Effects of Resveratrol in Goto-Kakizaki Rat, a Model of Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
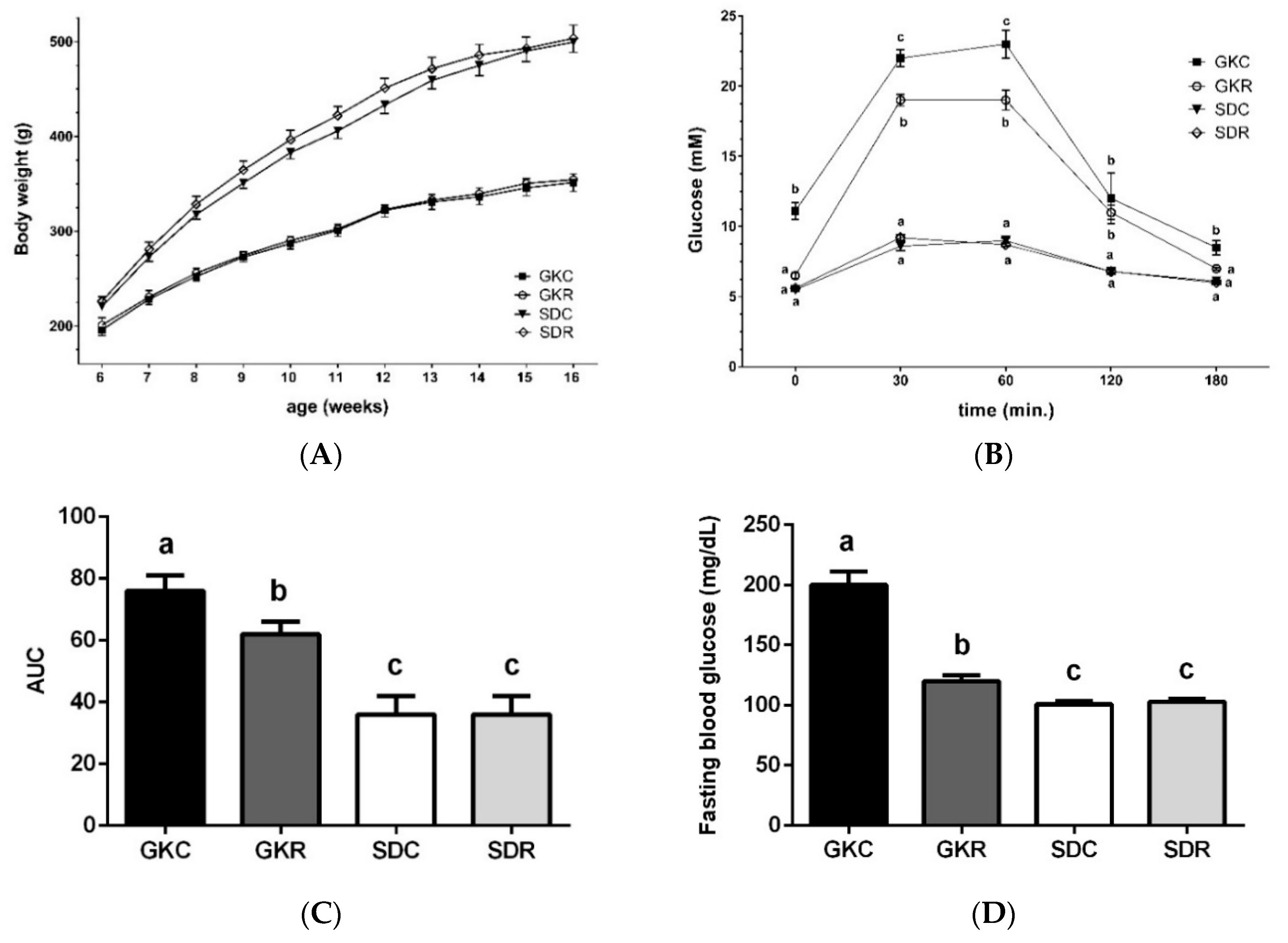
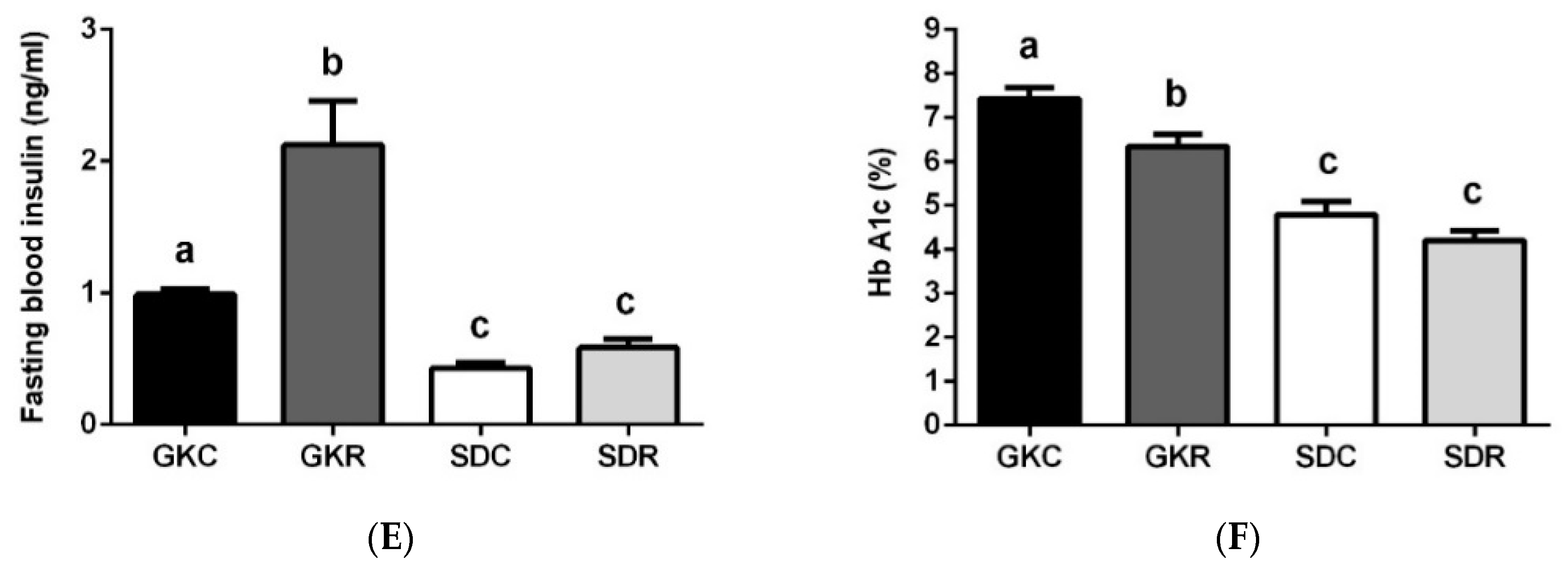
2.2. Glucose Tolerance Test and Fasting Blood Insulin Levels
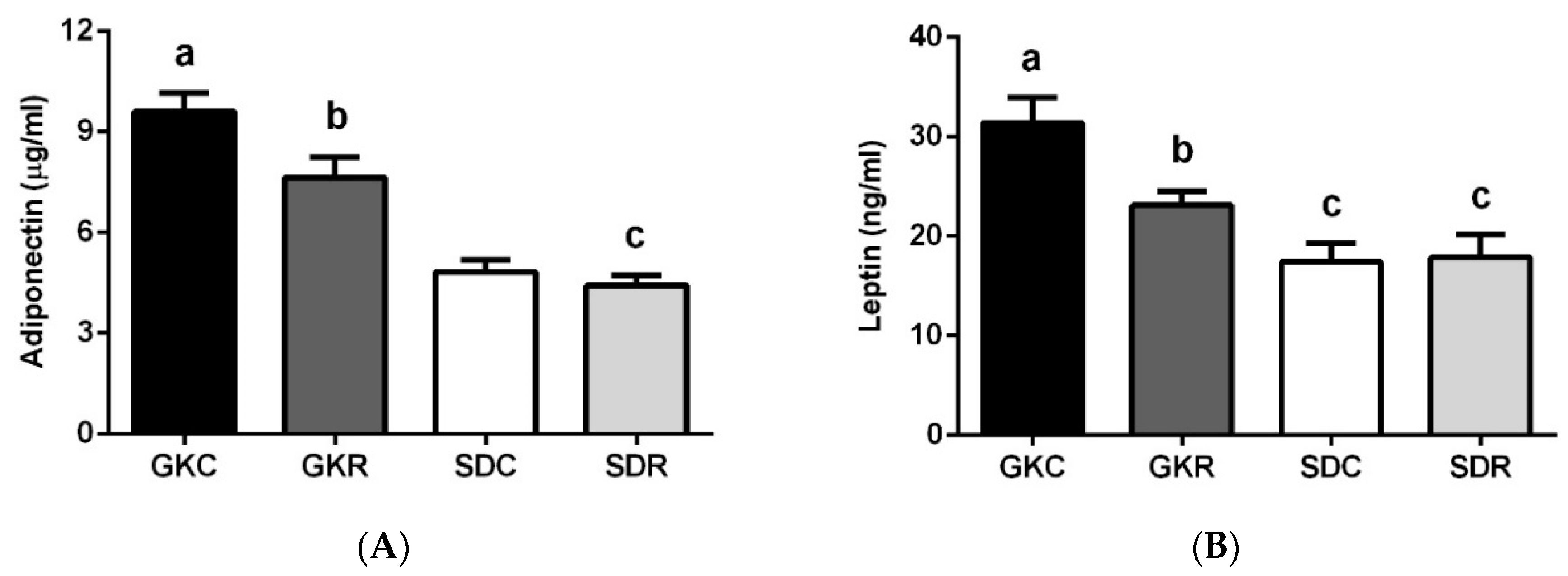
2.3. Blood Adipokine and HbA1c Levels
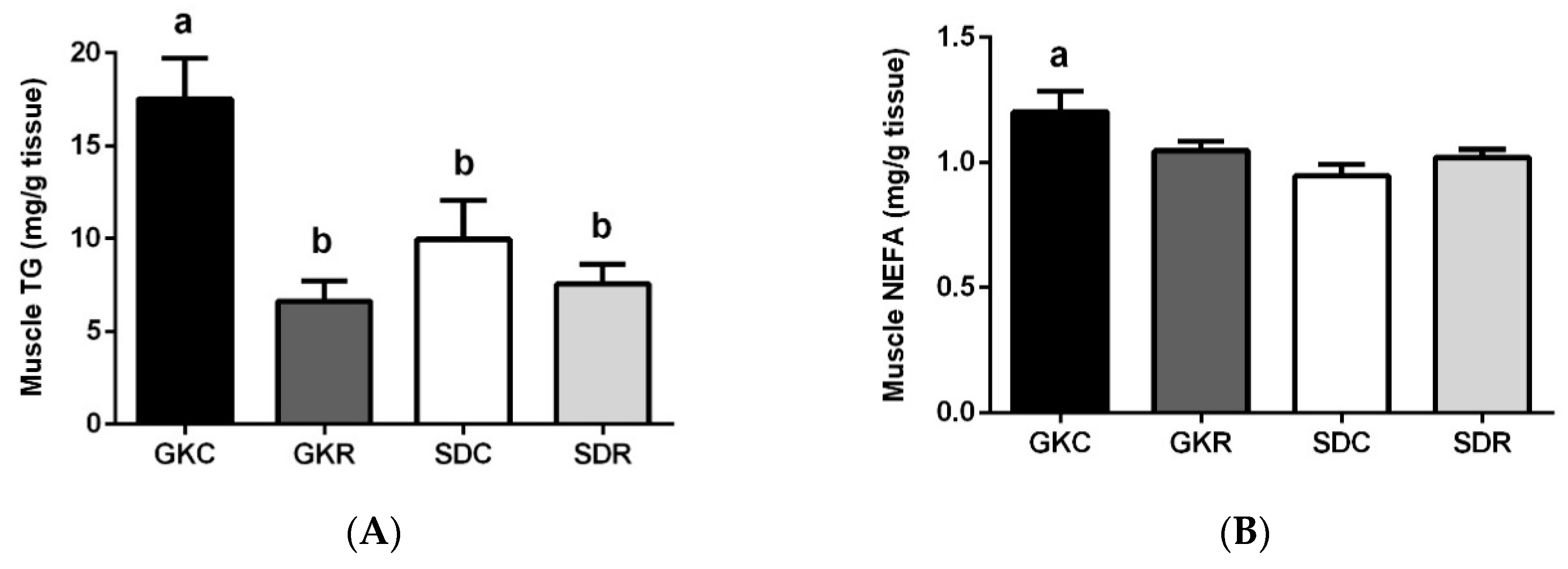
2.4. Liver and Muscle Lipids
2.5. Western Blot Immunodetection
2.6. Immunohistochemistry
2.7. Liver Histology
2.8. Statistical Analysis
3. Results
3.1. Resveratrol Improves Glucose Tolerance in GK Rats
3.2. Effects of Resveratrol on Blood Adipokine Levels
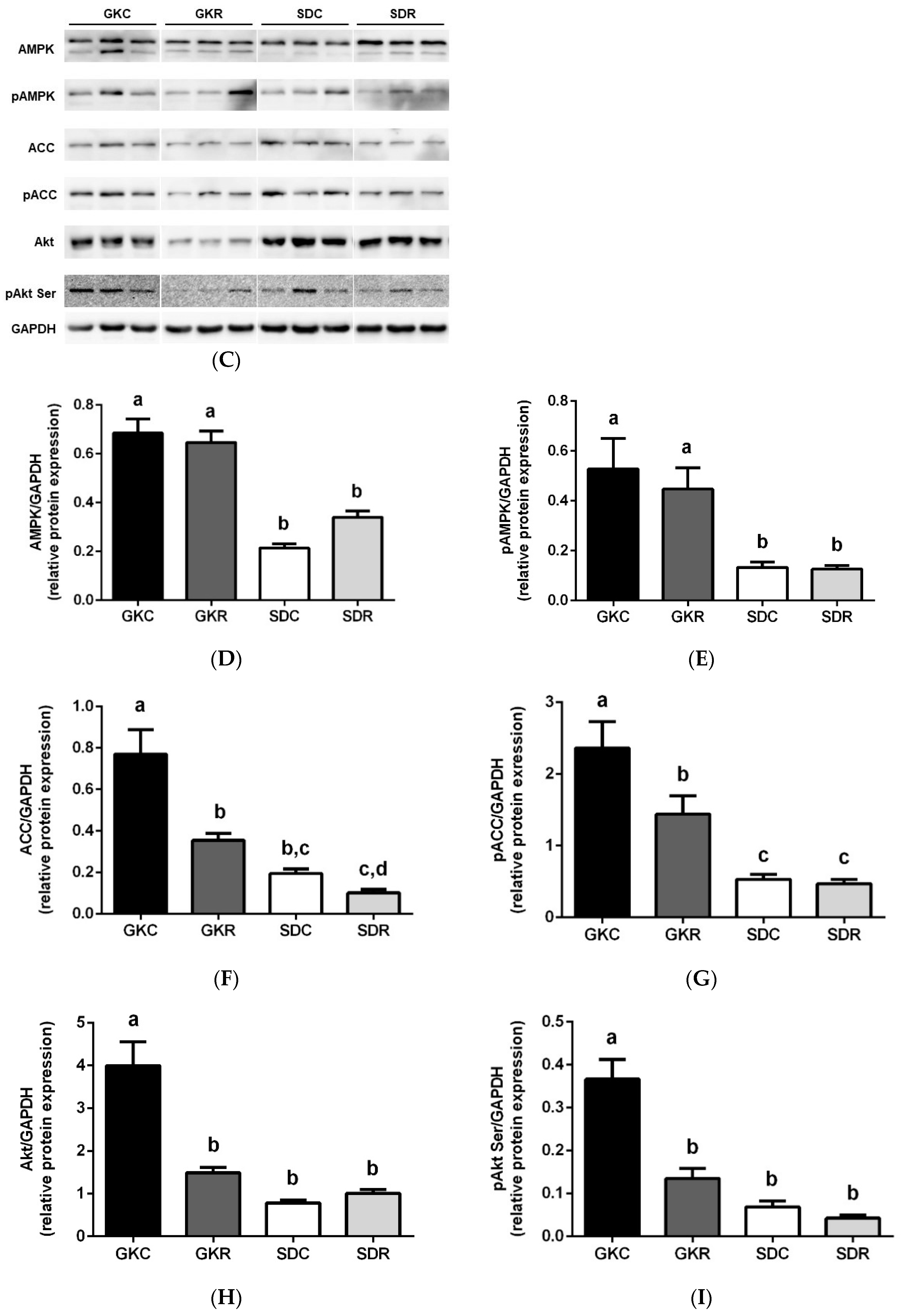
3.3. Effects of Resveratrol on Skeletal Muscle Lipids, Expression and Phosphorylation of AMPK, ACC and Akt in Muscle Tissue
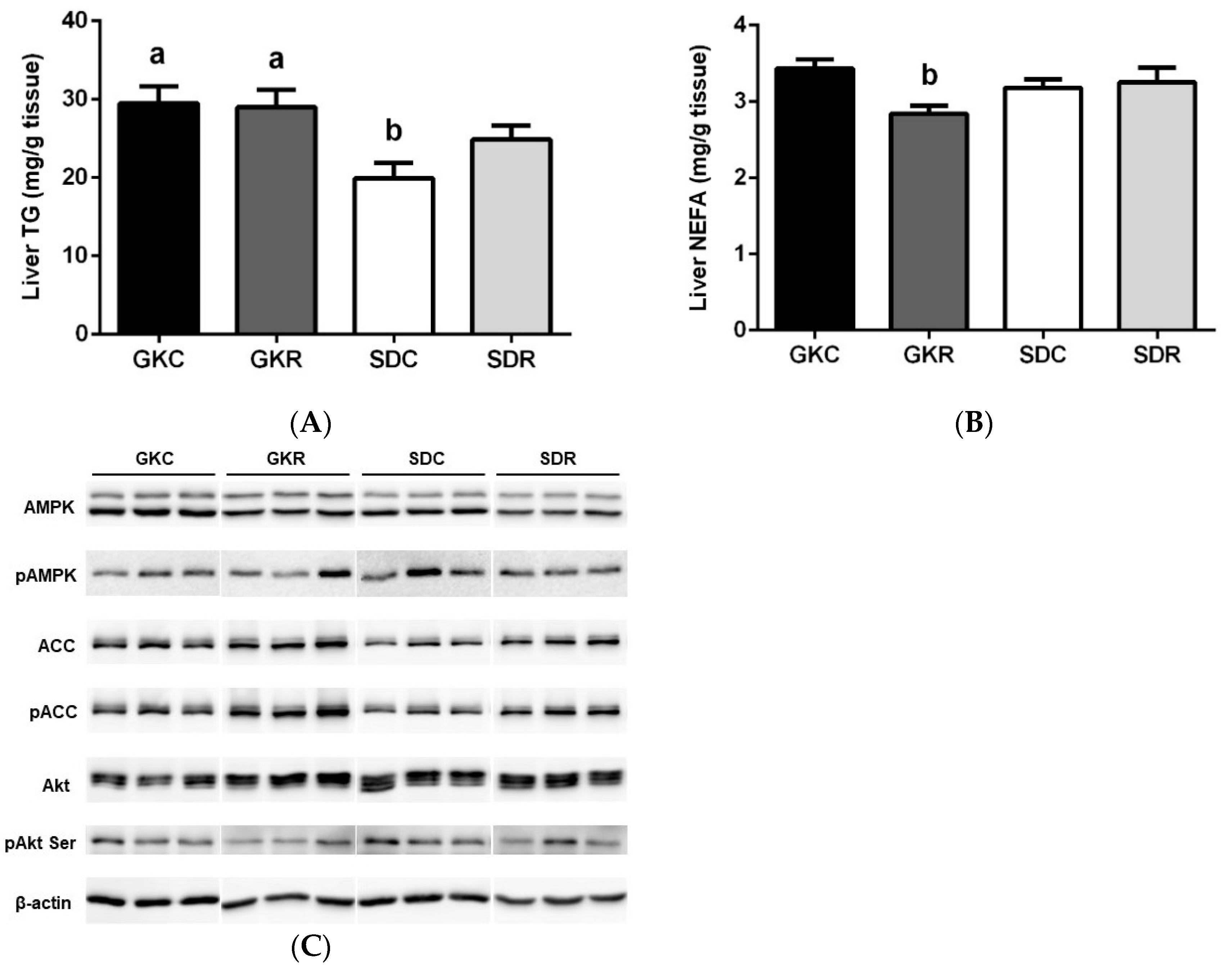
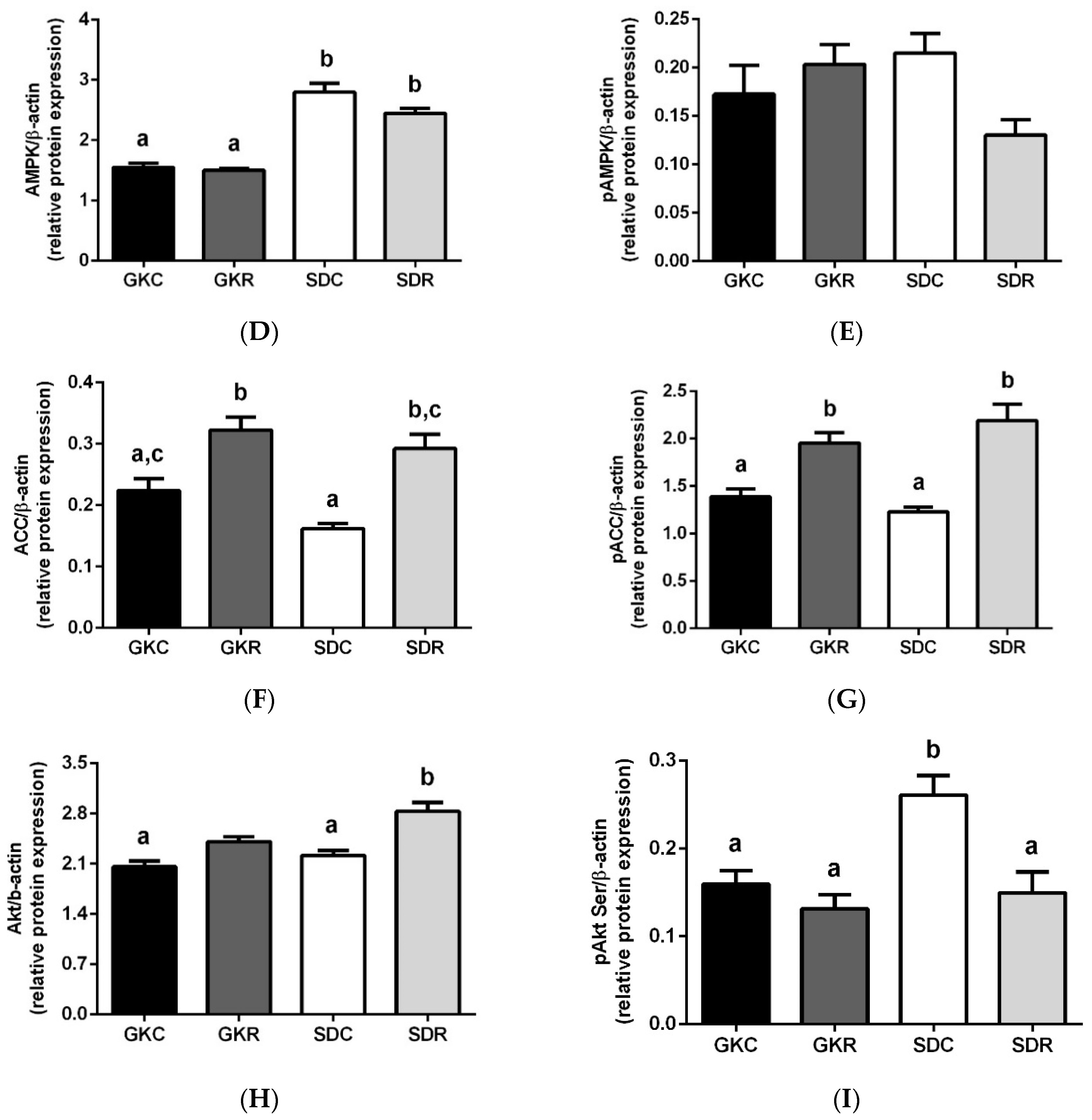
3.4. Effects of Resveratrol on Liver Lipids, Expression and Phosphorylation of AMPK, ACC and Akt in Liver Tissue
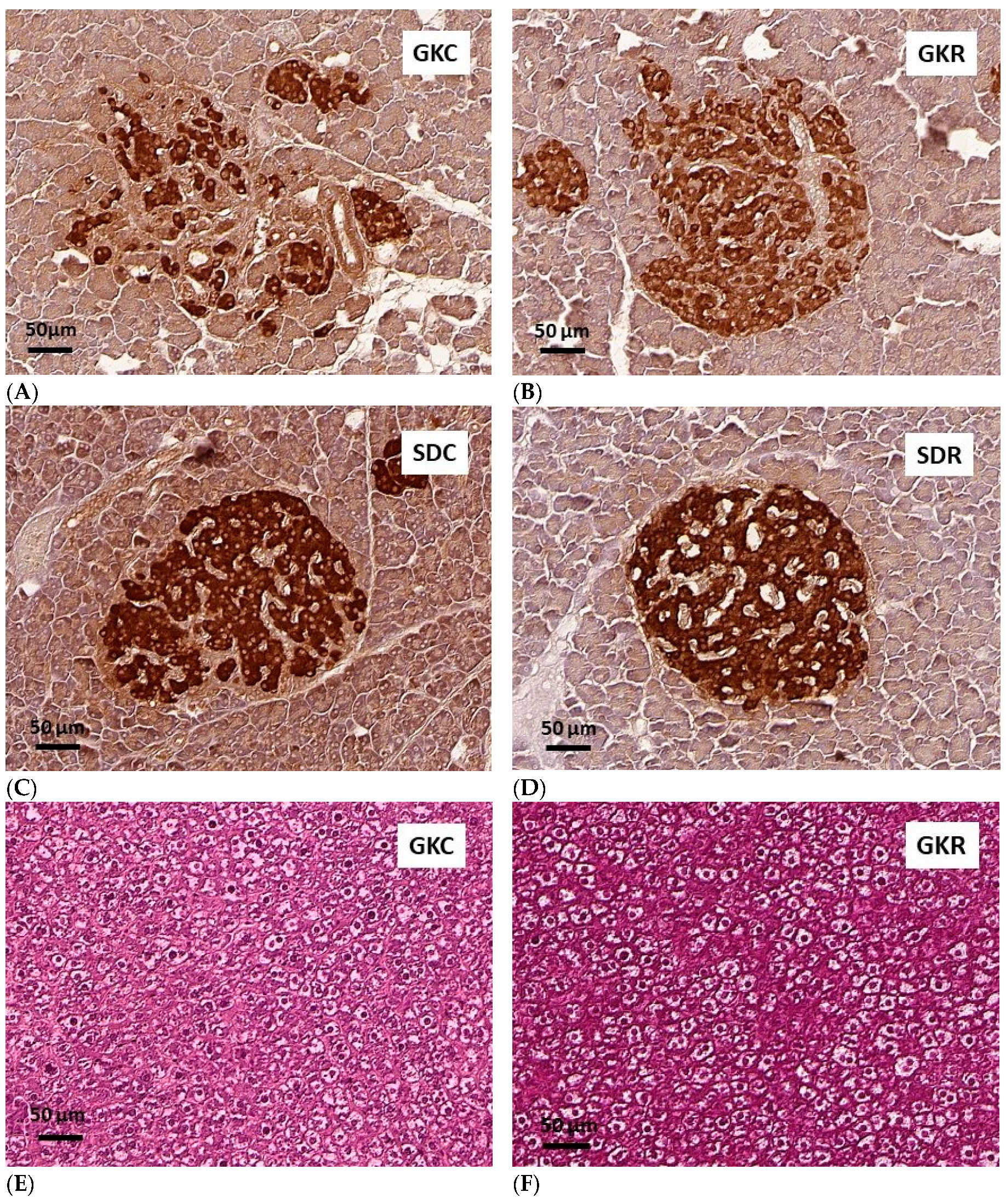
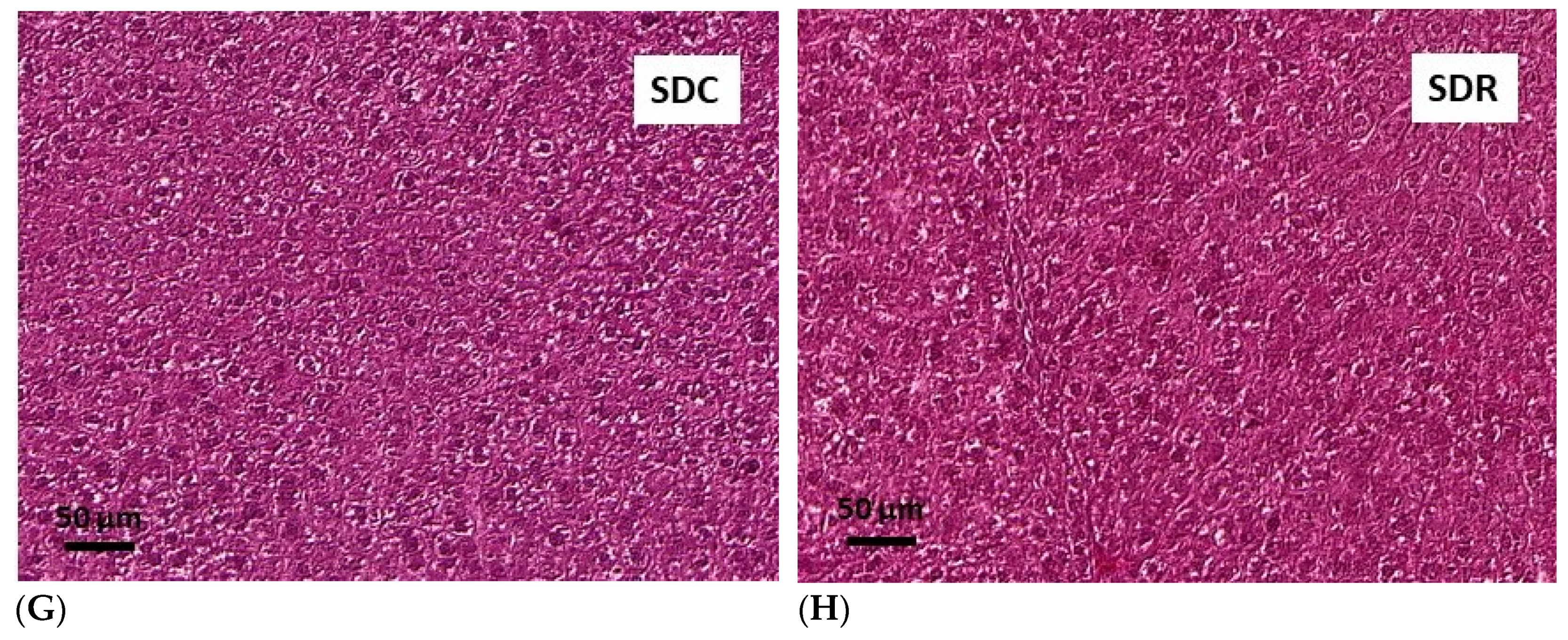
3.5. Effects of Resveratrol on Morphology of Pancreatic Islets and Liver Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biagi, M.; Bertelli, A.A. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta 2015, 1852, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Tishinsky, J.M.; Robinson, L.E.; Dyck, D.J. Insulin-sensitizing properties of adiponectin. Biochimie 2012, 94, 2131–2136. [Google Scholar] [CrossRef]
