Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics, Consent, and Permissions
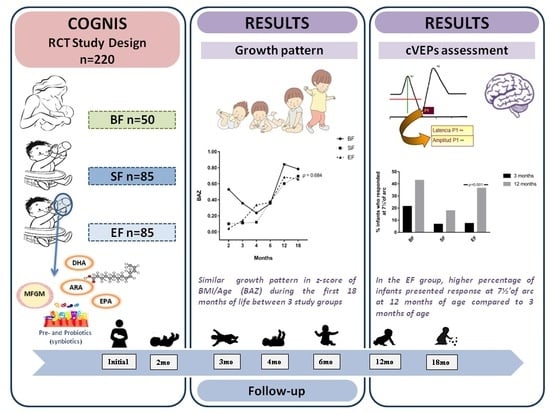
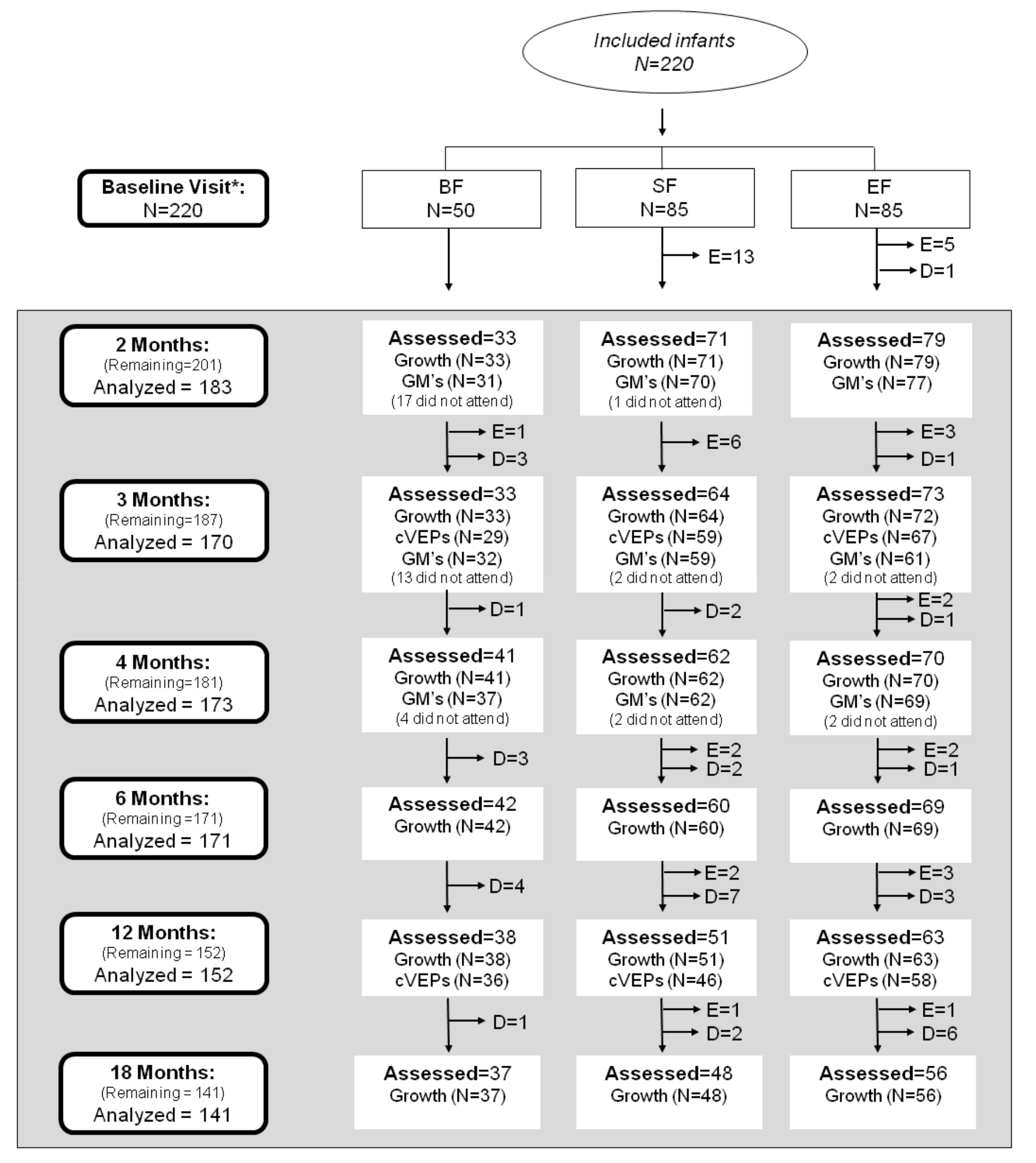
2.2. Study Design and Subjects
2.3. Data Collection
2.4. Assessments of Infant and Children Growth
2.5. Neurological Development: General Movements (GM’s) Test
2.6. Visual Function: Cortical Visual Evoked Potentials (cVEPs)
2.7. Statistical Analysis
3. Results
3.1. Parental and Newborn Characteristics of the COGNIS Study Participants
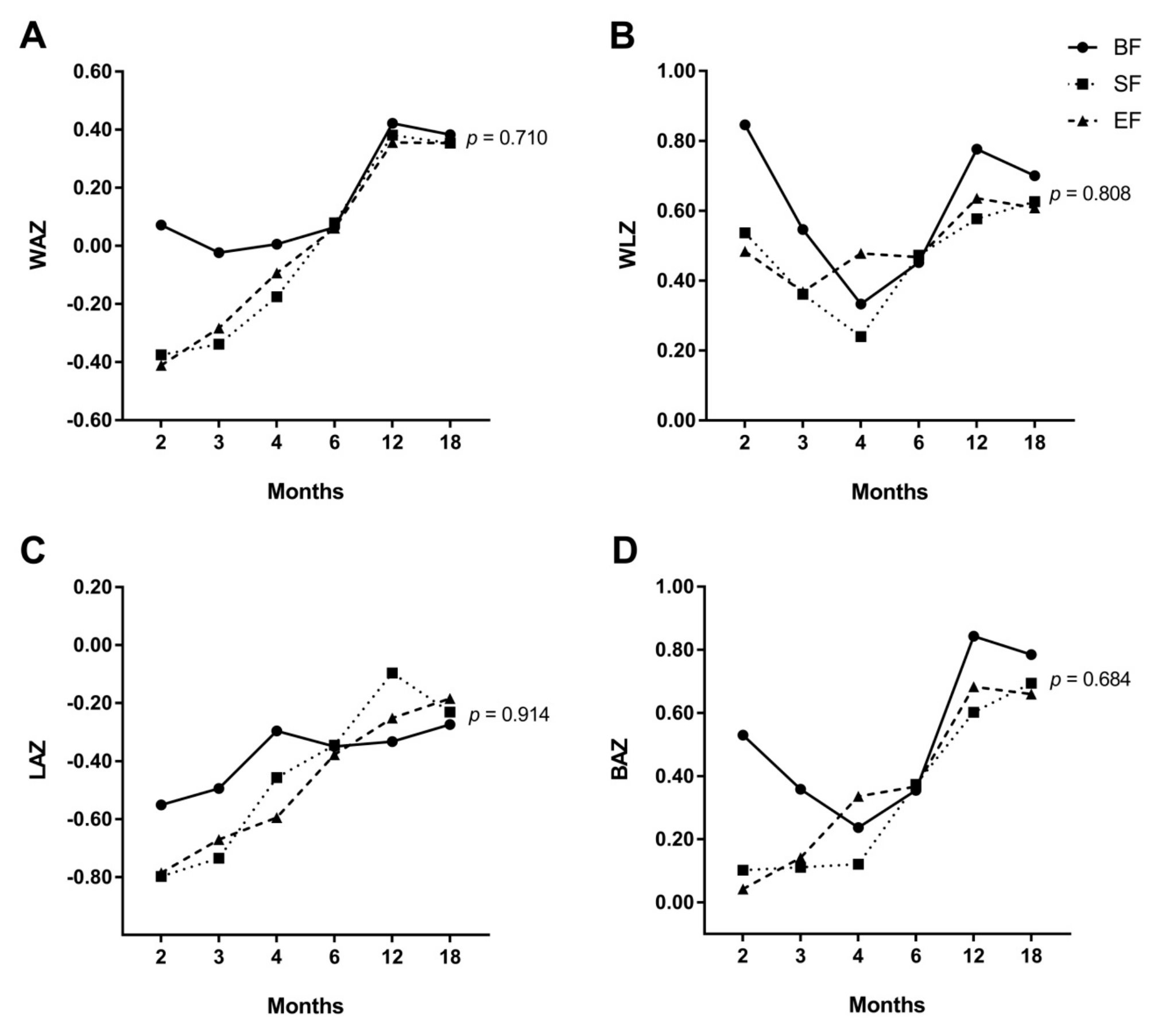
3.2. Effects of Experimental Formula on Infant’s Growth Up to 18 Months of Life
3.3. Results from GM’s
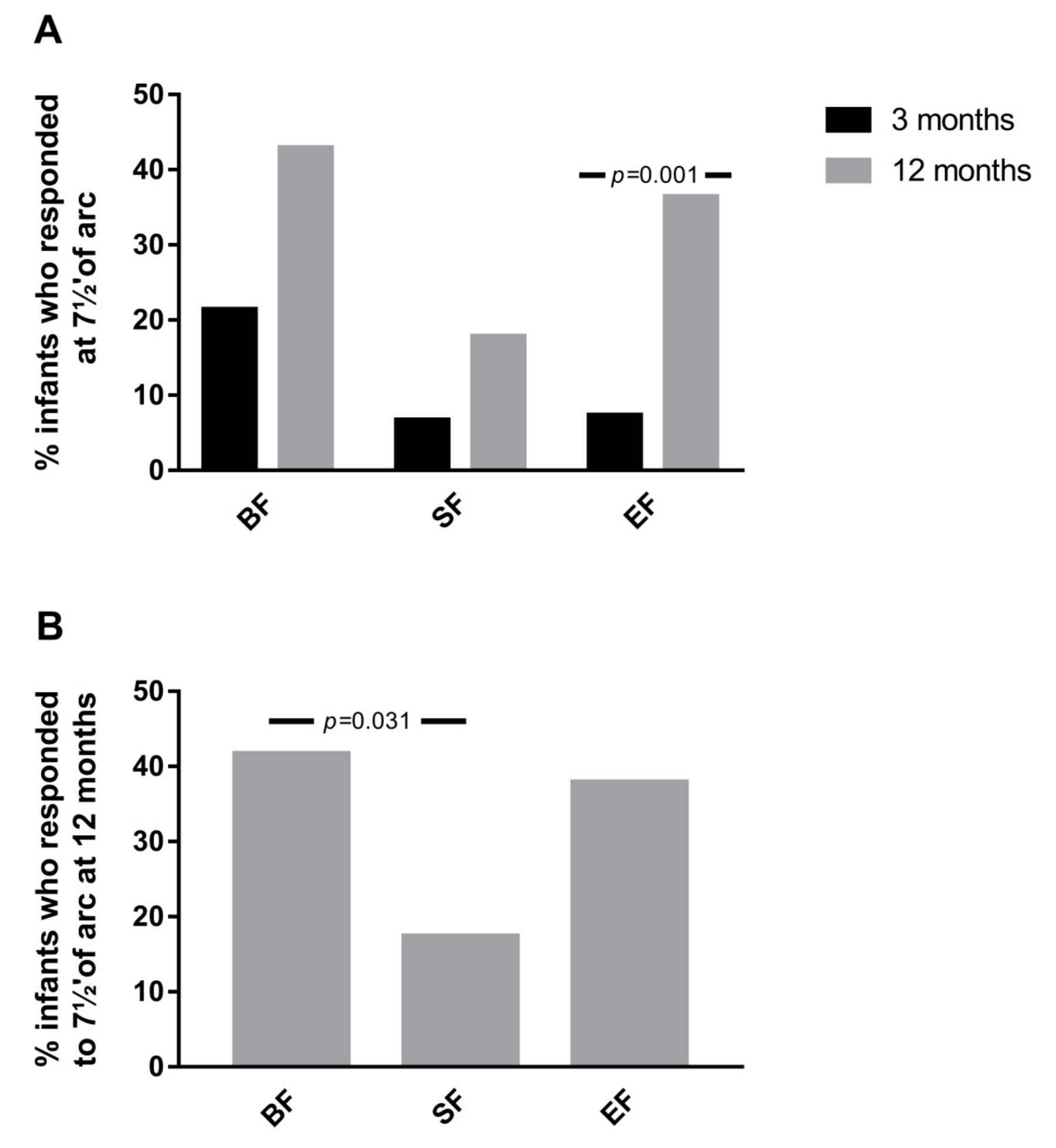
3.4. Results from Cortical Visual Evoked Potentials Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Statement
References
- Pecoraro, L.; Agostoni, C.; Pepaj, O.; Pietrobelli, A. Behind human milk and breastfeeding: Not only food. Int. J. Food Sci. Nutr. 2018, 69, 641–646. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and InfantFormula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Schoenmakers, I.; Laskey, M.A.; de Bono, S.; Ginty, F.; Goldberg, G.R. Nutrition and bone growth and development. Proc. Nutr. Soc. 2006, 65, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.-P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Qawasmi, A.; Landeros-Weisenberger, A.; Bloch, M.H. Meta-analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 2013, 131, e262–e272. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef]
- Richard, C.; Lewis, E.D.; Field, C.J. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl. Physiol. Nutr. Metab. 2016, 41, 461–475. [Google Scholar] [CrossRef]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Belkind-Gerson, J.; Carreon-Rodriguez, A.; Contreras-Ochoa, C.O.; Estrada-Mondaca, S.; Parra-Cabrera, M.S. Fatty acids and neurodevelopment. J. Pediatr. Gastroenterol. Nutr. 2008, 47 (Suppl. 1), S7–S9. [Google Scholar] [CrossRef]
