A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedures
2.3. Measurement of Body Weight and Gastrocnemius Muscle Thickness
2.4. Histopathological Analysis
2.5. Preparation of Chalcones from Roots of AK
2.6. Cell Culture, Myoblast Differentiation and Preparation of Conditioned Medium of CT26 Cancer Cells
2.7. MyoD-Reporter Gene Assay
2.8. Immunostaining of Myosin Heavy Chain (MHC)
2.9. Western Blot Analysis
2.10. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results
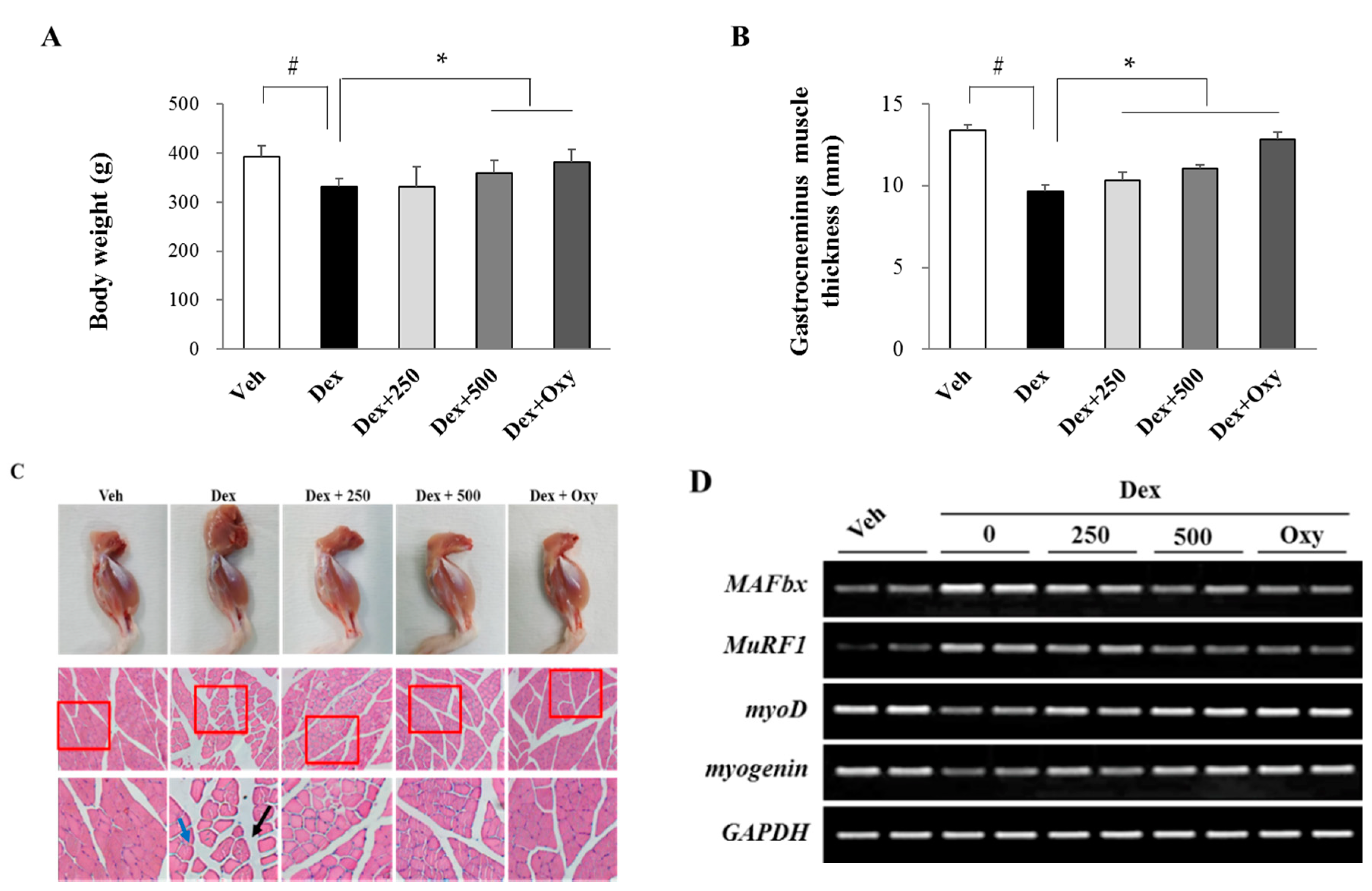
3.1. Ethanol Extract of Angelica keiskei Alleviates Dexamethasone-Induced Muscle Atrophy in Rats
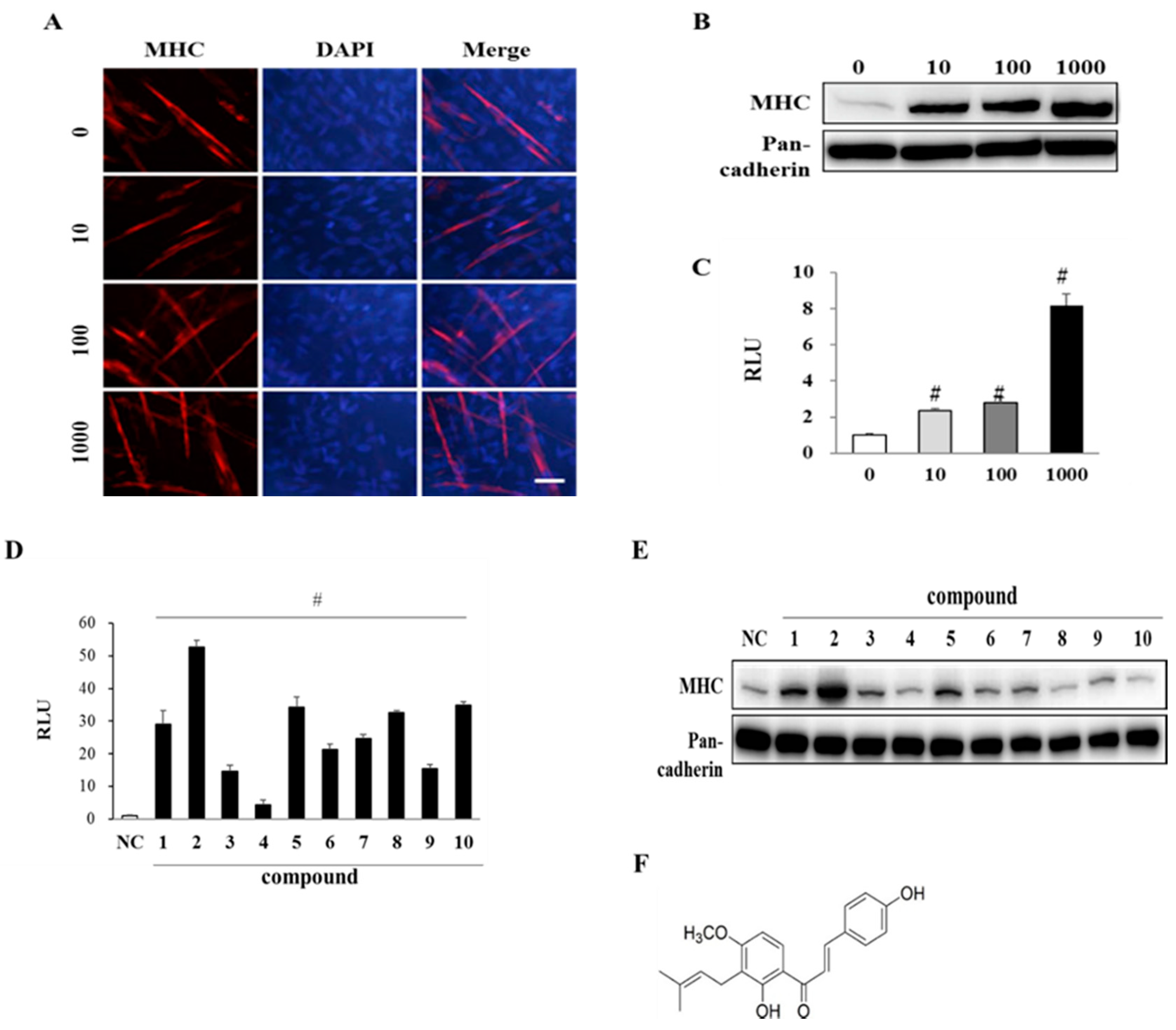
3.2. EAK and Its Components Promote Myogenesis
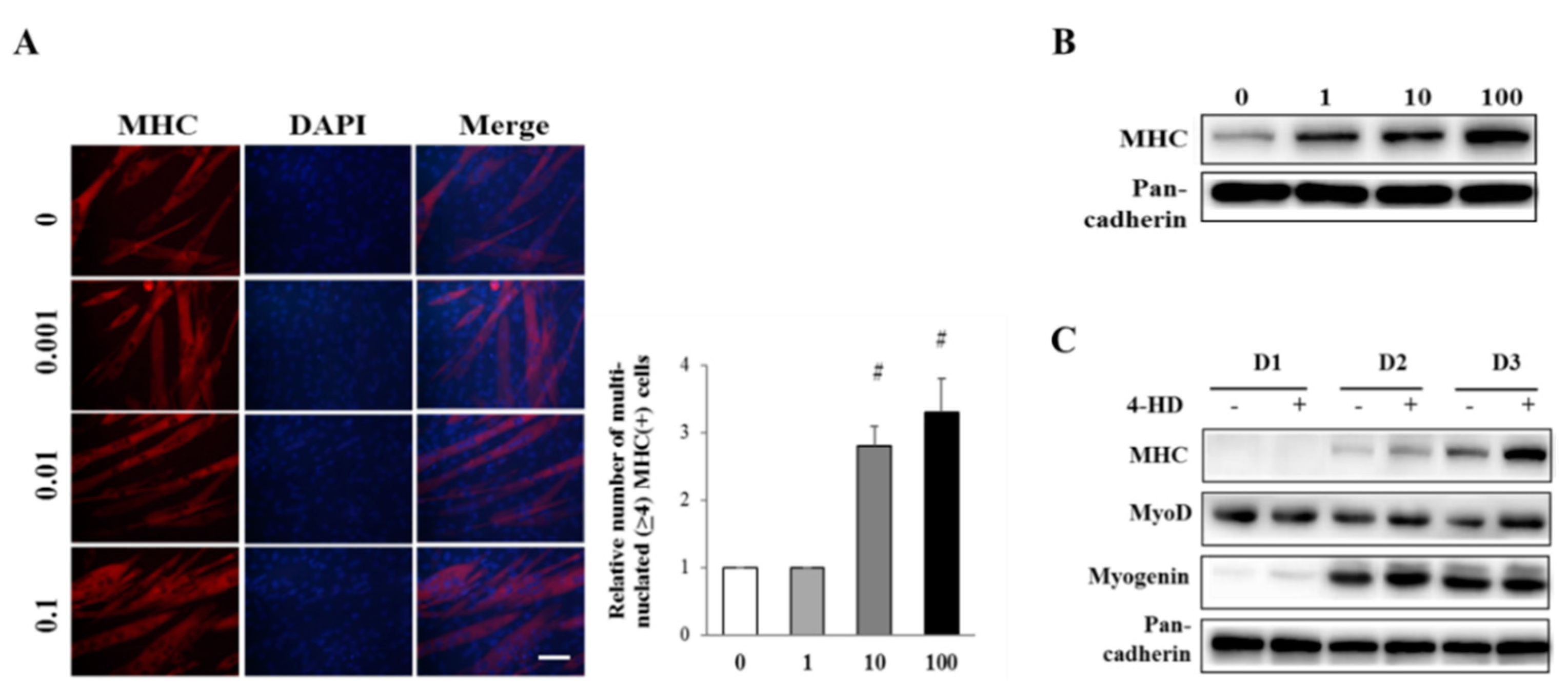
3.3. 4-HD Stimulates Myogenesis
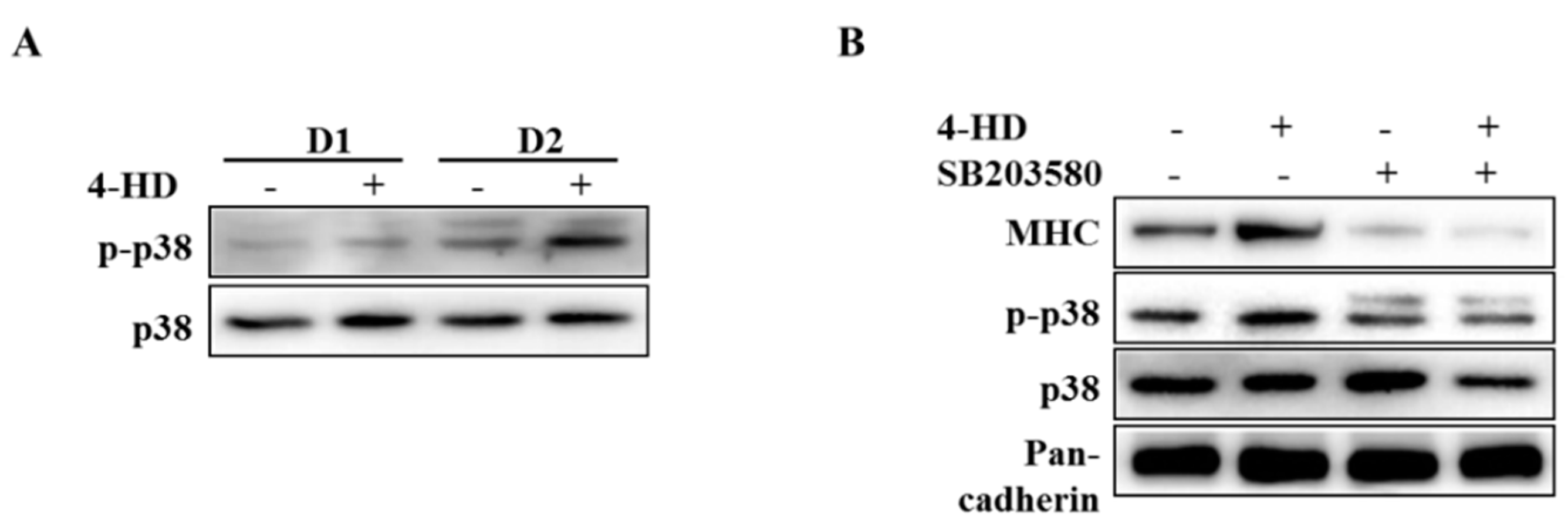
3.4. 4-HD Stimulates Myogenesis by p38 MAPK Activation
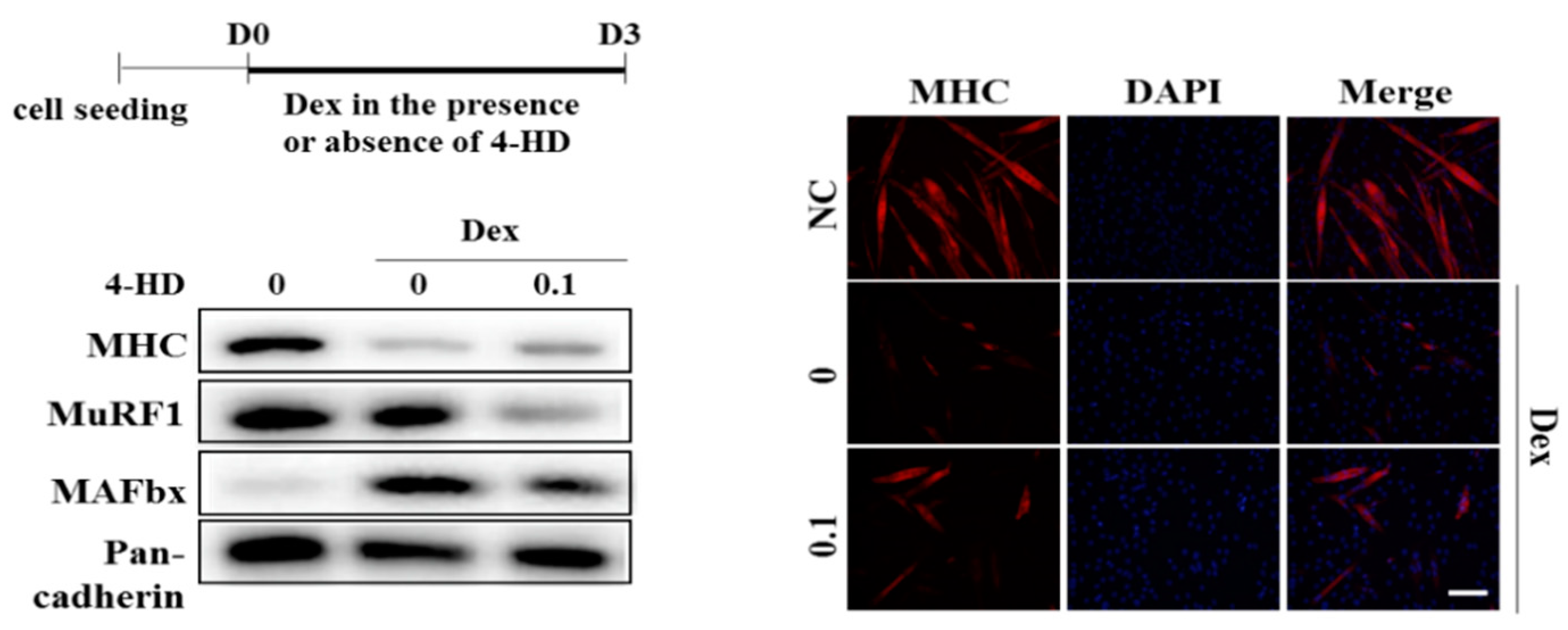
3.5. 4-HD Protects Against Muscle Wasting In vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dutt, V.; Gupta, S.; Dabur, R.; Injeti, E.; Mittal, A. Skeletal muscle atrophy: Potential therapeutic agents and their mechanisms of action. Pharm. Res. 2015, 99, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Shinde, A.M.; Hendifar, A.E. Pancreatic cancer cachexia: Current concepts and clinical management. In Frailty and Sarcopenia: Onset, Development and Clinical Challenges; IntechOpen: London, UK, 2017; Volume 133. [Google Scholar]
