of Vitamin D Administration on Intracellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 Levels in Hemodialysis Patients: A Placebo-controlled, Double-blinded Clinical Trial. J. Res. Pharm. Pract. 2017, 6, 16–20. [Google Scholar]
- Min, B. Effects of Vitamin D on Blood Pressure and Endothelial Function. Korean J. Physiol. Pharmacol. 2013, 17, 385–392. [Google Scholar] [CrossRef]
- Carbone, F.; Mach, F.; Vuilleumier, N.; Montecucco, F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J. Cardiol. 2014, 6, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, 2035. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Witham, M.D. Vitamin D and the cardiovascular system. Osteoporos. Int. 2013, 24, 2167–2180. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Khan, F.; Struthers, A.D.; Armitage, J.; Barchetta, I.; Bressendorff, I.; Cavallo, M.G.; Clarke, R.; Dalan, R.; Dreyer, G.; et al. Effect of Vitamin D Supplementation on Markers of Vascular Function: A Systematic Review and Individual Participant Meta-Analysis. J. Am. Hear. Assoc. 2018, 7, e008273. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, L.A.; Struthers, A.D.; Khan, F.; Jorde, R.; Scragg, R.; Macdonald, H.M.; Alvarez, J.A.; Boxer, R.S.; Dalbeni, A.; Gepner, A.D.; et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015, 175, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hussin, A.M.; Ashor, A.W.; Schoenmakers, I.; Hill, T.; Mathers, J.C.; Siervo, M. Effects of vitamin D supplementation on endothelial function: A systematic review and meta-analysis of randomised clinical trials. Eur. J. Nutr. 2017, 56, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Sanguankeo, A.; Congrete, S.; Jaruvongvanich, V. Effect of cholecalciferol supplementation on arterial stiffness: A systematic review and meta-analysis. Scand. Cardiovasc. J. 2016, 50, 230–235. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Zhou, J.C.; Zhu, Y.M.; Chen, Z.; Mo, J.L.; Xie, F.Z.; Wen, Y.H.; Guo, P.; Peng, J.; Xu, J.; Wang, J.; et al. Oral vitamin D supplementation has a lower bioavailability and reduces hypersecretion of parathyroid hormone and insulin resistance in obese Chinese males. Public Health Nutr. 2015, 18, 2211–2219. [Google Scholar] [CrossRef]
- Durand, M.J.; Dharmashankar, K.; Bian, J.T.; Das, E.; Vidovich, M.; Gutterman, D.D.; Phillips, S.A. Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension 2015, 65, 140–145. [Google Scholar] [CrossRef]
- Beyer, A.M.; Durand, M.J.; Hockenberry, J.; Gamblin, T.C.; Phillips, S.A.; Gutterman, D.D. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am. J. Physiol. Circ. Physiol. 2014, 307, 1587–1593. [Google Scholar] [CrossRef]
- Cai, H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc. Res. 2005, 68, 26–36. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Mott, M.M.; Hess, D.T.; Apovian, C.M.; McDonnell, M.E.; Duess, M.-A.; Kluge, M.A.; Fiscale, A.J.; Vita, J.A.; Gokce, N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity 2010, 18, 754–759. [Google Scholar] [CrossRef]
- Brook, R.D. Obesity, Weight Loss, and Vascular Function. Endocrine 2006, 29, 21–26. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: Findings from a systematic review. Int. J. Obes. 2008, 32, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Pinho, C.P.S.; da Silva Diniz, A.; de Arruda, I.K.G.; Leite, A.P.D.L.; Rodrigues, I.G. Effects of weight loss on adipose visceral and subcutaneous tissue in overweight adults. Clin. Nutr. 2018, 37, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevåg, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Bosnjak, J.J.; Ning, G.; Saito, T.; Miura, M.; Gutterman, D.D. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003, 92, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D Deficiency in Adults: When to Test and How to Treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Lespessailles, E.; Toumi, H. Vitamin D alteration associated with obesity and bariatric surgery. Exp. Boil. Med. 2017, 242, 1086–1094. [Google Scholar] [CrossRef]
- Riedt, C.S.; Brolin, R.E.; Sherrell, R.M.; Field, M.P.; Shapses, S.A. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity 2006, 14, 1940–1948. [Google Scholar] [CrossRef]
- Shaker, J.L.; Norton, A.J.; Woods, M.F.; Fallon, M.D.; Findling, J.W. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporos. Int. 1991, 1, 177–181. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European Guidelines on metabolic and bariatric surgery. Obes. Surg. 2013, 24, 42–55. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient—2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity 2013, 21, 1–27. [Google Scholar]


| Surgery (n = 15) | Post-Surgery (n = 8) | p-Value | |
|---|---|---|---|
| Age (years) | 37 ± 6 | 35 ± 6 | 0.455 |
| Sex | 2 ♂, 13 ♀ | 1 ♂, 7 ♀ | |
| Height (cm) | 164.9 ± 6.0 | 166.8 ± 7.7 | 0.518 |
| Body weight (kg) | 132.5 ± 10.4 | 119.2 ± 3.5 * | 0.002 |
| BMI (kg/m2) | 47.1 ± 6.3 | 40.7 ± 5.6 * | 0.025 |
| Waist circumference (cm) | 131.1 ± 12.6 | 115.4 ± 13.8 * | 0.012 |
| Surgery (n = 15) | Post-Surgery (n = 8) | p-Value | |
|---|---|---|---|
| Systolic BP (mm Hg) | 130.3 ± 13.5 | 119.6 ± 9.8 * | 0.042 |
| Diastolic BP (mm Hg) | 78.1 ± 9.1 | 66.7 ± 3.7 * | 0.003 |
| Heart rate (beats/min) | 85.2 ± 12.9 | 84.5 ± 10.8 | 0.897 |
| Total cholesterol (mg/dL) | 185.9 ± 20.8 | 168.7 ± 19.3 * | 0.044 |
| Triglycerides (mg/dL) | 78.8 ± 10.2 | 77.3 ± 16.5 | 0.789 |
| Glucose (mg/dL) | 87.4 ± 15.4 | 83.8 ± 13.3 * | 0.047 |
| Insulin (µIU/mL) | 10.1 ± 2.0 | 9.7 ± 1.3 | 0.616 |
| 1-25(OH)2D (ng/mL) | 13.8 ± 1.8 | 18.1 ± 2.2 * | 0.0003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.M.; Szczurek, M.; Hassan, C.; Masrur, M.; Gangemi, A.; Phillips, S.A. Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients. Nutrients 2019, 11, 2521. https://doi.org/10.3390/nu11102521
Mahmoud AM, Szczurek M, Hassan C, Masrur M, Gangemi A, Phillips SA. Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients. Nutrients. 2019; 11(10):2521. https://doi.org/10.3390/nu11102521
Chicago/Turabian StyleMahmoud, Abeer M., Mary Szczurek, Chandra Hassan, Mario Masrur, Antonio Gangemi, and Shane A. Phillips. 2019. "Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients" Nutrients 11, no. 10: 2521. https://doi.org/10.3390/nu11102521
APA StyleMahmoud, A. M., Szczurek, M., Hassan, C., Masrur, M., Gangemi, A., & Phillips, S. A. (2019). Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients. Nutrients, 11(10), 2521. https://doi.org/10.3390/nu11102521