- Spitsberg, V.L. Invited review: Bovine milk fat globule membrane as a potential nutraceutical. J. Dairy Sci. 2005, 88, 2289–2294. [Google Scholar] [CrossRef]
- Hernell, O.; Domellof, M.; Grip, T.; Lonnerdal, B.; Timby, N. Physiological Effects of Feeding Infants and Young Children Formula Supplemented with Milk Fat Globule Membranes. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 35–42. [Google Scholar] [PubMed]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Keunen, K.; van Elburg, R.M.; van Bel, F.; Benders, M.J.N.L. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr. Res. 2015, 77, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front. Cell. Neurosci. 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Salas Lorenzo, I.; Chisaguano Tonato, M.A.; de la Garza Puentes, A.; Nieto, A.; Herrmann, F.; Dieguez, E.; Castellote, I.A.; López-Sabater, C.M.; Rodríguez-Palmero, M.; Campoy, C. The Effect of an Infant Formula Supplemented with AA and DHA on Fatty Acid Levels of Infants with Different FADS Genotypes: The COGNIS Study. Nutrients 2019, 11, 602. [Google Scholar] [CrossRef]
- Nieto-Ruiz, A.; Diéguez, E.; Sepúlveda-Valbuena, N.; Herrmann, F.; Azarhyah, H.; Cerdó, T.; López-Torrecillas, F.; De-Castellar, R.; Rodríguez-Palmero, M.; Miranda, M.T.; et al. Effects of a new infant formula enriched with milk fat globule membrane (MFGM), synbiotics and other bioactive compounds on children behavioral development at 2.5 years old: The COGNIS Study. Eur. Child Adolesc. Psychiatry 2019. under review. [Google Scholar]
- WHO. Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- Hadders-Algra, M. General movements: A window for early identification of children at high risk for developmental disorders. J. Pediatr. 2004, 145, S12–S18. [Google Scholar] [CrossRef]