- De Ligt, M.; Timmers, S.; Schrauwen, P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim. Biophys. Acta 2015, 1852, 1137–1144. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjær, T.N.; Nøhr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 2015, 1852, 1124–1136. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Drygalski, K.; Konstantynowicz-Nowicka, K.; Berk, K.; Chabowski, A. Alternative treatment methods attenuate the development of NAFLD: A review of resveratrol molecular mechanisms and clinical trials. Nutrition 2017, 34, 108–117. [Google Scholar] [CrossRef]
- Szkudelska, K.; Deniziak, M.; Roś, P.; Gwóźdź, K.; Szkudelski, T. Resveratrol alleviates ethanol-induced hormonal and metabolic disturbances in the rat. Physiol. Res. 2017, 66, 135–145. [Google Scholar]
- Szkudelski, T.; Szkudelska, K. Potential of resveratrol in mitigating metabolic disturbances induced by ethanol. Biomed. Pharmacother. 2018, 101, 579–584. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-Zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on Blood Glucose, Insulin, Insulin Resistance, Triglyceride, and Periodontal Markers in Type 2 Diabetic Patients with Chronic Periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects with Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef]
- De Light, M.; Bruls, Y.M.H.; Hansen, J.; Habets, M.F.; Havekes, B.; Nascimento, E.B.M.; Moonen-Kornips, E.; Schaart, G.; Schrauwen-Hinderling, V.B.; van Marken Lichtenbelt, W.; et al. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol. Metab. 2018, 12, 39–47. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Beaudeux, J.L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef]
- Akash, M.S.; Rehman, K.; Chen, S. Goto-Kakizaki rats: Its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr. Diabetes Rev. 2013, 5, 387–396. [Google Scholar] [CrossRef]
