The Effect of the Yingyangbao Complementary Food Supplement on the Nutritional Status of Infants and Children: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Sources and Search Strategy
2.3. Data Extraction and Assessment of Methodological Quality
2.4. Statistical Analyses
3. Results
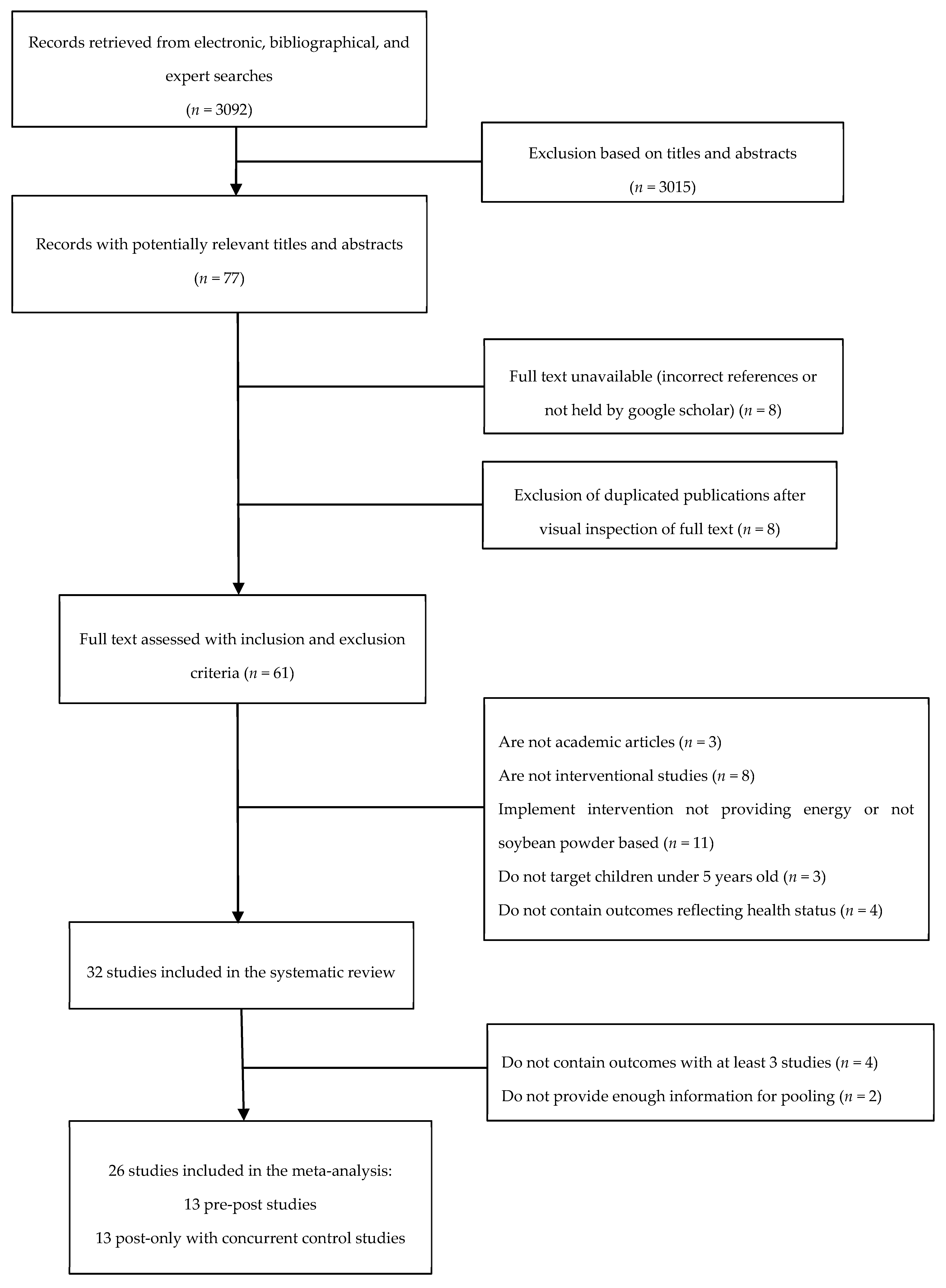
3.1. Literature Search and Selected Studies
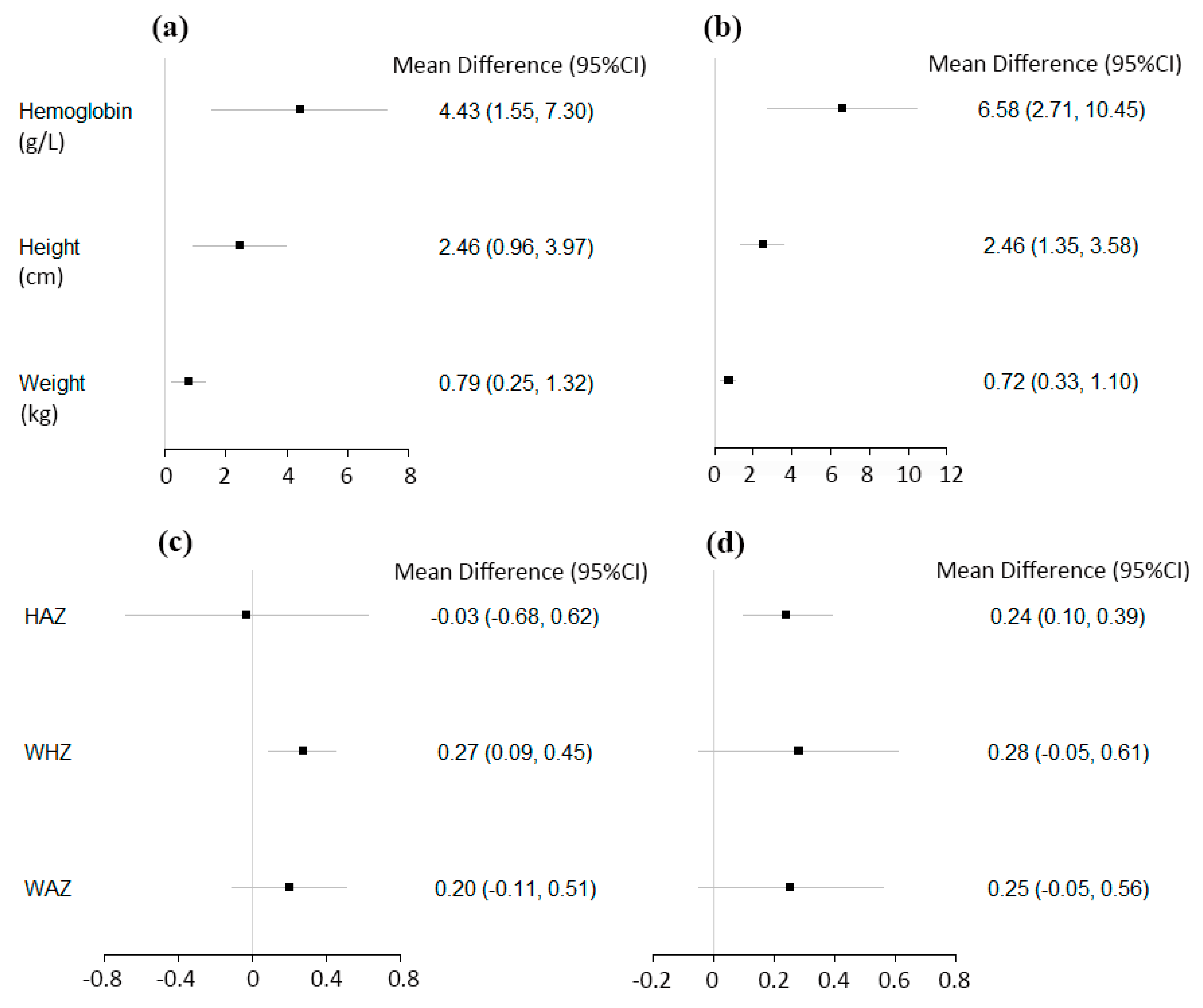