- McCulloch, D.L.; Skarf, B. Development of the human visual system: Monocular and binocular pattern VEP latency. Invest. Ophthalmol. Vis. Sci. 1991, 32, 2372–2381. [Google Scholar]
- Torres-Espinola, F.J.; Berglund, S.K.; Garcia, S.; Perez-Garcia, M.; Catena, A.; Rueda, R.; Saez, J.A.; Campoy, C. Visual evoked potentials in offspring born to mothers with overweight, obesity and gestational diabetes. PLoS ONE 2018, 13, e0203754. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Poston, L.; Godfrey, K.; Demmelmair, H. Early nutrition programming of long-term health. Proc. Nutr. Soc. 2012, 71, 371–378. [Google Scholar] [CrossRef]
- Assembly, F.W.H. Global Strategy for Infant and Young Child Feeding. The Optimal Duration of Exclusive Breastfeeding; WHO: Geneva, Switzerland, 2001; pp. 1–5. [Google Scholar]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- CDC. Breastfeeding Report Card: United States/2014. Available online: https://www.cdc.gov/breastfeeding/pdf/2014breastfeedingreportcard.pdf (accessed on 9 May 2019).
- Ahern, G.J.; Hennessy, A.A.; Ryan, C.A.; Ross, R.P.; Stanton, C. Advances in Infant Formula Science. Annu. Rev. Food Sci. Technol. 2019, 10, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am. J. Clin. Nutr. 1993, 58, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.; Anggraini, A.; Julia, M. Infant feeding practice on growth velocity in 4-6 month-olds. Paediatr. Indones. 2018, 58, 36–41. [Google Scholar] [CrossRef]
- Agostoni, C.; Grandi, F.; Giannì, M.L.; Silano, M.; Torcoletti, M.; Giovannini, M.; Riva, E. Growth patterns of breast fed and formula fed infants in the first 12 months of life: An Italian study. Arch. Dis. Child. 1999, 81, 395–399. [Google Scholar] [CrossRef][Green Version]
- Dorosty, A.R.; Emmett, P.M.; Cowin, S.D.; Reilly, J.J. Factors associated with early adiposity rebound. ALSPAC Study Team. Pediatrics 2000, 105, 1115–1118. [Google Scholar] [CrossRef]