- Kuwabara, W.M.T.; Panveloski-Costa, A.C.; Yokota, C.N.F.; Pereira, J.N.B.; Filho, J.M.; Torres, R.P.; Hirabara, S.M.; Curi, R.; Alba-Loureiro, T.C. Comparison of Goto-Kakizaki rats and high fat diet-induced obese rats: Are they reliable models to study type 2 diabetes mellitus? PLoS ONE 2017, 12, 189622. [Google Scholar] [CrossRef]
- Portha, B.; Lacraz, G.; Kergoat, M.; Homo-Delarche, F.; Giroix, M.H.; Bailbé, D.; Gangnerau, M.N.; Dolz, M.; Tourrel-Cuzin, C.; Movassat, J. The GK rat beta-cell: A prototype for the diseased human beta-cell in type 2 diabetes? Mol. Cell Endocrinol. 2009, 297, 73–85. [Google Scholar] [CrossRef]
- Kawai, J.; Ohara-Imaizumi, M.; Nakamichi, Y.; Okamura, T.; Akimoto, Y.; Matsushima, S.; Aoyagi, K.; Kawakami, H.; Watanabe, T.; Watada, H.; et al. Insulin exocytosis in Goto-Kakizaki rat beta-cells subjected to long-term glinide or sulfonylurea treatment. Biochem. J. 2008, 412, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Duncombe, W.G. The colorimetric micro-determination of non-esterified fatty acids in plasma. Clin. Chim. Acta 1964, 9, 122–125. [Google Scholar] [CrossRef]
- Ochiai, M.; Kuroda, T.; Matsuo, T. Increased muscular triglyceride content and hyperglycemia in Goto-Kakizaki rat are decreased by egg white hydrolysate. Int. J. Food Sci. Nutr. 2014, 65, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Crisóstomo, J.; Matafome, P.; Louro, T.; Nunes, E.; Seiça, R. Dietary restriction improves systemic and muscular oxidative stress in type 2 diabetic Goto-Kakizaki rats. J. Physiol. Biochem. 2011, 67, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E. Metabolic fluxes in skeletal muscle in relation to obesity and insulin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 391–403. [Google Scholar] [CrossRef]
