Urine Metabolites and Antioxidant Effect after Oral Intake of Date (Phoenix dactylifera L.) Seeds-Based Products (Powder, Bread and Extract) by Human
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Date Seed Products
2.3. Treatments
2.4. Blood and Urine Collection
2.5. Biochemical Parameters
2.6. Urine Analysis of Polyphenols Metabolites
2.7. Biomarkers of the Oxidative Status
2.8. Dietary Assessment
2.9. Statistical Analysis
3. Results
3.1. Participants and Polyphenols Consumption
3.2. Biochemical Assessment
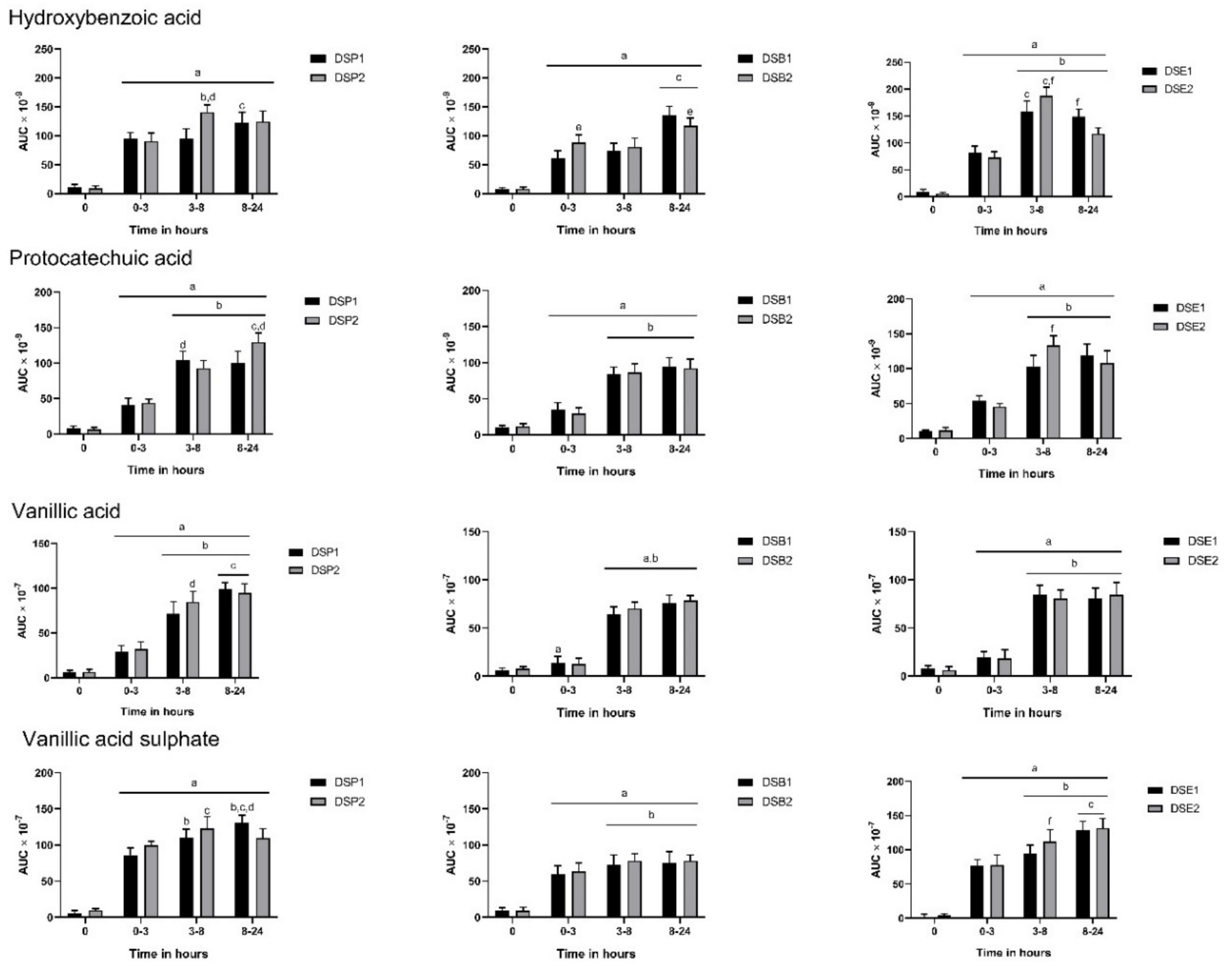
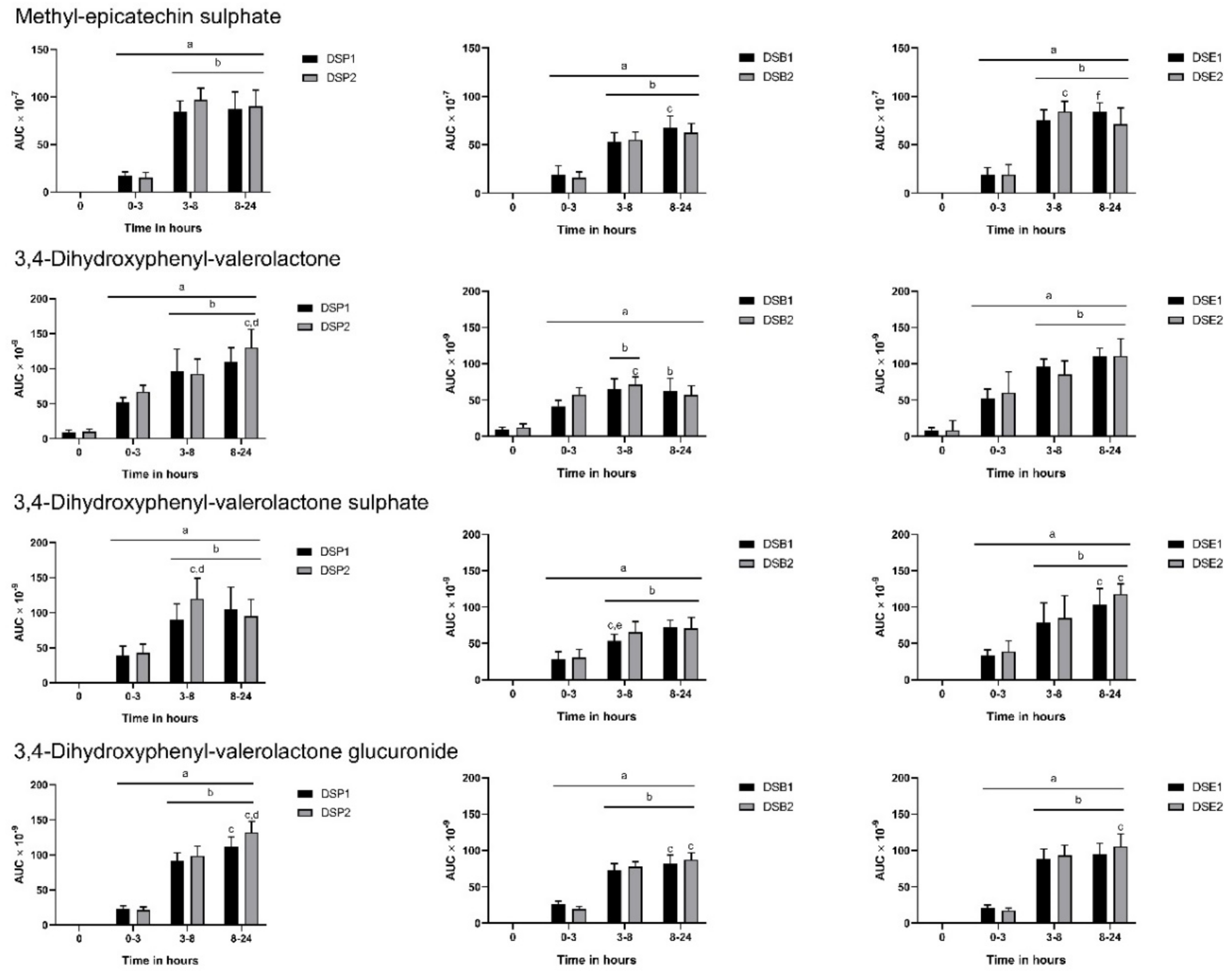
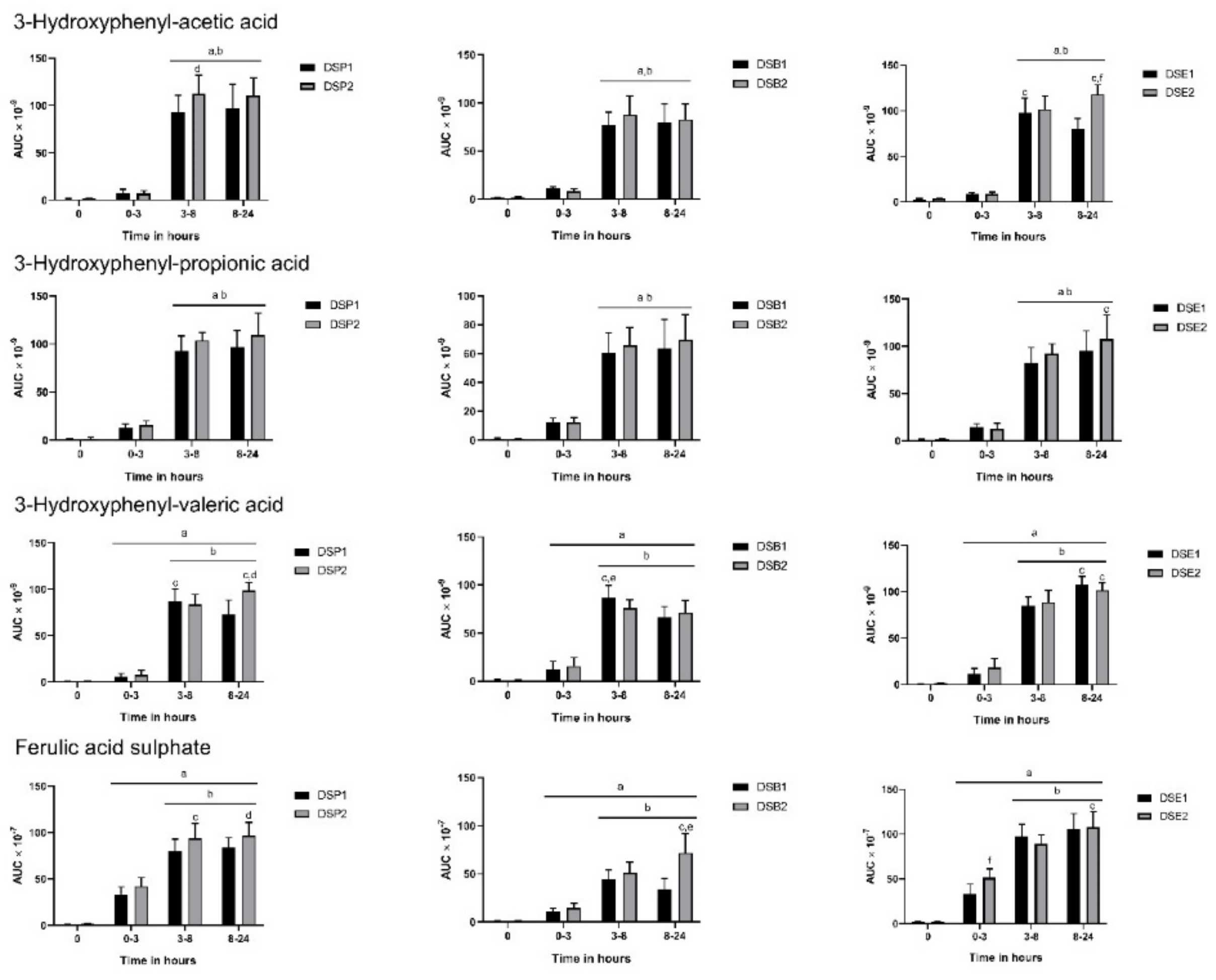
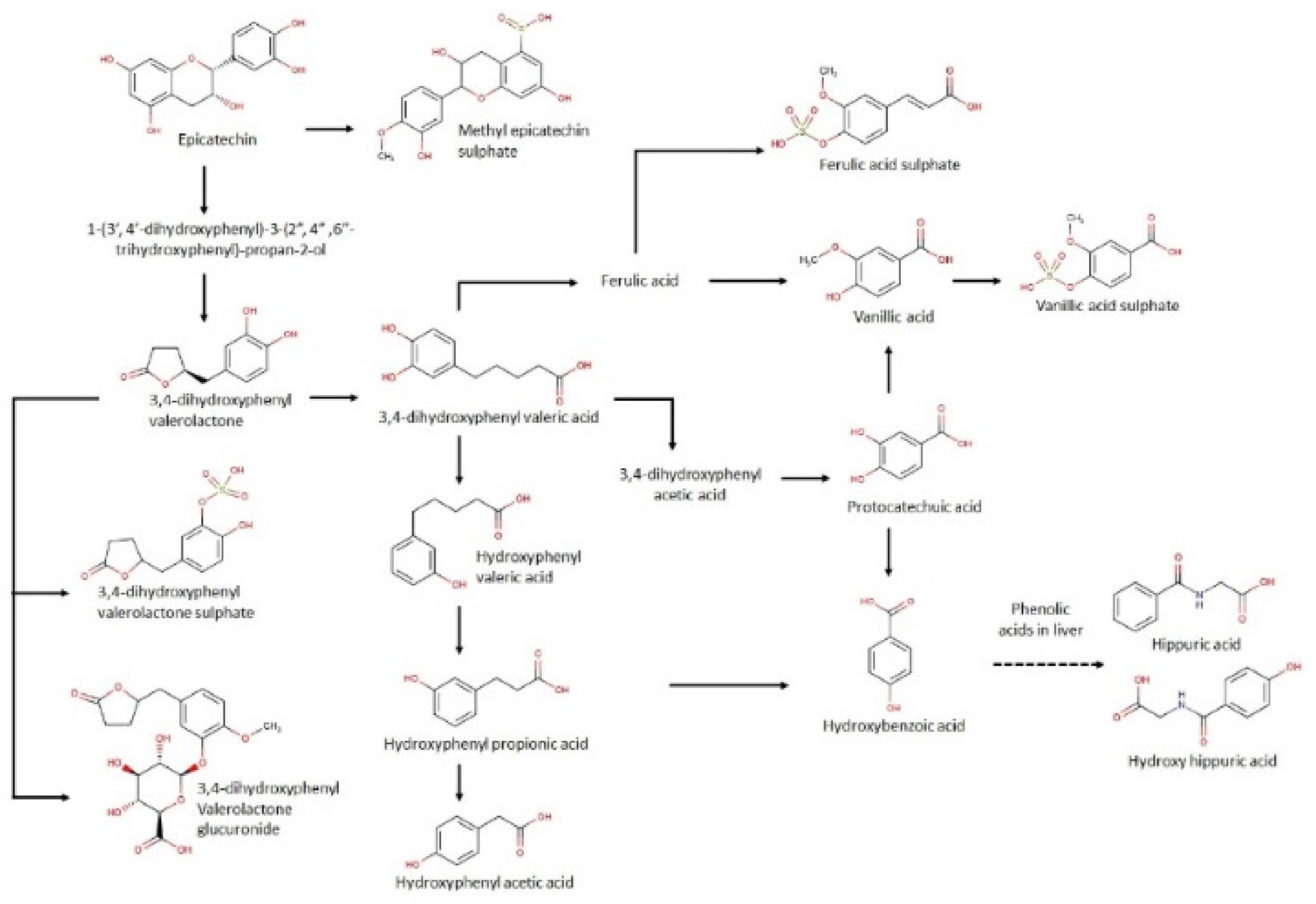
3.3. Urinary Metabolites
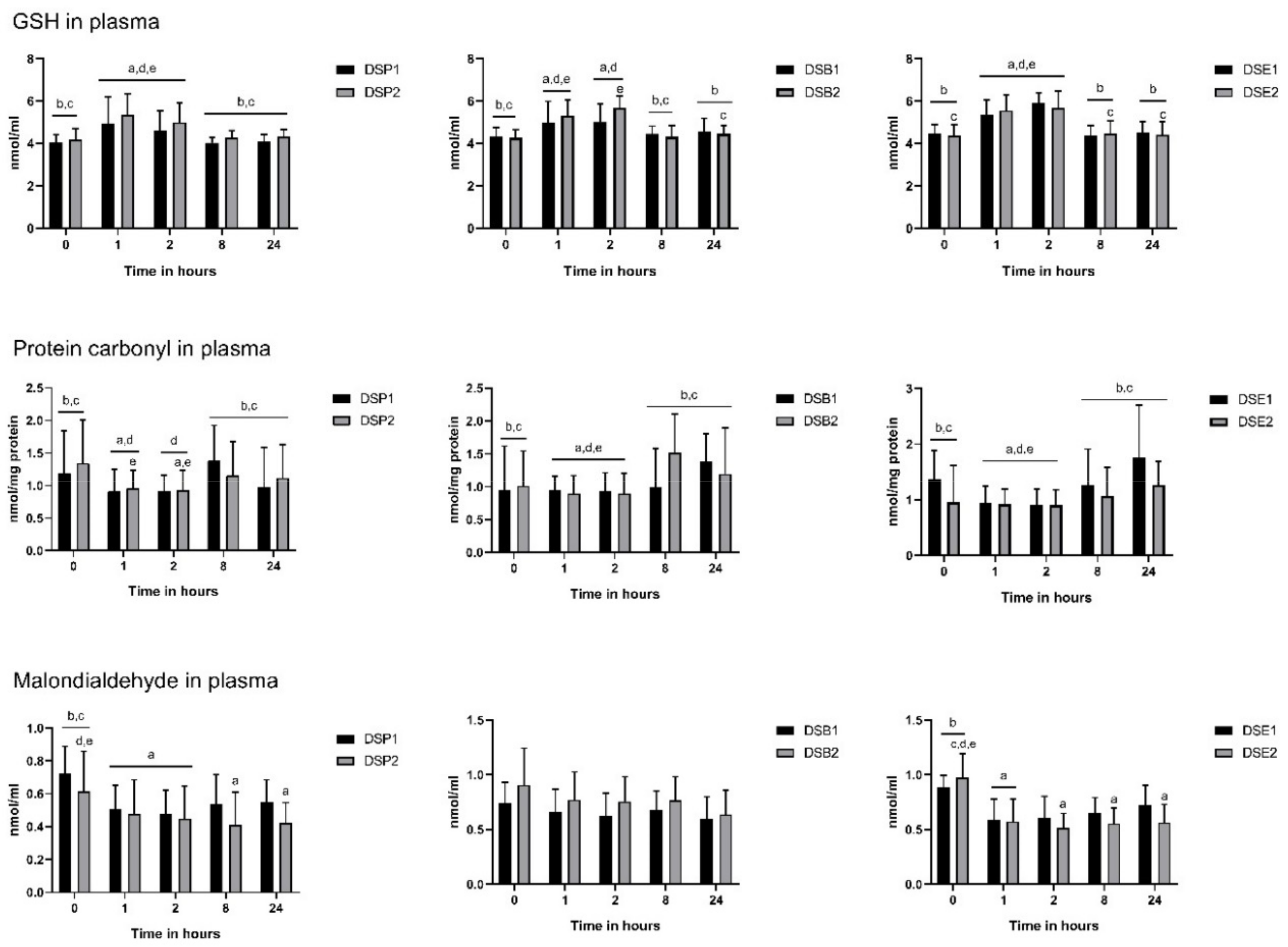
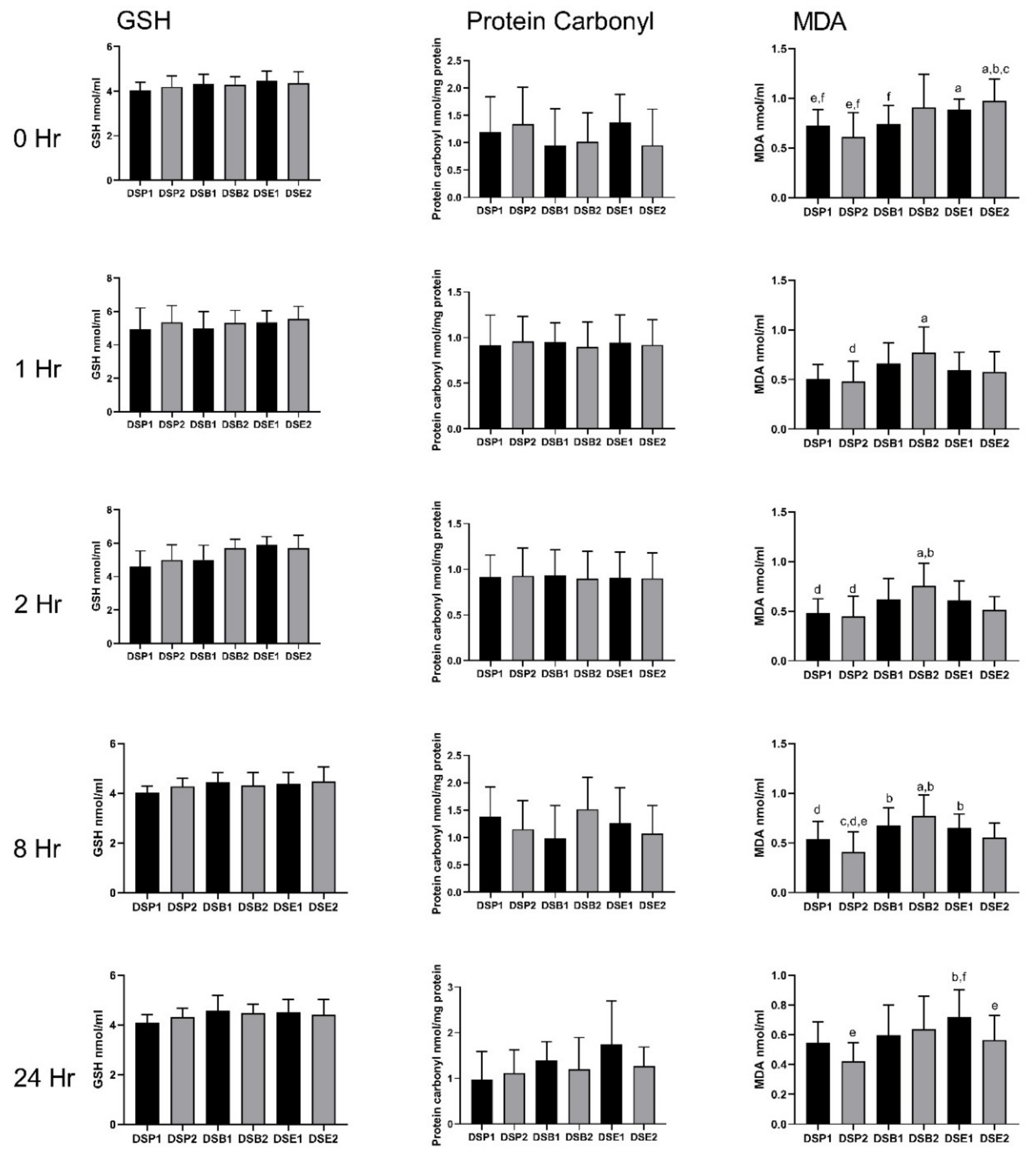
3.4. Biomarkers of Oxidative Stress
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Pérez-Jiménez, J.; Neveu, V.; Voß, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, Q.; Zhang, C.; Cao, W.; Fan, D.; Yang, H. Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties. Molecules 2016, 21, 832. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, M.J.; Aires, A.; Dias, C.; Almeida, J.A.; De Vasconcelos, M.; Santos, P.; Rosa, E.A. Evaluation of the potential of squash pumpkin by-products (seeds and shell) as sources of antioxidant and bioactive compounds. J. Food Sci. Technol. 2015, 52, 1008–1015. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. The Bioactivity of Pomegranate: Impact on Health and Disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef]
- Feringa, H.H.; Laskey, D.A.; Dickson, J.E.; Coleman, C.I. The Effect of Grape Seed Extract on Cardiovascular Risk Markers: A Meta-Analysis of Randomized Controlled Trials. J. Am. Diet. Assoc. 2011, 111, 1173–1181. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M.; Nassiri-Asl, M.; Nassiri-Asl, M. Review of the Pharmacological Effects ofVitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar]
- Gođevac, D.; Tešević, V.; Vajs, V.; Milosavljević, S.; Zdunić, G.; ĐOrđević, B.; Stanković, M. Chemical composition of currant seed extracts and their protective effect on human lymphocytes DNA. J. Food Sci. 2012, 77, C779–C783. [Google Scholar] [CrossRef]
