Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment and Testing Schedule
2.2. Electrophysiological Methods
2.3. Statistical Analysis
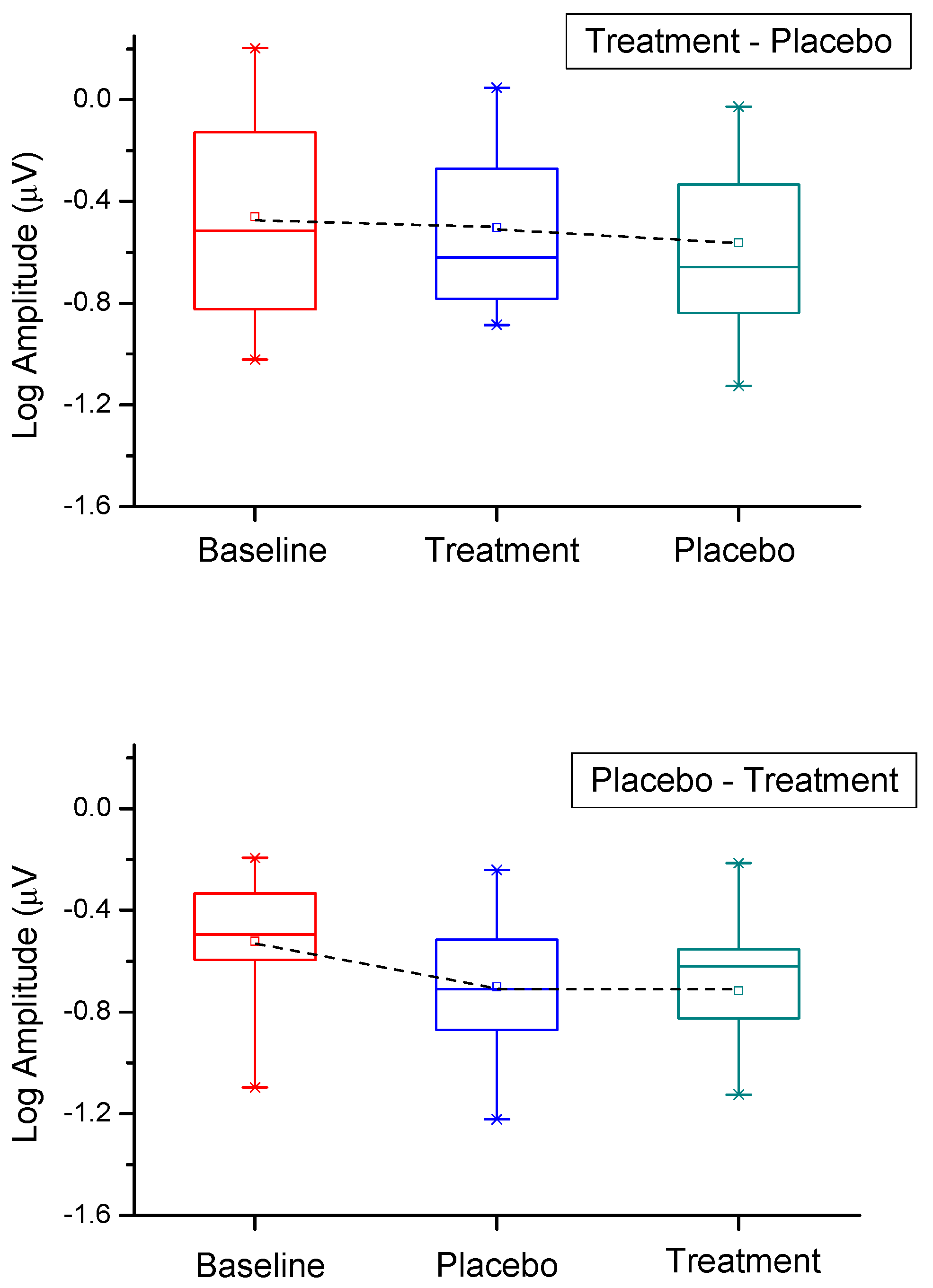
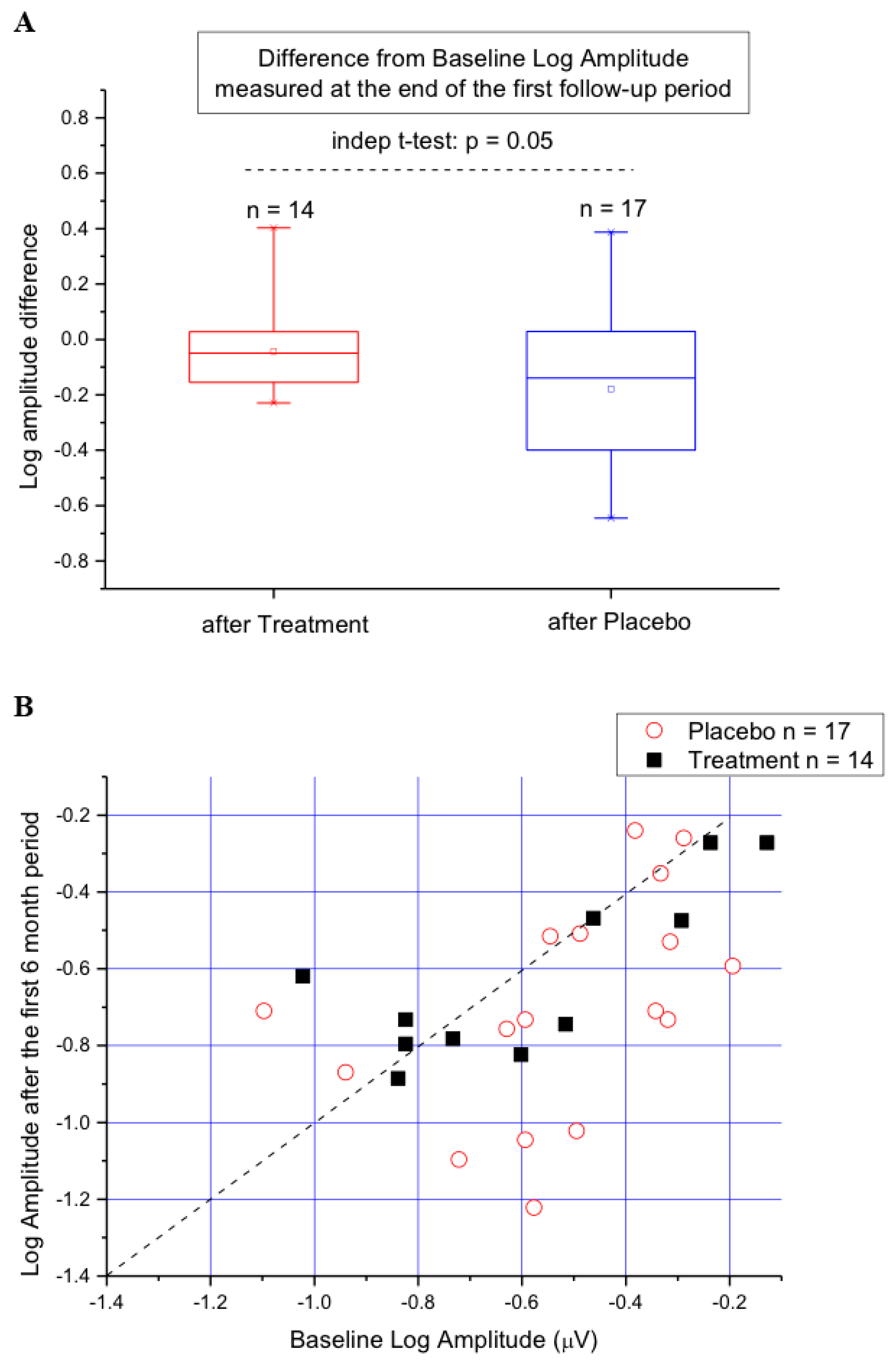
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| STG/FF | Stargardt disease/fundus flavimaculatus |
| S | Saffron |
| P | Placebo |
| ERG | electroretinogram |
| fERG | focal electroretinogram |
| FERG | high-frequency flicker ERG |
| RPE | retinal pigment epithelium |
| FF | fundus flavimaculatus (FF) |
| PE | phosphatidylethanolamine |
| ETDRS | Early Treatment Diabetic Retinopathy Study chart |
| SSCP | single-strand conformation polymorphism |
References
- Blacharski, P.A. Fundus flavimaculatus. In Retinal Dystrophies and Degenerations; Newsome, D.A., Ed.; Raven Press: New York, NY, USA, 1988; pp. 135–159. [Google Scholar]
- Noble, K.G.; Carr, R.E. Stargardt’s Disease and Fundus Flavimaculatus. Arch. Ophthalmol. 1979, 97, 1281–1285. [Google Scholar]
- Allikmets, R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 1997, 17, 122. [Google Scholar] [PubMed]
- Molday, L.L.; Rabin, A.R.; Molday, R.S. ABCR expression in foveal cone photoreceptors and its role in stargardt macular dystrophy. Am. J. Ophthalmol. 2000, 130, 689. [Google Scholar] [CrossRef]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Nakanishi, K.; Parish, C.A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Sun, H.; Nathans, J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J. Biol. Chem. 2001, 276, 11766–11774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Nakanishi, K.; Itagaki, Y.; Sparrow, J.R. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp. Eye Res. 2006, 82, 828–839. [Google Scholar] [CrossRef]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron Supplement Maintains Morphology and Function after Exposure to Damaging Light in Mammalian Retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef]
- Giaccio, M. Crocetin from Saffron: An Active Component of an Ancient Spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef]
- Ochiai, T.; Shimeno, H.; Mishima, K.-I.; Iwasaki, K.; Fujiwara, M.; Tanaka, H.; Shoyama, Y.; Toda, A.; Eyanagi, R.; Soeda, S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2007, 1770, 578–584. [Google Scholar] [CrossRef]
