Effects of Long-Versus Short-Term Exposure to the Mediterranean Diet on Skin Microvascular Function and Quality of Life of Healthy Adults in Greece and the UK
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design and Ethics
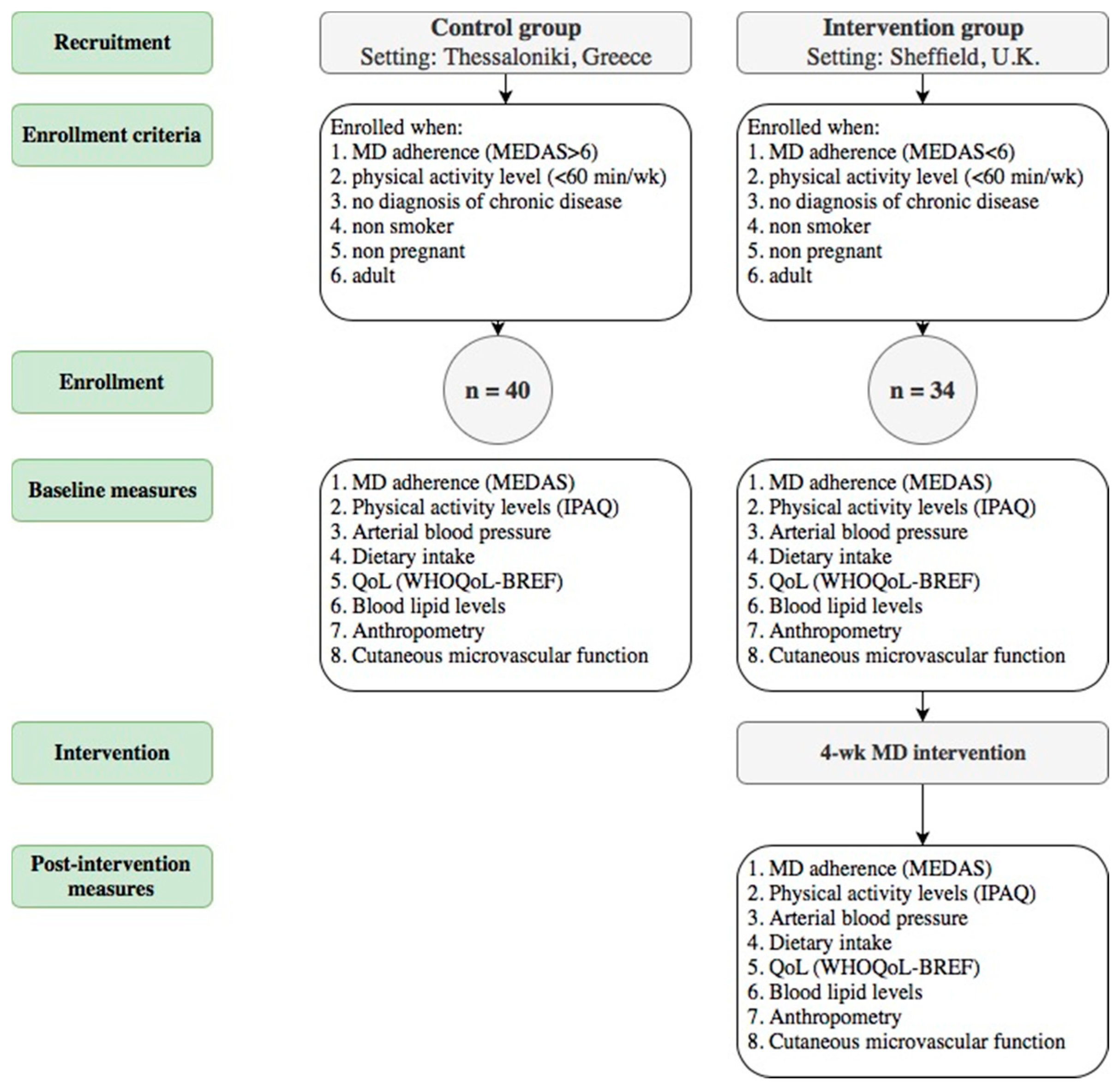
2.2. Study Population and Procedures
2.2.1. Mediterranean Diet Adherence
2.2.2. Physical Activity Levels, Anthropometry and Quality of Life
2.2.3. Blood Lipid Profile and Arterial Blood Pressure
2.2.4. Physiological Measures of CVD Risk—Laser Doppler Flowmetry (LDF)
2.2.5. Mediterranean Diet Intervention
2.3. Statistical Analysis
3. Results
3.1. Anthropometric and Physical Activity Measures
3.2. MD Adherence and Dietary Components
3.3. Cutaneous Vascular Conductance
3.4. Quality of Life and Lipidemic Profile
4. Discussion
4.1. Quality of Life
4.2. Compliance to the MD
4.3. Microvascular Function
4.4. Experimental Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Milner, M.; Klonizakis, M. Physiological effects of a short-term lifestyle intervention based on the Mediterranean diet: Comparison between older and younger healthy, sedentary adults. Nutrition 2018, 55–56, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Jug, B.; Lenasi, H. Clinical impact of exercise in patients with peripheral arterial disease. Vascular 2017, 25, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Alkhatib, A.; Middleton, G. Long-term effects of an exercise and Mediterranean diet intervention in the vascular function of an older, healthy population. Microvasc. Res. 2014, 95, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef]
- Rogerson, D.; Macas, D.; Milner, M.; Liu, Y.; Klonizakis, M. Contrasting Effects of Short-Term Mediterranean and Vegan Diets on Microvascular Function and Cholesterol in Younger Adults: A Comparative Pilot Study. Nutrients 2018, 10, 1897. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Mediterranean Diets: What Is So Special about the Diet of Greece? The Scientific Evidence. J. Nutr. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Maraki, M.I.; Giannopoulou, D.; Poulimeneas, D.; Sidossis, L.S.; Tsigga, M. Similar Mediterranean diet adherence but greater central adiposity is observed among Greek diaspora adolescents living in Istanbul, compared to Athens. Ethn. Health 2018, 23, 221–232. [Google Scholar] [CrossRef]
- Debbabi, H.; Bonnin, P.; Ducluzeau, P.H.; Leftheriotis, G.; Levy, B.I. Non-invasive Assessment of Endothelial Function in the Skin Microcirculation. Am. J. Hypertens. 2010, 23, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The Predimed Trial. PLoS ONE 2012, 7, e43134. [Google Scholar]