- Hughes, A.R.; Sherriff, A.; Ness, A.R.; Reilly, J.J. Timing of adiposity rebound and adiposity in adolescence. Pediatrics 2014, 134, e1354–e1361. [Google Scholar] [CrossRef]
- Koyama, S.; Ichikawa, G.; Kojima, M.; Shimura, N.; Sairenchi, T.; Arisaka, O. Adiposity rebound and the development of metabolic syndrome. Pediatrics 2014, 133, e114–e119. [Google Scholar] [CrossRef]
- Sidnell, A.; Greenstreet, E. Infant nutrition—Protein and its influence on growth rate. Nutr. Bull. 2009, 34, 395–400. [Google Scholar] [CrossRef]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [PubMed]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Bhutta, Z.A.; Cai, W.; Cruchet, S.; Guindi, M.E.; Fuchs, G.J.; Goddard, E.A.; van Goudoever, J.B.; Quak, S.H.; Kulkarni, B.; et al. Compositional Requirements of Follow-Up Formula for Use in Infancy: Recommendations of an International Expert Group Coordinated by the Early Nutrition Academy. Ann. Nutr. Metab. 2013, 62, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Campoy, C.; Escolano-Margarit, M.V.; Anjos, T.; Szajewska, H.; Uauy, R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br. J. Nutr. 2012, 107, S85–S106. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Sandre, P.C.; de Velasco, P.C.; Serfaty, C.A. The Impact of Low Omega-3 Fatty Acids Diet on the Development of the Visual System. In Handbook of Nutrition, Diet, and the Eye, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–395. ISBN 978-0-12-815245-4. [Google Scholar]
- Anjos, T.; Altmäe, S.; Emmett, P.; Tiemeier, H.; Closa-Monasterolo, R.; Luque, V.; Wiseman, S.; Pérez-García, M.; Lattka, E.; Demmelmair, H.; et al. Nutrition and neurodevelopment in children: Focus on NUTRIMENTHE project. Eur. J. Nutr. 2013, 52, 1825–1842. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Byard, R.W.; Simmer, K.; Gibson, R.A. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 1994, 60, 189–194. [Google Scholar] [CrossRef]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef]