- Laabich, A.; Vissvesvaran, G.P.; Lieu, K.L.; Murata, K.; McGinn, T.E.; Manmoto, C.C.; Sinclair, J.R.; Karliga, I.; Leung, D.W.; Fawzi, A.; et al. Protective Effect of Crocin against Blue Light- and White Light-Mediated Photoreceptor Cell Death in Bovine and Primate Retinal Primary Cell Culture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Tarantilis, P.A.; Tajmir-Riahi, H.A.; Polissiou, M.G. DNA interaction with saffron’s secondary metabolites safranal, crocetin, and dimethylcrocetin. DNA Cell Biol 2007, 26, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Zhu, Y.; Valter, K.; Bisti, S.; Eells, J.; Stone, J. Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol. Vis. 2010, 16, 1801–1822. [Google Scholar] [PubMed]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, M.C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of Saffron Supplementation on Retinal Flicker Sensitivity in Early Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Grigg, J.R.; McCluskey, P.; Hong, T.; Schlub, T.E.; Chang, A.A. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: a randomised clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Riazi, A.; Alishiri, A.A.; Hosseini, M.A.; Sahebkar, A.; Panahi, Y.; Zarchi, A.A.K. The impact of saffron (Crocus sativus) supplementation on visual function in patients with dry age-related macular degeneration. Ital. J. Med. 2016, 10, 196–201. [Google Scholar] [CrossRef]
- Lashay, A.; Sadough, G.; Ashrafi, E.; Lashay, M.; Movassat, M.; Akhondzadeh, S. Short-term Outcomes of Saffron Supplementation in Patients with Age-related Macular Degeneration: A Double-blind, Placebo-controlled, Randomized Trial. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2016, 5, 32–38. [Google Scholar]
- Falsini, B.; Fadda, A.; Iarossi, G.; Piccardi, M.; Canu, D.; Minnella, A.; Serrao, S.; Scullica, L. Retinal sensitivity to flicker modulation: reduced by early age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1498–1506. [Google Scholar]
- Falsini, B.; Galli-Resta, L.; Fadda, A.; Ziccardi, L.; Piccardi, M.; Iarossi, G.; Resta, G. Long-Term Decline of Central Cone Function in Retinitis Pigmentosa Evaluated by Focal Electroretinogram. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7701–7709. [Google Scholar] [CrossRef]