3.2. Continuous Hematologic and Anthropometric Outcomes
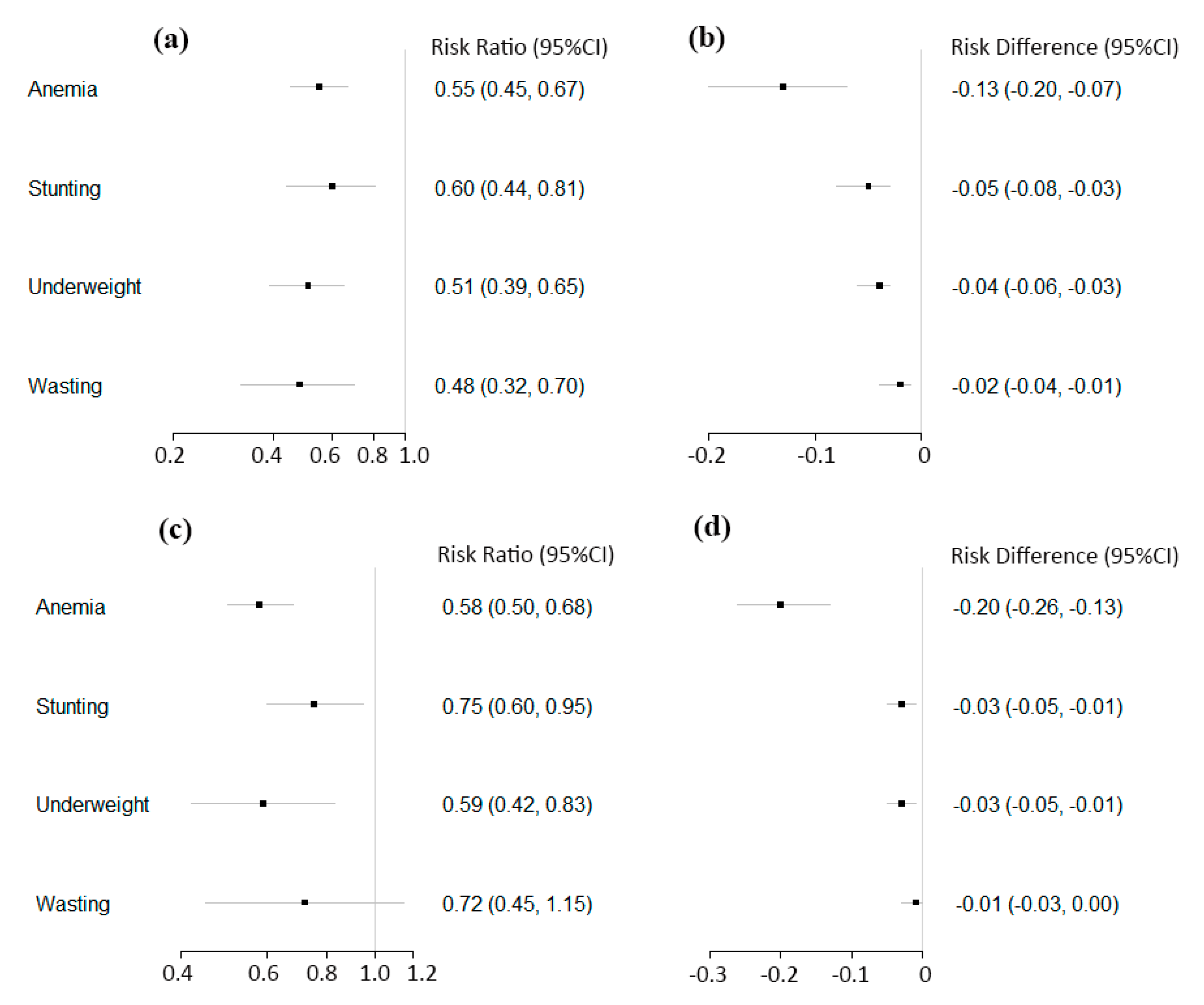
3.3. Anemia, Stunting, Underweight, and Wasting
3.4. Subgroup and Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistical Press: Beijing, China, 2017.
- The United Nations Development Programme (UNDP). World Population Prospects—Population Division—United Nations. Available online: https://esa.un.org/unpd/wpp/Download/Standard/Population/ (accessed on 2 August 2017).
- The World Bank. Prevalence of Stunting, Height For Age (% of Children Under 5) Data. Available online: https://data.worldbank.org/indicator/sh.sta.stnt.zs (accessed on 11 February 2019).
- The World Bank. Prevalence of Underweight, Weight for Age (% of Children under 5) | Data. Available online: https://data.worldbank.org/indicator/SH.STA.MALN.ZS (accessed on 11 February 2019).