- Cruz-Jentoft, A.J.; Landi, F.; Topinkova, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, Y.M.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide PYP15 protects against dexamethasone induced muscle atrophy through the downregulation of atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes. Mol. Med. Rep. 2017, 15, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.A.; Drujan, D.; Willis, M.S.; Murphy, L.O.; Corpina, R.A.; Burova, E.; Rakhilin, S.V.; Stitt, T.N.; Patterson, C.; Latres, E.; et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007, 6, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Lagirand-Cantaloube, J.; Cornille, K.; Csibi, A.; Batonnet-Pichon, S.; Leibovitch, M.P.; Leibovitch, S.A. Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS ONE 2009, 4, e4973. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin Attenuates Dexamethasone-Induced Muscle Atrophy by Improving Mitochondrial Function via the PGC-1alpha Pathway. Cell. Physiol. Biochem. 2018, 49, 758–779. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. A Review of the Medicinal Uses and Pharmacology of Ashitaba. Planta Med. 2016, 82, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Toyama, K.C.S. Ashitaba-Hachijojima Islands Reiso in the Early Modern Period. Kobe Health Welf. Univ. Anal. 2014, 15, 37–44. [Google Scholar]
- Chang, H.R.; Lee, H.J.; Ryu, J.H. Chalcones from Angelica keiskei attenuate the inflammatory responses by suppressing nuclear translocation of NF-kappaB. J. Med. Food 2014, 17, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sawada, K.; Yamamoto, N.; Ashida, H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways. Mol. Nutr. Food Res. 2013, 57, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.A.; Lee, H.; Park, S.Y.; Lim, Y.; Kwon, O.; Kim, J.Y.; Kim, D.; Jung, B.H. Analysis of plasma metabolic profiling and evaluation of the effect of the intake of Angelica keiskei using metabolomics and lipidomics. J. Ethnopharmacol. 2019, 243, 112058. [Google Scholar] [CrossRef] [PubMed]
- Maronpot, R.R. Toxicological assessment of Ashitaba Chalcone. Food Chem. Toxicol. 2015, 77, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tokushima, T.; Kawabata, K.; Yamamoto, N.; Miyamoto, M.; Ashida, H. Absorption and metabolism of 4-hydroxyderricin and xanthoangelol after oral administration of Angelica keiskei (Ashitaba) extract in mice. Arch. Biochem. Biophys. 2012, 521, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sawada, K.; Ikeda, K.; Fukuda, I.; Kawasaki, K.; Yamamoto, N.; Ashida, H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol. Nutr. Food Res. 2011, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Guo, T.; Bond, N.D.; Gavrilova, O. Increasing muscle mass to improve metabolism. Adipocyte 2013, 2, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef]