- Birch, E.E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.N.; Drover, J.R.; Castañeda, Y.S.; Minns, L.; Wheaton, D.K.H.; Mundy, D.; et al. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: A double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 2010, 91, 848–859. [Google Scholar] [CrossRef]
- Liao, K.; McCandliss, B.D.; Carlson, S.E.; Colombo, J.; Shaddy, D.J.; Kerling, E.H.; Lepping, R.J.; Sittiprapaporn, W.; Cheatham, C.L.; Gustafson, K.M. Event-related potential differences in children supplemented with long-chain polyunsaturated fatty acids during infancy. Dev. Sci. 2017, 20, e12455. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Lewis, G. Childhood internalizing behaviour: Analysis and implications. J. Psychiatr. Ment. Health Nurs. 2011, 18, 884–894. [Google Scholar] [CrossRef] [PubMed]
| GM’s Classification | |||
|---|---|---|---|
| Complexity | Variation | Fluidity | |
| Normal-optimal | +++ | +++ | + |
| Normal-suboptimal | ++ | ++ | - |
| Moderately abnormal | + | + | - |
| Definitively abnormal | - | - | - |
| BF (n = 50) | SF (n = 85) | EF (n = 85) | p1 | ||
|---|---|---|---|---|---|
| Parents Characteristics | |||||
| Maternal age (years) | 32 (30–36.25) | 32 (24.75–35.25) | 30.50 (26.25–34.75) | 0.068 | |
| Paternal age (years) | 35.07 ± 5.01 | 32.68 ± 6.89 | 33.31 ± 7.03 | 0.134 | |
| Maternal pBMI (kg/m2) | 23.90 (21.80–26.16) | 24.18 (21.75–27.61) | 23.68 (21.14–27.30) | 0.842 | |
| GA at delivery (weeks) | 39.5 (38–40.25) | 40 (38–40) | 40 (39–40) | 0.925 | |
| GWG (kg) | 6 (4–9) | 7 (3.5–10) | 6 (3–9.5) | 0.781 | |
| Siblings | No | 28 (56) | 33 (38.80) | 42 (49.40) | 0.128 |
| Yes | 22 (44) | 52 (61.20) | 43 (50.60) | ||
| Type of delivery | Vaginal | 37 (74) | 62 (72.90) | 60 (70.60) | 0.899 |
| Caesarean | 13 (26) | 23 (27) | 25 (29.40) | ||