- World Food Programme. 10 Facts about Nutrition in China. Available online: https://www.wfp.org/stories/10-facts-about-nutrition-china (accessed on 25 May 2018).
- Wang, J.; Chang, S.; Zhao, L.; Yu, W.; Zhang, J.; Man, Q.; He, L.; Duan, Y.; Wang, H.; Scherpbier, R.; et al. Effectiveness of community-based complementary food supplement (Yingyangbao) distribution in children aged 6–23 months in poor areas in China. PLoS ONE 2017, 12, e0174302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q.; Wang, W.; van Velthoven, M.H.; Chang, S.; Han, H.; Xing, M.; Chen, L.; Scherpbier, R.W. Effectiveness of complementary food supplements and dietary counselling on anaemia and stunting in children aged 6–23 months in poor areas of Qinghai Province, China: A controlled interventional study. BMJ Open 2016, 6, e011234. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Ying Yang Bao: Improving Complementary Feeding for Chinese Infants in Poor Regions. Nestle Nutrition Institute Workshop Series; 2017; pp. 131–138. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28315893 (accessed on 20 April 2018).
- Zhang, Q.; Sun, J.; Jia, X.; Huo, J. Meta analysis of the nutrition intervention effect of Yingyangbao on infants and young children in China. J. Hyg. Res. 2015, 44, 970–977. [Google Scholar]
- Wang, Y.; Chen, C.; Jia, M.; Fang, J.; Wang, F. Effect of food supplements on anemia in infants and young children. J. Hyg. Res. 2004, 33, 334–336. [Google Scholar]
- China Development Research Foundation. One Yuan Yingyangbao Program. 2018. Available online: https://www.cdrf.org.cn/gzbg/4447.jhtml (accessed on 26 September 2019).
- China’s Ministry of Health. Infant and Child Feeding Strategies. 2007. Available online: http://www.gov.cn/fwxx/jk/2007-08/01/content_703104.htm (accessed on 26 September 2019).
- Ministry of Education of the People’s Republic of China. Millions of Impoverished Children were Benefiting from Yingyangbao. Available online: http://www.moe.edu.cn/jyb_xwfb/s5147/201601/t20160112_227681.html (accessed on 4 September 2018).
- The State Council of the People’s Republic of China. Nutrition Strategy Plan for Chinese Citizens (2017–2030). Available online: http://www.mohrss.gov.cn/SYrlzyhshbzb/dongtaixinwen/shizhengyaowen/201707/t20170714_274000.html (accessed on 21 May 2018).
- The United Nations Children’s Fund (UNICEF). China’s Experience, Global Perspective. 2019. Available online: https://www.unicef.cn/media/8311/file/unicef-in-china-and-beyond-cn.pdf (accessed on 26 August 2019).
- Wang, Y.; Chen, C.; Wang, F.; Wang, K. Effects of nutrient fortified complements food supplements on growth of Chinese infants and young children in poor rural area in Gansu Province. J. Hyg. Res. 2007, 36, 78–81. [Google Scholar]
- Xu, J.; Li, Y.; Huo, J.; Sun, J.; Huang, J. Supplementing fortified soybean powder reduced anemia in infants and young children aged 6–24 months. Nutr. Res. 2019, 63, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Tramèr, M.R.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Impact of covert duplicate publication on meta-analysis: A case study. BMJ 1997, 315, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Intervention; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 22 February 2019).