- Khammuang, S.; Sarnthima, R. Antioxidant and antibacterial activities of selected varieties of thai mango seed extract. Pak. J. Pharm. Sci. 2011, 24, 37–42. [Google Scholar]
- Platat, C.; Habib, H.M.; Al Maqbali, F.D.; Jaber, N.N.; Ibrahim, W.H. Identification of Date Seeds Varieties Patterns to Optimize Nutritional Benefits of Date Seeds. J. Nutr. Food Sci. 2014, 8, 2. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, W.H. Nutritional quality evaluation of eighteen date pit varieties. Int. J. Food Sci. Nutr. 2009, 60, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sirisena, S.; Zabaras, D.; Ng, K.; Ajlouni, S. Characterization of Date (Deglet Nour) Seed Free and Bound Polyphenols by High-Performance Liquid Chromatography-Mass Spectrometry: HPLC characterization of date seed free and bound polyphenols. J. Food Sci. 2017, 82, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Platat, C.; Meudec, E.; Cheynier, V.; Ibrahim, W.H. Polyphenolic compounds in date fruit seed (Phoenix dactylifera): Characterisation and quantification by using UPLC-DAD-ESI-MS. J.Sci. Food Agric. 2014, 94, 1084–1089. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, W.H. Effect of date seeds on oxidative damage and antioxidant status in vivo. J. Sci. Food Agric. 2011, 91, 1674–1679. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.; Othman, A.; Al-Marzooqi, S.; Al-Bawardi, A.; Yasin, J.; Hilary, S.; Theyab, F.; Souka, U.; Al-Hammadi, S.; et al. Safety and protective effect of date (Phoenix Dactylifera) seed extract against oxidative damage in rat. Int. J. Food Nutr. Sci. 2015, 4, 21–28. [Google Scholar]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Meral, R.; Doğan, İ.S. Grape seed as a functional food ingredient in bread-making. Int. J. Food Sci. Nutr. 2013, 64, 372–379. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.M.; Hashim, I.B.; Kamal, H.; AlMaqbali, F.; Souka, U.; Ibrahim, W.H. Production of functional pita bread using date seed powder. J. Food Sci. Technol. 2015, 52, 6375–6384. [Google Scholar] [CrossRef] [Green Version]