- Galli-Resta, L.; Piccardi, M.; Ziccardi, L.; Fadda, A.; Minnella, A.; Marangoni, D.; Placidi, G.; Resta, G.; Falsini, B. Early Detection of Central Visual Function Decline in Cone-Rod Dystrophy by the Use of Macular Focal Cone Electroretinogram. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6560–6569. [Google Scholar] [CrossRef]
- Orita, M.; Iwahana, H.; Kanazawa, H.; Hayashi, K.; Sekiya, T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc. Natl. Acad. Sci. USA 1989, 86, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Piccardi, M.; Iarossi, G.; Fadda, A.; Merendino, E.; Valentini, P. Influence of short-term antioxidant supplementation on macular function in age-related maculopathy: a pilot study including electrophysiologic assessment. Ophthalmology 2003, 110, 51–60. [Google Scholar] [CrossRef]
- Parisi, V.; Canu, D.; Iarossi, G.; Olzi, D.; Falsini, B. Altered recovery of macular function after bleaching in Stargardt’s disease-fundus flavimaculatus: pattern VEP evidence. Invest. Ophthalmol. Vis. Sci. 2002, 43, 2741–2748. [Google Scholar]
- Porciatti, V.; Burr, D.C.; Morrone, M.C.; Fiorentini, A. The effects of ageing on the pattern electroretinogram and visual evoked potential in humans. Vis. Res. 1992, 32, 1199–1209. [Google Scholar] [CrossRef]
- Porciatti, V.; Ventura, L.M. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmol. 2004, 111, 161–168. [Google Scholar] [CrossRef]
- Falsini, B.; Serrao, S.; Fadda, A.; Iarossi, G.; Porrello, G.; Cocco, F.; Merendino, E. Focal electroretinograms and fundus appearance in nonexudative age-related macular degeneration. Quantitative relationship between retinal morphology and function. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Bach, M. [Reproducibility of the pattern electroretinogram]. Der Ophthalmol. 1997, 94, 217–221. [Google Scholar] [CrossRef]
- Fadda, A.; Falsini, B. Precision LED-based stimulator for focal electroretinography. Med. Boil. Eng. 1997, 35, 441–444. [Google Scholar] [CrossRef]
- Fadda, A.; Di Renzo, A.; Parisi, V.; Stifano, G.; Balestrazzi, E.; Riva, C.E.; Falsini, B. Lack of habituation in the light adapted flicker electroretinogram of normal subjects: A comparison with pattern electroretinogram. Clin. Neurophysiol. 2009, 120, 1828–1834. [Google Scholar] [CrossRef]
- Aleman, T.S.; Cideciyan, A.V.; Windsor, E.A.M.; Schwartz, S.B.; Swider, M.; Chico, J.D.; Sumaroka, A.; Pantelyat, A.Y.; Duncan, K.G.; Gardner, L.M.; et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1319–1329. [Google Scholar] [CrossRef]