- Ni, S.; Qin, X.; Chen, X.; Yue, C.; Liu, Y.; Zhang, F.; Liu, F. Study on nutrition intervention experiment of infants and young children in rural areas. Chinese J. Public Heal. 1995, 11, 337–339. [Google Scholar]
- Wang, Y.; Wang, F.-Z.; Wang, K.; Chen, C.-M.; Jin, M. Effect of nutritional fortified supplementary food supplement on intelligence development of infants and young children in poor rural areas of Gansu. J. Hyg. Res. 2006, 35, 772–774. [Google Scholar]
- Yu, D.; Wang, Y.; Wang, F. Effect of supplementary food supplement on respiratory tract infection and diarrhea in infants and young children in poor rural areas. J. Hyg. Res. 2007, 36, 355–357. [Google Scholar]
- Wang, Y.; Chen, C.; Wang, F.; Wang, K.; Yu, D. Effects of nutritional fortified supplementary food supplements on physical growth of infants and young children in poor rural areas of Gansu. J. Hyg. Res. 2007, 36, 78–81. [Google Scholar]
- Wang, Y.; Chen, C.; Wang, F.; Jia, M.; Wang, K. Effects of nutrient fortified complementary food supplements on anemia of infants and young children in poor rural of Gansu. Biomed. Env. Sci. 2009, 22, 194–200. [Google Scholar] [CrossRef]
- Chen, C.-M.; Wang, Y.-Y.; Chang, S.-Y. Effect of In-home Fortification of Complementary Feeding on Intellectual Development of Chinese Children. Biomed. Environ. Sci. 2010, 23, 83–91. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, H.; Zhao, L.; Tang, Z.; Ma, L.; Zhao, L.; Yu, W.; Jia, F.; Wang, Q. Interventional effect analysis of nutritional health status of infants aged 6–24 months in poor areas in 3 counties of Guangxi. Chinese J. Child Heal. Care 2010, 18, 638–640. [Google Scholar]
- Foundation CDR. China Development Research Foundation Early Childhood Development Project Qinghai Pilot Mid-term Evaluation. 2011. Available online: https://cdrf.org.cn/jjh/pdf/qinghai.pdf (accessed on 3 July 2018).
- Zhao, W.; Li, H.; Yang, H. Evaluation on the effect of nutrition intervention for children in poor rural areas of Gansu. Chinese J. Sch. Heal. 2012, 33, 257–258+262. [Google Scholar]
- Fan, S.; Li, J.; Zhang, Y.; Xu, C.; Zhao, L.; Ma, M.; M, H.; Ma, Q.; Z, L.; L, Y. Analysis of anemia and intervention effect of infants and young children in Hebei Province. Matern Child Heal Care China. 2013, 28, 2032–2033. [Google Scholar]
- Li, W.; Zhu, B.; Shao, J.; Zhu, Z. Effects of complementary food supplements on incidence of anemia among infants and young children in Zhejiang Province. Mod. Prev. Med. 2013, 40, 23. [Google Scholar]
- Ren, L. Effect evaluation of nutrition intervention for infants aged 6~24 months in Changle County. Mod Prev Med. 2014, 41, 1984–1986+1990. [Google Scholar]
- Li, S. Investigation report on implementation of early childhood nutrition intervention project in poor areas of Ledu District. Qinghai Med. J. 2014, 44, 51–52. [Google Scholar]
- Hu, Q.; Du, M.; Liang, C.; Zhang, R. Evaluation of the effect of infant nutrition for children aged 6 to 36 months in Shenzhen. Chinese J. Women Child Heal. 2016, 7, 26–29. [Google Scholar]
- Ding, X.; Zhang, F.; He, Q.; Mao, Z.; Li, R. Nutrition effectiveness of 6–18 months old infants in low-income rural areas in Jiangxi province. Mod. Prev. Med. 2016, 43, 3703–3705+3756. [Google Scholar]
- Li, S.; Zhang, K.; Qiu, J.; Cha, L.; Xiang, Y. Effect evaluation of nutritional intervention of infantile complementary food package in 6–24-month infants. Matern. Child Heal. Care China 2017, 32, 58–61. [Google Scholar]
- Shen, H.; Li, G. Study on comprehensive nutrition intervention of infants and young children in rural areas of Shaanxi. Chinese J Clin Res. 2011, 24, 857–858. [Google Scholar]
- Sun, J.; Dai, Y.; Zhang, S.; Huang, J.; Yang, Z.; Huo, J.; Chen, C. Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China. Matern. Child Nutr. 2011, 7, 96–111. [Google Scholar] [CrossRef]
- Wang, L.; Huo, J.; Sun, J.; Li, W.; Huang, J.; Huang, C.; Lai, S.; Hu, J.; Chen, C.-M.; Wang, Y. Effect of nutrition package on infants and young children aged 6 to 23 months after Wenchuan earthquake in Li County, Sichuan. J. Hyg. Res. 2011, 40, 61–64. [Google Scholar]