- Gemmink, A.; Goodpaster, B.H.; Schrauwen, P.; Hesselink, M.K.C. Intramyocellular lipid droplets and insulin sensitivity, the human perspective. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1242–1249. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Bitar, M.S.; Al-Saleh, E.; Al-Mulla, F. Oxidative stress--mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci. 2005, 77, 2552–2573. [Google Scholar] [CrossRef]
- Krook, A.; Kawano, Y.; Song, X.M.; Efendić, S.; Roth, R.A.; Wallberg-Henriksson, H.; Zierath, J.R. Improved glucose tolerance restores insulin-stimulated Akt kinase activity and glucose transport in skeletal muscle from diabetic Goto-Kakizaki rats. Diabetes 1997, 46, 2110–2114. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Rachek, L.I. Free fatty acids and skeletal muscle insulin resistance. Prog. Mol. Biol. Transl. Sci. 2014, 121, 267–292. [Google Scholar] [PubMed]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar]
- Kang, W.; Hong, H.J.; Guan, J.; Kim, D.G.; Yang, E.J.; Koh, G.; Park, D.; Han, C.H.; Lee, Y.J.; Lee, D.H. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: In vitro and in vivo experiments in rodents. Metabolism 2012, 61, 424–433. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Tung, H.C.; Chang, C.Y.; Tsai, Y.L.; Huang, J.P.; Yen, T.H.; Hung, L.M. Resveratrol exhibits differential protective effects on fast- and slow-twitch muscles in streptozotocin-induced diabetic rats. J. Diabetes 2014, 6, 60–67. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Yu, J.G.; Ofrecio, J.; Olefsky, J.M. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 2006, 55, 2277–2285. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Saha, A.K.; Vavvas, D.; Heydrick, S.J.; Kurowski, T.G. Lipid abnormalities in muscle of insulin-resistant rodents. The malonyl CoA hypothesis. Ann. N. Y. Acad. Sci. 1997, 827, 221–230. [Google Scholar] [CrossRef]
- Cahová, M.; Vavrínková, H.; Kazdová, L. Glucose-fatty acid interaction in skeletal muscle and adipose tissue in insulin resistance. Physiol. Res. 2007, 56, 1–15. [Google Scholar]
- Ruderman, N.B.; Cacicedo, J.M.; Itani, S.; Yagihashi, N.; Saha, A.K.; Ye, J.M.; Chen, K.; Zou, M.; Carling, D.; Boden, G.; et al. Malonyl-CoA and AMP-activated protein kinase (AMPK): Possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem. Soc. Trans. 2003, 31, 202–206. [Google Scholar] [CrossRef]
- Jørgensen, W.; Jelnes, P.; Rud, K.A.; Hansen, L.L.; Grunnet, N.; Quistorff, B. Progression of type 2 diabetes in GK rats affects muscle and liver mitochondria differently: Pronounced reduction of complex II flux is observed in liver only. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Steiler, T.L.; Galuska, D.; Leng, Y.; Chibalin, A.V.; Gilbert, M.; Zierath, J.R. Effect of hyperglycemia on signal transduction in skeletal muscle from diabetic Goto-Kakizaki rats. Endocrinology 2003, 144, 5259–5267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, J.; Feng, Z.; Liu, J.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Long, J.; Liu, J. Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: Ameliorating effects of (-)-epigallocatechin-3-gallate. J. Nutr. Biochem. 2012, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lam, K.S.; Wang, B.; Xu, A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 3–13. [Google Scholar] [CrossRef]
- Karahashi, M.; Hirata-Hanta, Y.; Kawabata, K.; Tsutsumi, D.; Kametani, M.; Takamatsu, N.; Sakamoto, T.; Yamazaki, T.; Asano, S.; Mitsumoto, A.; et al. Abnormalities in the metabolism of fatty acids and triacylglycerols in the liver of the Goto-Kakizaki rat: A model for non-obese type 2 diabetes. Lipids 2016, 51, 955–971. [Google Scholar] [CrossRef]
- Xue, B.; Sukumaran, S.; Nie, J.; Jusko, W.J.; Dubois, D.C.; Almon, R.R. Adipose tissue deficiency and chronic inflammation in diabetic Goto-Kakizaki rats. PLoS ONE 2011, 6, 17386. [Google Scholar] [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell. Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Engin, A. Adiponectin-resistance in obesity. Adv. Exp. Med. Biol. 2017, 960, 415–441. [Google Scholar]
- Barbu, A.; Hedlund, G.P.; Lind, J.; Carlsson, C. Pref-1 and adipokine expression in adipose tissues of GK and Zucker rats. Mol. Cell. Endocrinol. 2009, 299, 163–171. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, F.; Fujiwara, K.; Toriya, M.; Maejima, Y.; Nishio, T.; Toyoda, Y.; Nohara, K.; Yashiro, T.; Yada, T. Brain-derived neurotrophic factor in VMH as the causal factor for and therapeutic tool to treat visceral adiposity and hyperleptinemia in type 2 diabetic Goto-Kakizaki rats. Front. Synaptic Neurosci. 2013, 5, 7. [Google Scholar] [CrossRef]