- Bouvier, F.; Suire, C.; Mutterer, J.; Camara, B. Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell 2003, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Murakami, K.; Ulit, M.; Antonio, L.; Shirotori, M.; Morikawa, H.; Nakano, T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine 2011, 18, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, R.; Rapino, C.; Zerti, D.; Di Tommaso, M.; Battista, N.; Di Marco, S.; Bisti, S.; Maccarrone, M. Modulation of Type-1 and Type-2 Cannabinoid Receptors by Saffron in a Rat Model of Retinal Neurodegeneration. PLoS ONE 2016, 11, 0166827. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A Multitask Neuroprotective Agent for Retinal Degenerative Diseases. Antioxidants 2019, 8, 224. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Swider, M.; Aleman, T.S.; Tsybovsky, Y.; Schwartz, S.B.; Windsor, E.A.M.; Roman, A.J.; Sumaroka, A.; Steinberg, J.D.; Jacobson, S.G.; et al. ABCA4 disease progression and a proposed strategy for gene therapy. Hum. Mol. Genet. 2009, 18, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.B.; Mooney, D.J.; O’Connor, M.A. Retinal function in Stargardt’s disease and fundus flavimaculatus. Am. J. Ophthalmol. 1983, 96, 57–65. [Google Scholar] [CrossRef]
- Lachapelle, P.; Little, J.M.; Roy, M.S. The electroretinogram in Stargardt’s disease and fundus flavimaculatus. Doc. Ophthalmol. 1989, 73, 395–404. [Google Scholar] [CrossRef]
- Lois, N.; Holder, G.E.; Bunce, C.; Fitzke, F.W.; Bird, A.C. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch. Ophthalmol. (Chicago, Ill. 1960) 2001, 119, 359–369. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Swider, M.; Aleman, T.S.; Feuer, W.J.; Schwartz, S.B.; Russell, R.C.; Steinberg, J.D.; Stone, E.M.; Jacobson, S.G. Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Investig. Ophthalmol. Vis. Sci. 2012, 53, 841–852. [Google Scholar] [CrossRef] [PubMed]
| Patient # Sex | Acuity | Baseline | 1st CONTR | 2nd CONTR | Mutation I Allele | Mutation II Allele | |
|---|---|---|---|---|---|---|---|
| OD | OS | ||||||
| 2. F, 14 | 0.2 | 0.2 | B 11.04.11 | A 24.10.11 | 18.06.12 | IVS35 + 2t > c | IVS40 + 5g > a |
| 3. M, 11 | 0.1 | 0.1 | B 11.04.11 | A 10.10.11 | 16.04.12 | c432A > G; | Q767d > a G1961e |