- Dong, C.; Ge, P.; Ren, X.; Wang, J.; Fan, H.; Yan, X.; Yin, S.A. Prospective Study on the Effectiveness of Complementary Food Supplements on Improving Status of Elder Infants and Young Children in the Areas Affected by Wenchuan Earthquake. PLoS One. 2013, 9, e72711. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Xu, Z.; Chang, F.; Fu, P.; Zhang, J.; Zhang, T.; Zhang, H.; Li, J.; Song, P. Periods study of nutrition interventions about infants aged 6–24 months in Ningqiang country affected by Wenchuan earthquake. Chinese J. Child Heal. Care 2012, 20, 413–415. [Google Scholar]
- Li, L.; Chang, F.; Xu, Z.; Wang, L.; Full, G.; Fu, P.; Zhang, J.; Song, P. Half-year effect evaluation of infant nutrition package intervention for infants aged 6–24 months in Ningqiang, Shanxi. Chinese J. Child Heal. Care. 2012, 20, 395–397. [Google Scholar]
- Xu, Z.; Wang, L.; Chang, F.; Fu, P.; Zhang, J.; Zhang, H.; Li, J. Study on the effect of nutrition intervention on infants and young children aged 6 to 24 months in Ningqiang County of the earthquake-stricken area. Chinese J. Child Heal. Care. 2012, 20, 728–730. [Google Scholar]
- Liu, Z.; Yan, L.; Wu, T.; Lan, Z.; Xu, Y.; Lin, L. Study on Nutrition Improvement of Infants and Children in Earthquake-Stricken Areas. J. Prev. Med. Inf. 2013, 29, 107–110. [Google Scholar]
- Qin, J.; Ma, G. The effects of nutrition interventions about infants and children in Wulan County. Qinghai Med. J. 2014, 44, 63–64. [Google Scholar]
- Huo, J.; Sun, J.; Fang, Z.; Chang, S.; Zhao, L.; Fu, P.; Wang, J.; Huang, J.; Wang, L.; Begin, F.; et al. Effect of Home-Based Complementary Food Fortification on Prevalence of Anemia Among Infants and Young Children Aged 6 to 23 Months in Poor Rural Regions of China. Food Nutr. Bull. 2015, 36, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, H.; Su, X.; Zhou, X. Study on Nutritional Intervention Effect of Infants and Children in 6~24 Months in Chongqing Project Areas. Matern. Child Heal. Care China. 2016, 31, 2641–2643. [Google Scholar]
- Wu, P.; Shi, Y. Effect of children’s food supplement nutrition package on nutrition intervention of infants and young children in poor areas in China. Heal. Care Guid. 2017, 1, 114. [Google Scholar]
- Dewey, K.G.; Yang, Z.; Boy, E. Systematic review and meta-analysis of home fortification of complementary foods. Matern. Child Nutr. 2009, 5, 283–321. [Google Scholar] [CrossRef]
- Jack, S.J.; Ou, K.; Chea, M.; Chhin, L.; Devenish, R.; Dunbar, M.; Eang, C.; Hou, K.; Ly, S.; Khin, M.; et al. Effect of Micronutrient Sprinkles on Reducing Anemia. Arch. Pediatr. Adolesc. Med. 2012, 166, 842. [Google Scholar] [CrossRef]
- Smuts, C.M.; Lombard, C.J.; Benadé, A.J.S.; Dhansay, M.A.; Berger, J.; Hop, L.T.; López, d.R.G.; Untoro, J.; Karyadi, E.; Erhardt, J.; et al. Efficacy of a Foodlet-Based Multiple Micronutrient Supplement for Preventing Growth Faltering, Anemia, and Micronutrient Deficiency of Infants: The Four Country IRIS Trial Pooled Data Analysis. J. Nutr. 2005, 135, 631S–638S. [Google Scholar] [CrossRef]
- Zlotkin, S.H.; Schauer, C.; Christofides, A.; Sharieff, W.; Tondeur, M.C.; Hyder, S.M.Z. Micronutrient Sprinkles to Control Childhood Anaemia. PLoS Med. 2005, 2, e1. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Ruth, L.J.; Woodruff, B.A.; Mbakaya, C.; Mandava, U.; Flores-Ayala, R.; Jefferds, M.E.D.; Quick, R. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: A cluster-randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Hadi, Y.B.; Sheikh, S.S.; Bhutta, A.Z.; Prinzo, Z.W.; Bhutta, Z.A. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst. Rev. 2019, 5. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Suchdev, P.S.; Vist, G.E.; Walleser, S.; Peña-Rosas, J.P. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review). Evid. Based Child Health Cochrane Rev. J. 2013, 8, 112–201. [Google Scholar] [CrossRef] [PubMed]
- Samadpour, K.; Long, K.Z.; Hayatbakhsh, R.; Marks, G.C. Randomised comparison of the effects of Sprinkles and Foodlets with the currently recommended supplement (Drops) on micronutrient status and growth in Iranian children. Eur. J. Clin. Nutr. 2011, 65, 1287–1294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jyoti, V.; Sharma, S. Impact of micronutrients sprinkle on weight and height of children aged 6–36 months in Tonk district of Rajasthan state. Indian J. Community Health 2014, 26 (Suppl. 2), 294–299. [Google Scholar]
- The American Heart Association. Dietary Recommendations for Healthy Children American Heart Association. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/dietary-recommendations-for-healthy-children (accessed on 12 April 2019).