- Grammatikopoulou, M.G.; Gkiouras, K.; Theodoridis, X.; Tsisimiri, M.; Markaki, A.G.; Chourdakis, M.; Goulis, D.G. Food insecurity increases the risk of malnutrition among community-dwelling older adults. Maturitas 2019, 119, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mikkila, V.; Rasanen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Longitudinal changes in diet from childhood into adulthood with respect to risk of cardiovascular diseases: The Cardiovascular Risk in Young Finns Study. Eur. J. Clin. Nutr. 2004, 58, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Institute of Food Research (Great Britain); Public Health England; Royal Society of Chemistry (Great Britain). McCance and Widdowson’s the Composition of Foods, 7th ed.; Royal Society of Chemistry: London, UK, 2015; ISBN 9781849736367. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; Oja, P. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Ginieri-Coccossis, M.; Triantafillou, E.; Tomaras, V.; Soldatos, C.; Mavreas, V.; Christodoulou, G. Psychometric properties of WHOQOL-BREF in clinical and health Greek populations: Incorporating new culture-relevant items. Psychiatrike 2012, 23, 130–142. [Google Scholar]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A.; WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- Minson, C.T.; Berry, L.T.; Joyner, M.J. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J. Appl. Physiol. 2001, 91, 1619–1626. [Google Scholar] [CrossRef]
- Naseem, K. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Tew, G.A.; Klonizakis, M.; Saxton, J.M. Effects of ageing and fitness on skin-microvessel vasodilator function in humans. Eur. J. Appl. Physiol. 2010, 109, 173–181. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Gumber, A.; Crank, H.; Akil, M.; Klonizakis, M. The effects of upper and lower limb exercise on the microvascular reactivity in limited cutaneous systemic sclerosis patients. Arthr. Res. Ther. 2018, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Ferrans, C.E.; Zerwic, J.J.; Wilbur, J.E.; Larson, J.L. Conceptual model of health-related quality of life. J. Nurs. Scholarsh. 2005, 37, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Milte, C.M.; Thorpe, M.G.; Crawford, D.; Ball, K.; McNaughton, S.A. Associations of diet quality with health-related quality of life in older Australian men and women. Exp. Gerontol. 2015, 64, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Clonan, A.; Holdsworth, M. The challenges of eating a healthy and sustainable diet. Am. J. Clin. Nutr. 2012, 96, 459–460. [Google Scholar] [CrossRef]