| 4. F, 35 | 0.1 | 0.1 | A 11.04.11 | B 11.10.11 | 16.04.12 | c.52C > T; p.Arg18Trp | C7671 > A; p.Val767Asp |
| 5. F, 14 | 0.3 | 0.3 | A 18.04.11 | B 24.10.11 | 23.04.12 | c.1622T > C; p.Leu541Pro | ------ |
| 6. M, 12 | 0.3 | 0.3 | A 18.04.11 | B 24.10.11 | 23.04.12 | c.1622T > C; p.Leu541Pro | ------ |
| 8. M, 56 | 0.1 | 0.5 | B 18.04.11 | A 24.10.11 | 18.05.12 | Arg653His | Arg653His |
| 9. M, 61 | 0.9 | 0.9 | B 18.04.11 | A 24.10.11 | 18.05.12 | Arg653His | Arg653His |
| 11. M, 12 | 0.2 | 0.2 | A 02.05.11 | B 25.11.11 | 08.05.12 | c.5882G > A; p.Gly1961glu | c.6764G > T, p.Ser2255Ile |
| 12. F, 16 | 0.2 | 0.2 | B 09.05.11 | A 08.11.11 | 22.05.12 | c.3602T > G; p.Leu1201Arg | c.5530G > T; Gly1844Cys c.5722G > T; p.Glu1908X |
| 14. F, 78 | 0.1 | 0.1 | B 16.05.11 | A 28.11.11 | 21.05.12 | c.4793C > AAla1598Asp | (c.6184_6188delGTCT; p.Val2062fsX2113) |
| 18. F, 34 | 0.8 | 0.8 | B 20.06.11 | A 19.12.12 | 21.06.12 | c.2099G > A; p. W700X | c.4561C > T; P1486L |
| 19. F, 47 | 1.0 | 1.0 | A 11.07.11 | B 09.01.12 | 09.07.121 | c.2690C > T; p.Thr897Ile | ------ |
| 20. M, 43 | 0.1 | 0.1 | A 11.07.11 | B 09.01.12 | 09.07.12 | c.2690C > T; p.Thr897Ile | ------ |
| 21. M, 12 | 0.1 | 0.1 | B 11.07.11 | A 09.01.12 | 09.07.12 | c.4139C > T; Pro1380Leu | c.6005+1G > C |
| 23. M, 34 | 0.1 | 0.1 | B 20.06.11 | A 19.12.11 | 25.06.12 | c.61C > T; p.Gln21Ter | c.5882G > A; p.Gly1961Glu |
| 24. M, 16 | 0.1 | 0.9 | A 12.10.11 | B 23.04.12 | 29.10.12 | c.2461T > A; p.Trp821Arg c.2459A > G; p.Tyr850Cys | c.5882G > A; p.Gly1961Glu |
| 25. M, 29 | 0.1 | 0.1 | A 07.11.11 | B 02.05.12 | 12.11.12 | C768G > T; p.Val256splice | c.4437G>A;p.Trp1479X |
| 26. M, 32 | 0.1 | 0.1 | B 07.11.11 | A 28.05.12 | 03.12.12 | 5714 + 5G-A | 6088C > T |
| 27. F, 29 | 0.2 | 0.2 | A 08.11.11 | B 04.06.12 | 17.01.13 | Thr977Pro | IVS40 + 5G-A |
| 28. M, 27 | 0.2 | 0.2 | B 21.11.11 | A 21.05.12 | 22.11.12 | c.5065T > C; p.S1689P | c.5882G > A; p.G1961E |
| 29. F, 22 | 0.1 | 0.5 | A 13.12.11 | B 18.06.12 | 20.12.12 | 4709-4711delA | Gly1961Glu |
| 30. M, 42 | 0.1 | 0.2 | A 23.01.12 | B 27.06.12 | 07.01.13 | R152Q | R653C |
| 31. M, 10 | 0.2 | 0.2 | B 13.02.12 | A 06.08.12 | 11.02.13 | c.4200C>A;p.Tyr1400X | c.686T>C;p.Leu229Pro |
| 32. M, 17 | 0.3 | 0.3 | A 20.02.12 | B 03.09.12 | 18.03.13 | R18W | C1490Y |