| Smoking during pregnancy | Yes | 2 (4.70) | 13 (18.80) | 10 (13) | 0.098 |
| Postpartum Depression | Yes | 6 (12) | 21 (24.7) | 22 (26.2) | 0.131 |
| Maternal IQ (points) | 111 (99–118) a | 102 (92–111) b | 100 (86–108) b | <0.001 | |
| Maternal educational level | Primary | 1 (2) a | 19 (22.40) b | 19 (22.40) b | <0.001 |
| Secondary | 5 (10) a | 28 (32.90) b | 29 (34.10) b | ||
| VT | 16 (32) | 15 (17.60) | 21 (24.70) | ||
| University | 28 (56) a | 23 (27.10) b | 16 (18.80) b | ||
| Paternal IQ (points) | 108 (99–117) | 108 (96–117) | 102 (92–111) | 0.062 | |
| Paternal educational level | Primary | 6 (12.80) a | 28 (35) b | 36 (46.20) b | 0.003 |
| Secondary | 11 (23.40) | 25 (31.30) | 16 (20.50) | ||
| VT | 12 (25.50) | 13 (16.30) | 12 (15.40) | ||
| University | 18 (38.30) a | 14 (17.40) b | 14 (17.90) b | ||
| Place of residence | Urban | 15 (30) | 38 (44.70) | 28 (32.90) | 0.148 |
| Rural | 35 (70) | 47 (55.30) | 57 (67.10) | ||
| Newborn characteristics | |||||
| Sex (boy) | 21 (42) | 49 (57.6) | 51 (60) | 0.105 | |
| Birth weight (g) | 3321.20 ± 431.73 | 3266.25 ± 459.08 | 3347.76 ± 486.41 | 0.513 | |
| Birth length (cm) | 51 (49–51) | 50 (49–52) | 51 (49–52) | 0.431 | |
| Birth HC (cm) | 35 (33.25–35) | 35 (34–35.5) | 34.25 (34–35) | 0.481 | |
| Breastfeeding (days) | 420 (270–540) | 8 (0–22) | 7 (1–28) | <0.001 | |
| GM’s | Moderately Abnormal | Normal-Suboptimal | Normal-Optimal | p1 |
|---|---|---|---|---|
| 2 months of life | ||||
| BF (n = 31) | 10 (32.3) | 19 (61.3) | 2 (6.5) | 0.636 |
| SF (n = 70) | 23 (32.9) | 36 (51.4) | 11 (15.7) | |
| EF (n = 77) | 29 (37.7) | 40 (51.9) | 8 (10.4) | |
| 3 months of life | ||||
| BF (n = 32) | 10 (31.3) | 21 (65.6) | 1 (3.1) | 0.322 |
| SF (n = 59) | 11 (18.6) | 39 (66.1) | 9 (15.3) | |
| EF (n = 61) | 17 (27.9) | 38 (62.3) | 6 (9.8) | |
| 4 months of life | ||||