- Baba, K.; Nakata, K.; Taniguchi, M.; Kido, T.; Kozawa, M. Chalcones from Angelica keiskei. Phytochemistry 1990, 29, 3907–3910. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.J.; Bae, G.U.; Baek, N.I.; Ryu, J.H. Canadine from Corydalis turtschaninovii Stimulates Myoblast Differentiation and Protects against Myotube Atrophy. Int. J. Mol. Sci. 2017, 18, 2748. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, X.E.; Song, C.; Sun, S.; Yang, G.; Li, X. BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/beta-Catenin Signaling. Int. J. Mol. Sci. 2015, 16, 17734–17745. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hemker, M.L.; Xie, M.; Soukup, S.T.; Diel, P. Anabolic Activity of a Soy Extract and Three Major Isoflavones in C2C12 Myotubes. Planta Med. 2018, 84, 1022–1029. [Google Scholar] [CrossRef]
- Kim, D.W.; Curtis-Long, M.J.; Yuk, H.J.; Wang, Y.; Song, Y.H.; Jeong, S.H.; Park, K.H. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC-ESI MS/MS and their xanthine oxidase inhibition. Food Chem. 2014, 153, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Oeztuerk-Winder, F.; Ventura, J.J. The many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiation. Biochem. J. 2012, 445, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.H.; Jang, E.J.; Kim, Y.W.; Lee, J.H. Sulforaphane prevents dexamethasone-induced muscle atrophy via regulation of the Akt/Foxo1 axis in C2C12 myotubes. Biomed. Pharm. 2017, 95, 1486–1492. [Google Scholar] [CrossRef]
- Ohkura, N.; Atsumi, G.I.; Uehara, S.; Ohta, M.; Taniguchi, M. Ashitaba (Angelica keiskei) Exerts Possible Beneficial Effects on Metabolic Syndrome. OBM Integr. Complement. Med. 2018, 4. [Google Scholar] [CrossRef]