| 33. M, 22 | 0.4 | 0.4 | B 21.02.12 | A 10.09.12 | 09.03.13 | c.5882G>A;p.Gly1691Glu | (c.3994_4017dup;p,.Gln1332_cys1339dup) Val2062ThrfsX52 |
| 38. M, 15 | 0.2 | 0.2 | B 03.04.12 | A 15.10.12 | 22.04.13 | c.2791G>A;p.Val931Met | ------ |
| 39. M, 21 | 0.1 | 0.2 | A 04.04.12 | B 29.10.12 | 13.05.13 | Ser1064Pro | IVS35+2t>c |
| 42. F, 21 | 0.3 | 0.3 | B 17.04.12 | A 08.10.12 | 11.04.13 | Ivs13+1g>a | Ivs40+5g>40 |
| 43. M, 52 | 0.1 | 0.5 | B 23.04.12 | A 12.11.12 | 16.05.13 | c.3323G>A;p.Arg1018His | c.5882G>A;p.Gly1961Glu |
| 44. M, 35 | 0.4 | 0.4 | A 17.05.12 | B 13.12.12 | 20.06.13 | GlY1961Glu | IVS45+1g>c |
| 45. F, 56 | 0.8 | 0.1 | B 21.5.12 | A 05.11.12 | 02.05.13 | c.2549>G;P.Tyr850Cys c.2875A>G;p.Thr959Ala | c.5882G>A;p.Gly1961Glu |
| Baseline | SN1 | Noise I | 1st period | SN1 | Noise I | 2nd Period | SN1 | Noise I | |
|---|---|---|---|---|---|---|---|---|---|
| Pat. ID | Amp (ph) OD; OS | OD OS | OD OS | Amp (ph) OD; OS | OD OS | OD OS | Amp (ph) OD; OS | OD OS | OD OS |
| 2. | 0.63: 0.30 −160.5; −69.0 | 15.70 9.26 | 0.10 0.10 | 0.40; 0.49 −5.8; −177.3 | 0.86 6.12 | 0.17 0.27 | 0.24; 0.28 −67.3; 148.0 | 8.16 18.55 | 0.09 0.03 |
| 3. | 0.14; 0.50 −51.1; 10.7 | −11.61 10.93 | 0.54 0.13 | 0.12; 0.07 −108.4; 146.1 | −4.23 −10.51 | 0.20 10.23 | 0.09; 0.28 152.1; −86.6 | −1.05 4.43 | 0.10 0.17 |
| 4. | 0.33; 0.35 23.6; 163.0 | 6.30 3.09 | 0.16 0.25 | 0.13; 0.55 10.6; 44.6 | 13.49 11.21 | 0.03 n.c. | 0.39; 0.05 −32.1; 20.5 | 7.19 −8.92 | 0.17 0.13 |
| 5. | 0.97;0.71 27.1; −161.4 | 4.41 n.c. | 0.29 n.c. | 0.70; 0.63 19.6; −132.9 | 21.56 14.17 | 0.06 0.12 | 0.84; 0.84 48.0; −164.6 | 13.32 14.73 | 0.18 0.15 |
| 6. | 0.8; 0.858 2.0; −146.0 | 9.92 n.c. | 0.28 n.c. | 0.90; 0.92 42.1; −129.1 | 22.73 14.98 | 0.06 0.11 | 0.32; 0.61 42.2; 150.6 | 12.78 14.98 | 0.07 0.11 |
| 8. | 0.34; 0.17 −53.1; 30.2 | 9.04 1.70 | 0.12 0.10 | 0.12; 0.06 −179.2; 113.2 | −1.00 8.18 | 0.04 0.02 | 0.09; 0.08 −103.6; 70.9 | 10.57 2.64 | 0.01 0.04 |
| 9. | 0.63; 0.65 −54.1; −173.3 | 9.73 4.05 | 0.21 0.41 | 0.28; 0.23 −80.5; −175.8 | 6.79 −1.39 | 0.13 0.27 | 0.04; 0.11 −153.5; 115.0 | −11.34 −2.65 | 0.13 0.14 |
| 11. | 0.79; 0.70 −173.0; 18.5 | 14.86 10.57 | 0.14 0.21 | 0.75; 0.32 12.6; −177.8 | 10.47 4.35 | 0.23 0.19 | 0.40; 0.33 22.4; −142.2 | 10.70 10.07 | 0.12 0.10 |
| 12. | 0.18; 0.33 −139.3; 116.7 | −0.04 7.10 | 0.18 0.15 | 0.19; 0.18 99.1; −36.5 | 9.00 9.54 | 0.07 0.06 | 0.20; 0.38 −124.2; −124.5 | 19.63 12.61 | 0.02 0.09 |