| BF (n = 37) | 15 (40.5) | 20 (54.1) | 2 (5.4) | 0.453 |
| SF (n = 62) | 21 (33.9) | 31 (50) | 10 (16.1) | |
| EF (n = 69) | 20 (29) | 37 (53.6) | 12 (17.4) | |
| 3 Months of Life | 12 Months of Life | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | X ± SD | Punadj | Padj | n | X ± SD | Punadj | Padj | ||
| Latencies (ms) | |||||||||
| P100 120′ of arc | BF | 29 | 117.48 ± 14.46 b | 0.059 | 0.052 | 36 | 106.22 ± 6.70 | 0.759 | 0.470 |
| SF | 59 | 125.68 ± 19.10 a,b | 45 | 106.40 ± 8.83 | |||||
| EF | 67 | 127.12 ± 19.27 a | 58 | 107.34 ± 8.22 | |||||
| P100 60′ of arc | BF | 29 | 122.03 ± 17.90 b | 0.028 | 0.012 | 36 | 108.69 ± 7.13 | 0.402 | 0.225 |
| SF | 58 | 128.74 ± 16.48 a,b | 46 | 112.00 ± 14.51 | |||||
| EF | 67 | 133.16 ± 20.55 a | 54 | 110.80 ± 9.60 | |||||
| P100 30′ of arc | BF | 29 | 127.34 ± 20.85 b | 0.029 | 0.015 | 36 | 113.75 ± 7.52 | 0.186 | 0.090 |
| SF | 55 | 137.62 ± 20.43 a,b | 43 | 115.21 ± 11.90 | |||||
| EF | 65 | 139.80 ± 21.68 a | 57 | 117.75 ± 11.19 | |||||
| P100 15′ of arc | BF | 24 | 136.7 ± 17.65 b | 0.021 | 0.040 | 29 | 116.41 ± 8.04 a | 0.004 | 0.004 |
| SF | 38 | 143.63 ± 30.31 a,b | 28 | 124.00 ± 13.92 b | |||||
| EF | 51 | 153.16 ± 22.66 a | 43 | 124.91 ± 9.95 b | |||||
| P100 7½′ of arc | BF | 6 | 161.00 ± 19.89 | 0.142 | 0.445 | 15 | 128.73 ± 6.72 a | 0.061 | 0.031 |
| SF | 4 | 183.25 ± 37.21 | 8 | 136.38 ± 9.41 a,b | |||||
| EF | 6 | 104.50 ± 92.84 | 22 | 136.14 ± 11.28 b | |||||
| Amplitudes (µV) | |||||||||
| P100 120′ of arc | BF | 29 | 23.24 ± 11.95 | 0.103 | 0.085 | 36 | 21.09 ± 10.58 a | 0.007 | 0.003 |
| SF | 59 | 18.04 ± 8.62 | 45 | 15.77 ± 8.21 b | |||||
| EF | 67 | 20.14 ± 11.54 | 58 | 15.86 ± 7.20 b | |||||
| P100 60′ of arc | BF | 29 | 27.73 ± 14.85 a | 0.004 | 0.007 | 36 | 19.40 ± 8.55 | 0.204 | 0.189 |
| SF | 58 | 20.09 ± 10.86 b | 46 | 16.40 ± 8.17 | |||||
| EF | 67 | 18.97 ± 11.60 b | 54 | 16.57 ± 8.39 | |||||