- Hilary, S.; Tomas-Barberan, F.A.; Martinez-Blazquez, J.A.; Kizhakkayil, J.; Souka, U.; Al-Hammadi, S.; Habib, H.; Ibrahim, W.; Platat, C. Polyphenol characterisation of Phoenix dactylifera L. (date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. Under Review.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Hilary, S.; Habib, H.; Souka, U.; Ibrahim, W.; Platat, C. Bioactivity of arid region honey: an in vitro study. BMC Complement. Altern. Med. 2017, 17, 177. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A.; Castegna, A.; Drake, J.; Pocernich, C.; Butterfield, D.A. Protein Carbonyl Levels– An Assessment of Protein Oxidation. In Methods in Biological Oxidative Stress; Springer: Berlin/Heidelberg, Germany, 2003; pp. 161–168. [Google Scholar]
- Mirmiran, P.; Noori, N.; Zavareh, M.B.; Azizi, F. Fruit and vegetable consumption and risk factors for cardiovascular disease. Metabolism 2009, 58, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Al Meqbaali, F.T.; Habib, H.; Othman, A.; Al-Marzooqi, S.; Al-Bawardi, A.; Pathan, J.Y.; Hilary, S.; Souka, U.; Al-Hammadi, S.; Ibrahim, W.; et al. The antioxidant activity of date seed: preliminary results of a preclinical in vivo study. Emir. J. Food Agric. 2017, 29, 822. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; De Diego, E.H.; Riomoros-Arranz, M.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Effects of acute intake of grape/pomegranate pomace dietary supplement on glucose metabolism and oxidative stress in adults with abdominal obesity. Int. J. Food Sci. Nutr. 2019, 1–12. [Google Scholar] [CrossRef]
- García-Alonso, J.; Ros, G.; Vidal-Guevara, M.L.; Periago, M.J. Acute intake of phenolic-rich juice improves antioxidant status in healthy subjects. Nutr. Res. 2006, 26, 330–339. [Google Scholar] [CrossRef]
- Rios, L.Y.; Gonthier, M.-P.; Rémésy, C.; Mila, I.; Lapierre, C.; A Lazarus, S.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, M.-P.; Cheynier, V.; Donovan, J.L.; Manach, C.; Morand, C.; Mila, I.; Lapierre, C.; Rémésy, C.; Scalbert, A. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J. Nutr. 2003, 133, 461–467. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Isabel Bravo, F.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B. A comparative study on the bioavailability of phenolic compounds from organic and nonorganic red grapes. Food Chem. 2019, 299, 125092. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.-P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free. Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Scheline, R.R. CRC Handbook of Mammalian Metabolism of Plant Compounds; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Ahmad, M.; Baba, W.N.; Shah, U.; Gani, A.; Gani, A.; Masoodi, F.A. Nutraceutical Properties of the Green Tea Polyphenols. J. Food Process. Technol. 2014, 5, 1–5. [Google Scholar]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure−Activity Relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.Y.; Waterhouse, A.L. Proanthocyanidin metabolism, a mini review. Nutr. Aging 2014, 2, 111–116. [Google Scholar]
- Ward, N.C.; Croft, K.D.; Puddey, I.B.; Hodgson, J.M. Supplementation with Grape Seed Polyphenols Results in Increased Urinary Excretion of 3-Hydroxyphenylpropionic Acid, an Important Metabolite of Proanthocyanidins in Humans. J. Agric. Food Chem. 2004, 52, 5545–5549. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Dominguez, C.; Ruiz, E.; Gussinyé, M.; Carrascosa, A. Oxidative Stress at Onset and in Early Stages of Type 1 Diabetes in Children and Adolescents. Diabetes Care 1998, 21, 1736–1742. [Google Scholar] [CrossRef]
- Lacroix, S.; Badoux, J.K.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A comutationally driven analysis of the polyphenol-protein interactome. Sci. Rep. 2018, 8, 2232. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.-S.; Dong, L.-L. Inhibitory effect of polysaccharides from pumpkin on advanced glycation end-products formation and aldose reductase activity. Food Chem. 2012, 130, 821–825. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Yin, D.; Chen, S.; Gao, X. Coptis chinensisPolysaccharides Inhibit Advanced Glycation End Product Formation. J. Med. Food 2016, 19, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Zhao, C.N.; Gan, R.Y.; Xu, X.Y.; Wei, X.L.; Corke, H.; Atanasov, A.G.; Li, H.B. Effects and Mechanisms of Tea and Its Bioactive Compounds for the Prevention and Treatment of Cardiovascular Diseases: An Updated Review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Rosa, C.D.; Dos Santos, C.A.; Leite, J.I.A.; Caldas, A.P.S.; Bressan, J. Impact of nutrients and food components on dyslipidemias: what is the evidence? Adv. Nutr. 2015, 6, 703–711. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hariri, M. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol. Res. 2018, 129, 141–150. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of quercetin supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef]

| Treatment Number | Product | Dose | Polyphenols Content of the Product (g/kg) | Total Average Dose of Polyphenols * (g) |
|---|---|---|---|---|
| DSP1 | Date seed powder | 0.25 g/kg body weight | 49.75 | 0.74 |
| DSP2 | Date seed powder | 0.5 g/kg body weight | 99.5 | 1.48 |
| DSB1 | Date seed pita bread | 10% date seed powder, 6 loaves of 60 g each | 13.48 | 1.79 |
| DSB2 | Date seed pita bread | 15% date seed powder, 6 loaves of 60 g each | 26.96 | 2.69 |
| DSE1 | Date seed extract | 30 mg/kg body weight | 41.11 | 0.075 |
| DSE2 | Date seed extract | 60 mg/kg body weight | 82.22 | 0.15 |
| Total (n = 14) | Female (n = 8) | Male (n = 6) | |
|---|---|---|---|
| Age (y) | 25.43 ± 5.11 | 26.75 ± 4.50 | 23.67 ± 5.75 |
| Height (cm) | 163.21 ± 10.44 | 156.14 ± 5.63 | 172.65 ± 7.20 * |
| Weight (kg) | 59.43 ± 6.97 | 54.81 ± 4.02 | 65.58 ± 4.92 * |
| BMI (kg/m2) | 22.32 ± 1.79 | 22.51 ± 1.58 | 22.07 ± 2.16 |
| Polyphenols consumption (g/d) | 3.09 ± 1.93 | 3.58 ± 2.35 | 2.27 ± 0.89 |
| Glucose (mg/dl) | Total Cholesterol (mg/dl) | LDL-Cholesterol (mg/dl) | HDL-Cholesterol (mg/dl) | Triglycerides (mg/dl) | |
|---|---|---|---|---|---|
| DSP1 | |||||
| Baseline | 91.07 ± 6.68 | 171.70 ± 25.46 | 107.66 ± 24.19 | 45.16 ± 10.16 | 72.49 ± 21.70 |
| 24 h | 93.99 ± 7.01 | 169.77 ± 25.04 | 104.55 ± 24.92 | 46.56 ± 9.61 | 78.27 ± 30.76 |
| DSP2 | |||||
| Baseline | 86.32 ± 12.98 | 169.58 ± 21.79 | 98.83 ± 25.93 | 46.71 ± 16.74 | 71.50 ± 25.72 |