| 14. | 0.24; 0.33 140.0; 176.5 | 4.53 2.75 | 0.14 0.24 | 0.41; 0.20 −89.7; −42.6 | 1.12 −0.76 | n.c. n.c. | 0.37; 0.40 145.2; −133.5 | 17.16 0.77 | 0.05 0.37 |
| 18. | 0.10; 0.28 −33.7; 167.4 | −5.02 17.98 | 0.11 0.03 | 0.13; 0.03 −40.1; 135.2 | 7.83 −5.87 | 0.05 0.07 | 0.11; 0.19 −47.9; −0.9 | 7.02 17.08 | 0.05 0.03 |
| 19. | 1.33; 1.86 −3.3; −174.4 | n.c. 20.35 | n.c. 0.18 | 1.22; 1.01 −9.5; −159.8 | 21.99 16.39 | 0.07 0.15 | 0.70; 0.48 110.4; −81.0 | 23.78 18.27 | 0.05 0.06 |
| 20. | 0.1; 0.089 3.6; 56.9 | 2.93 9.98 | 0.13 0.25 | 0.32; 0.16 −20.3; 19.5 | 11.61 −5.72 | 0.08 0.30 | 0.20; 0.07 −36.5; −25.6 | 4.49 −6.04 | 0.12 0.14 |
| 21. | 0.37; 0.46 −9.4; −173.6 | 11.85 11.55 | 0.10 0.12 | 0.89; 0.26 −15.3; −144.8 | 10.01 13.00 | 0.28 0.06 | 0.05; 0.12 −47.9; 127.6 | 5.25 0.20 | 0.01 0.05 |
| 23. | 0.50; 0.53 41.6; −141.6 | 9.34 22.92 | 0.17 0.04 | 0.57; 0.53 −11.7; −177.4 | 7.33 16.68 | 0.25 0.08 | 0.23; 0.28 −60.1; 137.7 | 10.32 16.82 | 0.07 0.04 |
| 24. | 0.10; 0.20 −55.4; 163.3 | 1.50 7.72 | 0.08 0.08 | 0.24; −9.0 0.13; −141.1 | 6.45 6.69 | 0.12 0.06 | 0.21; 0.05 −30.6; 85.1 | 23.02 −3.01 | 0.01 0.07 |
| 25. | 0.14; 0.16 −121.4; 146.4 | −0.02 −2.6 | 0.14 0.10 | 0.15; 0.17 26.7; −80.2 | 4.94 6.98 | 0.09 0.07 | 0.09; 0.06 29.8; 64.9 | 1.92 −4.92 | 0.08 0.10 |
| 26. | 0.32; 0.21 12.5; 84.4 | 2.93 −0.36 | 0.23 0.22 | 0.10; 0.02 −12.3; −53.3 | −536 −13.28 | 0.18 0.11 | 0.04; 0.11 −159.6; −90.5 | −8.29 5.79 | 0.11 0.06 |
| 27. | 0.46; 0.15 48.7; −124.1 | 6.60 1.69 | 0.23 0.12 | 0.16; 0.20 −43.7; 142.4 | 10.77 −3.07 | 0.05 0.06 | 0.34; 0.04 −0.4; 42.1 | 8.41 0.46 | 0.13 0.04 |
| 28. | 0.59; 0.37 35.3; −120.8 | 18.07 22.20 | 0.07 0.03 | 0.29; 0.08 −171.2; −12.6 | 14.59 4.81 | 0.05 0.05 | 0.31; 0.18 −53.3; 104.6 | 10.66 11.73 | 0.10 0.05 |
| 29. | 0.57; 0.45 32.9; −155.2 | 12.33 3.98 | 0.14 0.28 | 0.49; 0.18 −46.8; 167.1 | 18.43 3.78 | 0.06 0.12 | 0.19; 0.18 16.4; 70.6 | 2.43 −3.11 | 0.15 0.26 |
| 30. | 0.22; 0.07 49.4; 172.2 | 11.65 7.71 | 0.06 0.03 | 0.15; 0.11 −63.9; 148.0 | 16.07 7.63 | 0.02 0.04 | 0.22; 0.07 49.4; 172.2 | 11.65 7.71 | 0.06 0.03 |
| 31. | 0.45; 0.46 34.7; −126.2 | 13.83 12.98 | 0.09 0.12 | 0.21; 0.18 −35.7; 165.8 | 10.43 14.03 | 0.06 0.04 | 0.29; 0.27 −18.2; 170.6 | 9.68 10.01 | 0.10 0.08 |