| P100 30′ of arc | BF | 29 | 23.64 ± 15.78 a | 0.086 | 0.014 | 36 | 15.83 ± 8.82 | 0.505 | 0.311 |
| SF | 55 | 16.52 ± 9.08 b | 43 | 13.28 ± 7.28 | |||||
| EF | 65 | 17.04 ± 9.59 b | 57 | 14.33 ± 11.52 | |||||
| P100 15′ of arc | BF | 24 | 19.84 ± 12.57 a | 0.046 | 0.005 | 29 | 13.72 ± 10.63 | 0.201 | 0.288 |
| SF | 38 | 13.22 ± 8.51 b | 28 | 10.49 ± 6.01 | |||||
| EF | 52 | 12.82 ± 6.97 b | 43 | 12.92 ± 6.68 | |||||
| P100 7½′ of arc | BF | 6 | 13.83 ± 5.35 | 0.181 | 0.923 | 15 | 14.12 ± 10.37 | 0.789 | 0.645 |
| SF | 4 | 14.28 ± 5.76 | 8 | 13.00 ± 5.97 | |||||
| EF | 5 | 6.02 ± 10.06 | 22 | 12.45 ± 4.53 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto-Ruiz, A.; García-Santos, J.A.; Bermúdez, M.G.; Herrmann, F.; Diéguez, E.; Sepúlveda-Valbuena, N.; García, S.; Miranda, M.T.; De-Castellar, R.; Rodríguez-Palmero, M.; et al. Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients 2019, 11, 2456. https://doi.org/10.3390/nu11102456
Nieto-Ruiz A, García-Santos JA, Bermúdez MG, Herrmann F, Diéguez E, Sepúlveda-Valbuena N, García S, Miranda MT, De-Castellar R, Rodríguez-Palmero M, et al. Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients. 2019; 11(10):2456. https://doi.org/10.3390/nu11102456
Chicago/Turabian StyleNieto-Ruiz, Ana, José Antonio García-Santos, Mercedes G. Bermúdez, Florian Herrmann, Estefanía Diéguez, Natalia Sepúlveda-Valbuena, Salomé García, Maria Teresa Miranda, Roser De-Castellar, María Rodríguez-Palmero, and et al. 2019. "Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial" Nutrients 11, no. 10: 2456. https://doi.org/10.3390/nu11102456
APA StyleNieto-Ruiz, A., García-Santos, J. A., Bermúdez, M. G., Herrmann, F., Diéguez, E., Sepúlveda-Valbuena, N., García, S., Miranda, M. T., De-Castellar, R., Rodríguez-Palmero, M., Catena, A., & Campoy, C. (2019). Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients, 11(10), 2456. https://doi.org/10.3390/nu11102456