- Papazoglou, I.; Berthou, F.; Vicaire, N.; Rouch, C.; Markaki, E.M.; Bailbe, D.; Portha, B.; Taouis, M.; Gerozissis, K. Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model. Mol. Cell. Endocrinol. 2012, 350, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. The role of leptin in the control of insulin-glucose axis. Front. Neurosci. 2013, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Dłużewicz, K.; Sadoch, J.; Szkudelska, K. Effects of the activation of heme oxygenase-1 on hormonal and metabolic changes in rats fed a high-fat diet. Biomed. Pharmacother. 2017, 87, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Z. Resistin’s, obesity and insulin resistance: The continuing disconnect between rodents and humans. J. Endocrinol. Investig. 2016, 39, 607–615. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Jadhav, A. Up-regulating the Hemeoxygenase Systems Enhances Insulin Sensivity and Improves Glucose Metabolism in Insulin-Resistant Diabetes in Goto-Kakizaki Rats. Endocrinology 2009, 150, 2627–2636. [Google Scholar] [CrossRef]
- Dong-Sun, K.; Thea-Wha, K.; Ju-Seop, K. Chromium picolinate supplementation improves insulin sensitivity in Goto-Kakizaki diabetic rats. J. Trace Elem. Med. Biol. 2004, 17, 243–247. [Google Scholar]
- Ndisang, J.F.; Lane, N.; Jadhav, A. Upregulation of the heme oxygenase system ameliorates postprandial and fasting hyperglycemia in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 1029–1041. [Google Scholar] [CrossRef]
- Liu, T.; Duan, W.; Nizigiyimana, P.; Gao, L.; Liao, Z.; Xu, B.; Liu, L.; Lei, M. Alpha-mangostin attenuates diabetic nephropathy in associacion with suppression of acid sphingomyelinase and endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2018, 496, 394–400. [Google Scholar] [CrossRef]