- Middleton, G.; Keegan, R.; Smith, M.F.; Alkhatib, A.; Klonizakis, M. Brief Report: Implementing a Mediterranean Diet Intervention into a RCT: Lessons Learned from a Non-Mediterranean Based Country. J. Nutr. Health Aging 2015, 19, 1019–1022. [Google Scholar] [CrossRef]
- Van Diepen, S.; Scholten, A.M.; Korobili, C.; Kyrli, D.; Tsigga, M.; Van Dieijen, T.; Kotzamanidis, C.; Grammatikopoulou, M.G. Greater Mediterranean diet adherence is observed in Dutch compared with Greek university students. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 534–540. [Google Scholar] [CrossRef]
- Casas, R.; Estruch, R.; Sacanella, E. The Protective Effects of Extra Virgin Olive Oil on Immune-mediated Inflammatory Responses. Endocr. Metab. Immune. Disord. Drug Targets 2017, 18, 23–35. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef]
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef]
- Masana, L.; Camprubi, M.; Sarda, P.; Sola, R.; Joven, J.; Turner, P.R. The Mediterranean-type diet: Is there a need for further modification? Am. J. Clin. Nutr. 1991, 53, 886–889. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Steeves, J.A.; Tudor-Locke, C.; Murphy, R.A.; King, G.A.; Fitzhugh, E.C.; Harris, T.B. Classification of occupational activity categories using accelerometry: NHANES 2003–2004. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferre, M.; Babio, N.; Martinez-Gonzalez, M.A.; Corella, D.; Ros, E.; Martin-Pelaez, S.; Estruch, R.; Aros, F.; Gomez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar]
- De Lorgeril, M.; Salen, P. Mediterranean diet and n-3 fatty acids in the prevention and treatment of cardiovascular disease. J. Cardiovasc. Med. 2007, 8, S38–S41. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Di Lullo, L.; Ronco, C.; Zannini, C.; La Manna, G. Folic Acid and Homocysteine in Chronic Kidney Disease and Cardiovascular Disease Progression: Which Comes First? Cardiorenal Med. 2017, 7, 255–266. [Google Scholar] [CrossRef]
- Bryan, N.S. Functional Nitric Oxide Nutrition to Combat Cardiovascular Disease. Curr. Atheroscler. Rep. 2018, 20, 21. [Google Scholar] [CrossRef]
- Bandini, A.; Orlandi, S.; Manfredi, C.; Evangelisti, A.; Barrella, M.; Bevilacqua, M.; Bocchi, L. Modelling of thermal hyperemia in the skin of type 2 diabetic patients. J. Healthc. Eng. 2013, 4, 541–554. [Google Scholar] [CrossRef]
- Salvat-Melis, M.; Carpentier, P.H.; Minson, C.T.; Boignard, A.; McCord, G.R.; Paris, A.; Moreau-Gaudry, A.; Cracowski, J.-L. Digital thermal hyperaemia impairment does not relate to skin fibrosis or macrovascular disease in systemic sclerosis. Rheumatology 2006, 45, 1490–1496. [Google Scholar] [CrossRef][Green Version]