| 24 h | 91.01 ± 7.31 | 168.94 ± 21.09 | 98.91 ± 21.00 | 48.12 ± 13.70 | 76.04 ± 17.62 |
| DSB1 | |||||
| Baseline | 87.67 ± 8.81 | 160.74 ± 27.68 | 97.07 ± 22.96 | 48.15 ± 17.35 | 71.95 ± 23.81 |
| 24 h | 88.84 ± 9.64 | 162.61 ± 29.94 | 92.99 ± 23.84 | 45.98 ± 15.76 | 71.20 ± 15.08 |
| DSP2 | |||||
| Baseline | 85.13 ± 9.85 | 165.47 ± 24.49 | 99.18 ± 23.44 | 47.07 ± 15.36 | 75.91 ± 22.74 |
| 24 h | 88.26 ± 11.38 | 169.26 ± 31.13 | 97.12 ± 25.40 | 46.39 ± 13.42 | 78.45 ± 19.06 |
| DSE1 | |||||
| Baseline | 84.78 ± 8.46 | 158.45 ± 27.20 | 92.54 ± 24.56 | 45.60 ± 15.92 | 80.94 ± 37.63 |
| 24 h | 89.56 ± 5.41 * | 168.28 ± 29.16 * | 100.16 ± 25.99 * | 47.47 ± 14.41 | 79.99 ± 28.65 |
| DSE2 | |||||
| Baseline | 84.74 ± 6.45 | 164.69 ± 20.28 | 88.17 ± 24.96 | 46.61 ± 19.31 | 65.19 ± 19.62 |
| 24 h | 83.70 ± 13.05 | 166.85 ± 23.81 | 91.25 ± 24.54 | 43.97 ± 16.25 | 84.37 ± 39.80 |
| Creatinine (µmol/l) | Albumin (g/dl) | Urea (mmol/l) | Creatine Kinase (U/dl) | Lactate Dehydrogenase (U/l) | GGT (U/l) | AST (U/l) | ALT (U/l) | ALP (U/l) | |
|---|---|---|---|---|---|---|---|---|---|
| DSP1 | |||||||||
| Baseline | 61.70 ± 13.54 | 4.67 ± 0.24 | 4.22 ± 1.13 | 94.70 ± 47.47 | 165.27 ± 56.72 | 14.26 ± 6.86 | 18.05 ± 4.05 | 14.72 ± 7.12 | 57.01 ± 14.01 |
| 24 h | 62.30 ± 12.70 | 4.64 ± 0.29 | 3.91 ± 0.80 | 82.08 ± 35.26 * | 136.77 ± 14.11 | 14.03 ± 6.84 | 16.57 ± 3.59 | 14.93 ± 8.81 | 59.23 ± 12.35 |
| DSP2 | |||||||||
| Baseline | 56.90 ± 17.55 | 4.31 ± 0.67 | 4.06 ± 1.27 | 83.69 ± 44.95 | 126.00 ± 32.23 | 12.93 ± 7.76 | 15.85 ± 2.46 | 13.83 ± 6.08 | 55.71 ± 16.25 |
| 24 h | 60.93 ± 13.61 | 4.64 ± 0.36 | 4.1186 ± 1.10 | 77.19 ± 27.98 | 115.78 ± 24.65 | 15.04 ± 8.54 * | 16.25 ± 3.26 | 14.26 ± 6.05 | 62.68 ± 16.31 * |
| DSB1 | |||||||||
| Baseline | 57.99 ± 16.71 | 4.50 ± 0.76 | 3.91 ± 1.06 | 83.87 ± 42.99 | 120.10 ± 17.04 | 13.93 ± 11.64 | 15.82 ± 3.50 | 13.06 ± 5.13 | 57.71 ± 19.43 |
| 24 h | 59.31 ± 15.53 | 4.31 ± 0.66 | 3.74 ± 0.95 | 74.85 ± 30.25 | 95.77 ± 33.12 * | 14.00 ± 10.68 | 14.64 ± 2.70 | 12.01 ± 4.90 | 57.32 ± 15.74 |
| DSB2 | |||||||||