| 32. | 0.30; 0.20 −42.0; 135.5 | 9.26 −0.96 | 0.10 0.22 | 0.18; 0.12 −95.9; 85.9 | 10.72 4.90 | 0.05 0.07 | 0.38; 0.33 −92.2; 118.0 | 7.84 4.85 | 0.16 0.19 |
| 33. | 0.07; 0.16 −3.22; −9.3 | 0.60 4.32 | 0.06 0.10 | 0.20; 0.07 −17.4; −87.7 | 12.79 2.59 | 0.05 0.05 | 021; 0.10 −61.8; 136.7 | 4.76 7.40 | 0.12 0.04 |
| 38. | 0.57; 0.40 −17.4; 137.2 | 14 n.c. | 12 n.c. | 0.35; 0.24 −20.0; −149.0 | 12.18 11.13 | 0.09 0.07 | 0.65; 0.57 −76; −84 | 26.77 11.13 | 0.03 0.15 |
| 39. | 0.20; 0.17 −145.8; −68.8 | −3.99 6.96 | 0.32 0.08 | 0.16; 0.17 47.0; 67.5 | 6.41 0.94 | 0.08 0.15 | 0.44; 0.35 −36.1;−22.2 | 14.02 16.3 | 0.09 0.06 |
| 42. | 0.07; 0.09 34.7; −47.9 | 2.73 2.38 | 0.04 0.07 | 0.21; 0.18 −20.1; 127.0 | 4.14 8.12 | 0.09 0.07 | 0.27; 0.21 −48.9; −157.9 | 3.89 3.67 | 0.17 0.14 |
| 43. | 0.34; 0.27 −71; −140 | 12.50 n.c. | 0.08 n.c. | 0.32; 0.30 −2.7; −29 | 4.31 12.69 | 0.20 0.07 | 0.17; 0.41 −41.7; −28.5 | 15 20.5 | 0.03 0.04 |
| 44. | 0.49; 0.46 −157; −142 | 10.31 30.1 | 0.08 0.01 | 0.16; 0.28 −87.6; −6.6 | 20.66 6.83 | 0.01 0.10 | 0.39; 0.30 −20.9; −10.6 | 4.31 11.11 | n.c. 0.11 |
| 45. | 0.16; 0.31 13.4; −168.1 | 11.13 17.47 | 0.04 0.08 | 0.27; 0.08 −27.9; −84.6 | 21.91 −4.51 | 0.02 −0.13 | 0.17; 0.18 −80.9; −57 | 25.21 11.43 | 0.01 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccardi, M.; Fadda, A.; Martelli, F.; Marangoni, D.; Magli, A.; Minnella, A.M.; Bertelli, M.; Di Marco, S.; Bisti, S.; Falsini, B. Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy. Nutrients 2019, 11, 2461. https://doi.org/10.3390/nu11102461
Piccardi M, Fadda A, Martelli F, Marangoni D, Magli A, Minnella AM, Bertelli M, Di Marco S, Bisti S, Falsini B. Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy. Nutrients. 2019; 11(10):2461. https://doi.org/10.3390/nu11102461
Chicago/Turabian StylePiccardi, Marco, Antonello Fadda, Francesco Martelli, Dario Marangoni, Adriano Magli, Angelo M. Minnella, Matteo Bertelli, Stefano Di Marco, Silvia Bisti, and Benedetto Falsini. 2019. "Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy" Nutrients 11, no. 10: 2461. https://doi.org/10.3390/nu11102461
APA StylePiccardi, M., Fadda, A., Martelli, F., Marangoni, D., Magli, A., Minnella, A. M., Bertelli, M., Di Marco, S., Bisti, S., & Falsini, B. (2019). Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy. Nutrients, 11(10), 2461. https://doi.org/10.3390/nu11102461