- Trichopoulou, A.; Naska, A.; Orfanos, P.; Trichopoulos, D. Mediterranean diet in relation to body mass index and waist-to-hip ratio: The Greek European Prospective Investigation into Cancer and Nutrition Study. Am. J. Clin. Nutr. 2005, 82, 935–940. [Google Scholar] [CrossRef]
- Norman, P.E.; Powell, J.T. Vitamin D and Cardiovascular Disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Morrone, L.F.; Bolasco, P.; Camerini, C.; Cianciolo, G.; Cupisti, A.; Galassi, A.; Mazzaferro, S.; Russo, D.; Russo, L.; Cozzolino, M. Vitamin D in patients with chronic kidney disease: A position statement of the Working Group “Trace Elements and Mineral Metabolism” of the Italian Society of Nephrology. J. Nephrol. 2016, 29, 305–328. [Google Scholar] [CrossRef] [PubMed]
| Control Group (n = 40) | Intervention Group (n = 34) | ||
|---|---|---|---|
| Single visit | Baseline | 4 Weeks | |
| Gender (Male/Female ratio) (n) | 29/11 | 11/23 * | |
| Age (years) | 37 ± 11 | 40 ± 17 | |
| Height (cm) | 171 ± 9 | 169 ± 1 | |
| IPAQ Sitting Time (min) | 480 ± 270 | 475 ± 120 | 465 ± 118 |
| Body Weight (kg) | 68.9 ± 15.1 | 72.0 ± 12.9 | 71.0 ± 11.3 |
| BMI (kg/m2) | 23.4 ± 4.6 | 25.2 ± 6.1 | 24.6 ± 5.5 |
| Waist circumference (cm) | 78 ± 15 | 81 ± 9 | 80 ± 8 |
| Waist/hip ratio | 0.65 ± 0.1 | 0.66 ± 0.1 | 0.64 ± 0.2 |
| Physical activity occupational category (sedentary/low/moderate/intense) (n) | 28/8/4/0 | 24/7/1/0 | |
| Calf circumference (cm) | 49 ± 6 | 51 ± 6 | 50 ± 5 |
| Resting heart rate (bpm) | 70 ± 10 | 69 ± 8 | 68 ± 7 |
| Resting SBP (mm Hg) | 120 ± 11 | 118 ± 12 | 117 ± 11 |
| Resting DBP (mm Hg) | 78 ± 8 | 75 ± 11 | 74 ± 10 |
| Body Fat (% of body weight) | 30.0 ± 9.0 | 29.0 ± 9.0 | 28.1 ± 8.2 |
| Measurement Phase: | Time Point: |
|---|---|
| Baseline | The arithmetical mean of the last 2 min of the first 5 min period. |
| Initial peak | The arithmetical mean of the highest consecutive 30-s period within the distinct initial hyperemic response. |
| Plateau | The arithmetical mean of the last 2 min of heating at 42 °C. |
| Maximum | The arithmetical mean of the last 2 min of heating at 44 °C. |
| MD Adherence, Food-Group and Nutrient Intake | Control Group (n = 40) | Intervention Group (n = 34) | |
|---|---|---|---|
| Single Visit | Baseline | 4 Weeks | |
| MEDAS score | 9 ± 1 | 5 ± 1 | 11 ± 1 *,++ |
| Energy Intake (kcal/day) | 1742 ± 353 | 1865 ± 323 | 1732 ± 432 |
| Carbohydrates (g/day) | 155 ± 45.6 | 213 ± 33 | 172.3 ± 55 * |
| Fiber (g/day) | 17.9 ± 8.4 | 17.0 ± 6.1 | 23.5 ± 7.2 *,++ |
| Protein (g/day) | 56.7 ± 18.7 | 70.5 ± 20.5 | 74.6 ± 20.1 ++ |
| Fat (g/day) | 102.8 ± 21.5 | 86 ± 21 | 71 ± 23 *,++ |
| Saturated fat (g/day) | 24.2 ± 8.2 | 32.0 ± 7.5 | 20 ± 9.1 *,+ |
| MUFA (g/day) | 62.4 ± 11.0 | 27.2 ± 9.0 | 32.3 ± 9.5 + |