| Baseline | 58.55 ± 16.69 | 4.40 ± 0.60 | 3.82 ± 1.03 | 87.82 ± 47.43 | 124.77 ± 27.93 | 13.35 ± 8.11 | 15.64 ± 4.30 | 12.61 ± 6.85 | 57.42 ± 16.82 |
| 24 h | 60.25 ± 16.88 | 4.38 ± 0.72 | 3.75 ± 0.83 | 79.81 ± 33.55 | 98.17 ± 23.88 * | 14.13 ± 9.26 | 15.13 ± 4.83 | 12.72 ± 8.17 | 57.78 ± 16.12 |
| DSE1 | |||||||||
| Baseline | 60.84 ± 15.33 | 4.31 ± 0.62 | 3.83 ± 1.24 | 82.75 ± 29.33 | 125.04 ± 79.58 | 12.74 ± 8.37 | 15.94 ± 6.16 | 11.89 ± 7.02 | 54.79 ± 16.10 |
| 24 h | 60.56 ± 14.71 | 4.66 ± 0.39 * | 3.64 ± 0.86 | 79.64 ± 27.69 | 106.56 ± 25.37 | 14.01 ± 7.76 | 15.18 ± 2.94 | 11.89 ± 6.26 | 56.89 ± 12.55 |
| DSE2 | |||||||||
| Baseline | 51.57 ± 16.15 | 4.20 ± 0.61 | 3.47 ± 1.31 | 77.84 ± 39.22 | 110.04 ± 35.85 | 11.33 ± 6.26 | 13.71 ± 3.53 | 11.23 ± 5.30 | 48.96 ± 17.24 |
| 24 h | 56.91 ± 17.40 | 4.42 ± 0.57 | 3.76 ± 1.02 | 69.80 ± 27.41 | 107.91 ± 35.63 | 13.63 ± 10.47 | 13.09 ± 3.60 | 12.01 ± 6.75 | 56.99 ± 15.75 |
| Compound | Formula | Pseudo-molecular ion (M-H) Exact | Retention Time (min) | Score | Error |
|---|---|---|---|---|---|
| Hydroxybenzoic acid 1 | C7 H6 O3 | 137.0244 | 3.090 | 99.49 ± 0.05 | 1.43 ± 2.45 |
| Hydroxybenzoic acid 2 | C7 H6 O3 | 137.0244 | 4.464 | 97.58 ± 0.84 | 2.86 ± 0.10 |
| Protocatechuic acid 1 | C7 H6 O4 | 153.0193 | 4.640 | 96.98 ± 1.06 | 3.73 ± 3.83 |
| Protocatechuic acid 2 | C7 H6 O5 | 153.0193 | 5.352 | 95.31 ± 2.06 | 1.64 ± 2.64 |
| Vanillic acid 1 | C8 H8 O4 | 167.035 | 3.300 | 95.39 ± 3.67 | 1.66 ± 3.93 |
| Vanillic acid 2 | C8 H8 O4 | 167.035 | 4.735 | 93.64 ± 3.29 | 0.23 ± 3.31 |
| Vanillic acid sulphate | C8 H8 O7 S | 246.9918 | 3.368 | 93.26 ± 2.10 | 2.19 ± 1.98 |
| Ferulic acid sulphate | C10 H6 O7 S | 273.0074 | 4.618 | 93.78 ± 2.44 | 2.31 ± 2.18 |
| Methyl epicatechin sulphate | C16 H6 O9 S | 383.0442 | 5.623 | 89.13 ± 3.55 | 0.01 ± 2.04 |
| 3,4-dihydroxyphenyl valerolactone | C11 H12 O4 | 207.0663 | 5.250 | 97.03 ± 1.76 | 2.56 ± 0.57 |
| 3,4-dihydroxyphenyl valerolactone glucuronide | C17 H20 O10 | 383.0984 | 5.126 | 97.30 ± 2.47 | 3.95 ± 2.44 |
| 3,4-dihydroxyphenyl valerolactone sulphate | C11 H12 O7 S | 287.0231 | 5.273 | 96.60 ± 2.40 | 1.50 ± 0.89 |
| 3-hydroxyphenyl acetic acid | C8 H8 O3 | 151.0401 | 6.006 | 91.47 ± 0.83 | 2.95 ± 2.55 |