- Patriti, A.; Aisa, M.C.; Annetti, C.; Sidoni, A.; Galli, F.; Ferri, I.; Gulla, N.; Donini, A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-Kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery 2007, 142, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, W.; Liu, S.; Zhang, G.; Sun, D.; Hu, S. Myocardial insulin signaling and glucose transport are up-regulated in Goto-Kakizaki type 2 diabetic rats after ileal transposition. Obes. Surg. 2012, 22, 493–501. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudelska, K.; Deniziak, M.; Hertig, I.; Wojciechowicz, T.; Tyczewska, M.; Jaroszewska, M.; Szkudelski, T. Effects of Resveratrol in Goto-Kakizaki Rat, a Model of Type 2 Diabetes. Nutrients 2019, 11, 2488. https://doi.org/10.3390/nu11102488
Szkudelska K, Deniziak M, Hertig I, Wojciechowicz T, Tyczewska M, Jaroszewska M, Szkudelski T. Effects of Resveratrol in Goto-Kakizaki Rat, a Model of Type 2 Diabetes. Nutrients. 2019; 11(10):2488. https://doi.org/10.3390/nu11102488
Chicago/Turabian StyleSzkudelska, Katarzyna, Marzanna Deniziak, Iwona Hertig, Tatiana Wojciechowicz, Marianna Tyczewska, Magdalena Jaroszewska, and Tomasz Szkudelski. 2019. "Effects of Resveratrol in Goto-Kakizaki Rat, a Model of Type 2 Diabetes" Nutrients 11, no. 10: 2488. https://doi.org/10.3390/nu11102488
APA StyleSzkudelska, K., Deniziak, M., Hertig, I., Wojciechowicz, T., Tyczewska, M., Jaroszewska, M., & Szkudelski, T. (2019). Effects of Resveratrol in Goto-Kakizaki Rat, a Model of Type 2 Diabetes. Nutrients, 11(10), 2488. https://doi.org/10.3390/nu11102488