| PUFA (g/day) | 12.0 ± 4.3 | 11.9 ± 4.4 | 13.0 ± 6.5 |
| Olive oil (servings/day) | 4.5 ± 0.5 | 0.4 ± 0.4 | 2.1 ± 1.5 *,++ |
| Fruit and vegetables (servings/day) | 7.2 ± 2.1 | 3.3 ± 2 | 6.9 ± 3.3 * |
| Measure | Control Group (n = 40) | Intervention Group (n = 34) | |
|---|---|---|---|
| Single Visit | Baseline | 4 Weeks | |
| Baseline CVC (APU/mmHg) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.2 |
| Initial peak CVC (APU/mmHg) | 2.7 ± 0.6 | 2.0 ± 0.6 | 2.8 ± 0.8 + |
| Plateau CVC (APU/mmHg) | 3.8 ± 0.8 | 2.2 ± 0.6 | 3.1 ± 1.2 * |
| Baseline CVCmax (%) | 3.0 ± 2.3 | 8.9 ± 5.2 | 7.3 ± 8.5 * |
| Initial peak CVCmax (%) | 56.7 ± 8.2 | 73.1 ± 15.2 | 75.5 ± 16.1 * |
| Plateau CVCmax (%) | 79.4 ± 9.6 | 86.1 ± 16.2 | 82.8 ± 9.7 |
| Variables | Control Group (n = 40) | Intervention Group (n = 34) | |
|---|---|---|---|
| Baseline | Baseline | 4 weeks | |
| QoL domains | |||
| Physical | 16.2 ± 1.8 | 13.7 ± 1.4 | 15.9 ± 1.2 + |
| Psychological | 15.5 ± 2.3 | 13.9 ± 1.8 | 14.4 ± 2.3 |
| Social | 15.6 ± 2.2 * | 13.1 ± 3.9 | 14.6 ± 1.7 |
| Environmental | 13.9 ± 1.9 | 13.3 ± 1.6 | 13.7 ± 1.6 |
| Lipidemic profile | |||
| TC (mmol/L) | 4.1 ± 1.1 | 4.1 ± 0.6 | 4.2 ± 0.6 |
| HDL (mmol/L) | 1.6 ± 0.7 | 1.4 ± 0.5 | 1.5 ± 0.3 |
| TC/HDL (ratio) | 2.8 ± 1.1 | 3.6 ± 1.9 | 3.0 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klonizakis, M.; Grammatikopoulou, M.G.; Theodoridis, X.; Milner, M.; Liu, Y.; Chourdakis, M. Effects of Long-Versus Short-Term Exposure to the Mediterranean Diet on Skin Microvascular Function and Quality of Life of Healthy Adults in Greece and the UK. Nutrients 2019, 11, 2487. https://doi.org/10.3390/nu11102487
Klonizakis M, Grammatikopoulou MG, Theodoridis X, Milner M, Liu Y, Chourdakis M. Effects of Long-Versus Short-Term Exposure to the Mediterranean Diet on Skin Microvascular Function and Quality of Life of Healthy Adults in Greece and the UK. Nutrients. 2019; 11(10):2487. https://doi.org/10.3390/nu11102487
Chicago/Turabian StyleKlonizakis, Markos, Maria G. Grammatikopoulou, Xenophon Theodoridis, Marianne Milner, Yingshan Liu, and Michael Chourdakis. 2019. "Effects of Long-Versus Short-Term Exposure to the Mediterranean Diet on Skin Microvascular Function and Quality of Life of Healthy Adults in Greece and the UK" Nutrients 11, no. 10: 2487. https://doi.org/10.3390/nu11102487
APA StyleKlonizakis, M., Grammatikopoulou, M. G., Theodoridis, X., Milner, M., Liu, Y., & Chourdakis, M. (2019). Effects of Long-Versus Short-Term Exposure to the Mediterranean Diet on Skin Microvascular Function and Quality of Life of Healthy Adults in Greece and the UK. Nutrients, 11(10), 2487. https://doi.org/10.3390/nu11102487