- Dedieu, S.; Mazeres, G.; Cottin, P.; Brustis, J.J. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int. J. Dev. Biol. 2002, 46, 235–241. [Google Scholar]
- Ferri, P.; Barbieri, E.; Burattini, S.; Guescini, M.; D’Emilio, A.; Biagiotti, L.; Del Grande, P.; De Luca, A.; Stocchi, V.; Falcieri, E. Expression and subcellular localization of myogenic regulatory factors during the differentiation of skeletal muscle C2C12 myoblasts. J. Cell Biochem. 2009, 108, 1302–1317. [Google Scholar] [CrossRef]
- Yasuda, M.; Kawabata, K.; Miyashita, M.; Okumura, M.; Yamamoto, N.; Takahashi, M.; Ashida, H.; Ohigashi, H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J. Agric. Food Chem. 2014, 62, 462–467. [Google Scholar] [CrossRef]
- Lassar, A.B. The p38 MAPK family, a pushmi-pullyu of skeletal muscle differentiation. J. Cell Biol. 2009, 187, 941–943. [Google Scholar] [CrossRef]
- Jeong, J.; Park, C.H.; Kim, I.; Kim, Y.H.; Yoon, J.M.; Kim, K.S.; Kim, J.B. Korean mistletoe (Viscum album coloratum) extract regulates gene expression related to muscle atrophy and muscle hypertrophy. BMC Complement. Altern. Med. 2017, 17, 68. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Grobet, L.; Pirottin, D.; Farnir, F.; Poncelet, D.; Royo, L.J.; Brouwers, B.; Christians, E.; Desmecht, D.; Coignoul, F.; Kahn, R.; et al. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis 2003, 35, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.; Toraldo, G.; Aversa, Z.; Smith, I.; Castillero, E.; Renaud, G.; Qaisar, R.; Larsson, L.; Jasuja, R.; Hasselgren, P.O. Loss of muscle strength during sepsis is in part regulated by glucocorticoids and is associated with reduced muscle fiber stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R1090–R1099. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Du, R.; Yang, Y.Q.; Zhang, H.Q.; Li, Q.; Liu, L.; Guan, H.; Hou, J.; An, X.R. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 2013, 94, 84–89. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward Primer | Reverse Primer | Accession Number |
|---|---|---|---|
| MAFbx MuRF1 GAPDH | CGACCTGCCTGTGTGCTTAC GGTGCCTACTTGCTCCTTGT TGCACCACCAACTGCTTAG | CTTGCGAATCTGCCTCTCTG CTGGTGGCTATTCTCCTTGG GGCATGGACTGTGGTCATGAG | BC027211 NC_000070 BC0960 42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweon, M.; Lee, H.; Park, C.; Choi, Y.H.; Ryu, J.-H. A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy. Nutrients 2019, 11, 2419. https://doi.org/10.3390/nu11102419
Kweon M, Lee H, Park C, Choi YH, Ryu J-H. A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy. Nutrients. 2019; 11(10):2419. https://doi.org/10.3390/nu11102419
Chicago/Turabian StyleKweon, Minson, Hyejin Lee, Cheol Park, Yung Hyun Choi, and Jae-Ha Ryu. 2019. "A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy" Nutrients 11, no. 10: 2419. https://doi.org/10.3390/nu11102419
APA StyleKweon, M., Lee, H., Park, C., Choi, Y. H., & Ryu, J.-H. (2019). A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy. Nutrients, 11(10), 2419. https://doi.org/10.3390/nu11102419