| 3-hydroxyphenyl propionic acid | C9 H10 O3 | 165.0557 | 4.968 | 92.76 ± 0.78 | 1.89 ± 2.80 |
| 3-hydroxyphenyl valeric acid | C11 H14 O3 | 193.087 | 6.979 | 90.11 ± 0.63 | 2.65 ± 3.78 |
| 3,4-dihydroxyphenyl valeric acid | C11 H14 O4 | 209.0819 | 10.407 | 95.02 ± 1.11 | 0.56 ± 3.56 |
| 3,4-dihydroxyphenyl valeric acid sulphate | C11 H14 O7 S | 289.0387 | 10.863 | 92.48 ± 4.85 | 0.91 ± 0.46 |
| Hippuric acid | C9 H9 NO3 | 178.051 | 4.921 | 98.47 ± 0.28 | 1.87 ± 1.86 |
| Hydroxy hippuric acid 1 | C9 H9 NO4 | 194.0459 | 3.703 | 95.91 ± 1.71 | 0.58 ± 0.76 |
| Hydroxy hippuric acid 2 | C9 H9 NO4 | 194.0459 | 4.505 | 94.49 ± 0.78 | 3.28 ± 3.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platat, C.; Hilary, S.; Tomas-Barberan, F.A.; Martinez-Blazquez, J.A.; Al-Meqbali, F.; Souka, U.; Al-Hammadi, S.; Ibrahim, W. Urine Metabolites and Antioxidant Effect after Oral Intake of Date (Phoenix dactylifera L.) Seeds-Based Products (Powder, Bread and Extract) by Human. Nutrients 2019, 11, 2489. https://doi.org/10.3390/nu11102489
Platat C, Hilary S, Tomas-Barberan FA, Martinez-Blazquez JA, Al-Meqbali F, Souka U, Al-Hammadi S, Ibrahim W. Urine Metabolites and Antioxidant Effect after Oral Intake of Date (Phoenix dactylifera L.) Seeds-Based Products (Powder, Bread and Extract) by Human. Nutrients. 2019; 11(10):2489. https://doi.org/10.3390/nu11102489
Chicago/Turabian StylePlatat, Carine, Serene Hilary, Francisco A. Tomas-Barberan, J. Alberto Martinez-Blazquez, Fatima Al-Meqbali, Usama Souka, Suleiman Al-Hammadi, and Wissam Ibrahim. 2019. "Urine Metabolites and Antioxidant Effect after Oral Intake of Date (Phoenix dactylifera L.) Seeds-Based Products (Powder, Bread and Extract) by Human" Nutrients 11, no. 10: 2489. https://doi.org/10.3390/nu11102489
APA StylePlatat, C., Hilary, S., Tomas-Barberan, F. A., Martinez-Blazquez, J. A., Al-Meqbali, F., Souka, U., Al-Hammadi, S., & Ibrahim, W. (2019). Urine Metabolites and Antioxidant Effect after Oral Intake of Date (Phoenix dactylifera L.) Seeds-Based Products (Powder, Bread and Extract) by Human. Nutrients, 11(10), 2489. https://doi.org/10.3390/nu11102489