- Arimond, M.; Zeilani, M.; Jungjohann, S.; Brown, K.H.; Ashorn, P.; Allen, L.H.; Dewey, K.G. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: Experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern. Child Nutr. 2015, 11, 31–61. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Pena-Rosas, J.P.; Boy-Mena, E.; Sachdev, H.S. Lipid based nutrient supplements (LNS) for treatment of children (6 months to 59 months) with moderate acute malnutrition (MAM): A systematic review. PLoS ONE 2017, 12, e0182096. [Google Scholar] [CrossRef]
- Chaparro, C.M.; Dewey, K.G. Use of lipid-based nutrient supplements (LNS) to improve the nutrient adequacy of general food distribution rations for vulnerable sub-groups in emergency settings. Matern. Child Nutr. 2010, 6, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Home Fortification. Complementary Food Supplements (CFS). Available online: http://www.hftag.org/page.asp?content_id=33984 (accessed on 26 September 2019).
- Martins, V.J.; Toledo Florêncio, T.M.; Grillo, L.P.; Do Carmo, P.F.; Martins, P.A.; Clemente, A.P.G.; Santos, C.D.; Vieira, M.D.F.A.; Sawaya, A.L. Long-lasting effects of undernutrition. Int. J. Environ. Res. Public Health 2011, 8, 1817–1846. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Ying Yang Bao Story. 2015. Available online: http://www.chinanutri.cn/xwzx_238/xyxw/201510/t20151019_121165.html (accessed on 12 April 2019).
- The World Health Organization (WHO). Interventions by Global Target. Available online: https://www.who.int/elena/global-targets/en/ (accessed on 11 June 2019).
| First Author, Year | Target Age (Months) | Intervention Group 1 | Control Group | Intervention Duration (Months) | Age When Outcomes Measured (Months) | Outcomes Measured 2 |
|---|---|---|---|---|---|---|
| Shuhua Ni, 1995 [22] | 5–13 | 2 counties; n = 76; YYB-C | 2 counties; n = 77; Blank control | 8 months | 13 | Height, weight, Kaup index, Vervaeck index, urine hydroxyproline index |
| Yuying Wang, 2004 [10] | 4–12 | 5 counties; n = 670; YYB-B | 5 counties; n = 307; Energy matched rice flour | 12 months | 16–24 | Hb concentration and prevalence of anemia |
| Yuying Wang, 2006 [23] | 4–12 | 5 counties; n = 232; YYB-B | 5 counties; n = 116; Energy matched rice flour | Until 24 months of age for each child | 24 | Developmental quotient |
| Dongmei Yu, 2007 [24] | 4–12 | 5 counties; n = 978; YYB-B | 5 counties; n = 500; Energy matched rice flour | 12 months | 16–24 | Prevalence of diarrhea and respiratory infection in past two weeks |
| Yuying Wang, 2007 [25] | 4–12 | 5 counties; n = 978; YYB-B | 5 counties; n = 500; Energy matched rice flour | Until 24 months of age | 24 | WAZ, HAZ |
| Yuying Wang, 2009 [26] | 6–12 | 5 counties; n = 978; YYB-B | 5 counties; n = 500; Energy matched rice flour | 12 months | 18–24 | Hb concentration and prevalence of anemia |
| Chunming Chen, 2010 [27] | 4–12 | 5 counties; n = 232; YYB-B | 5 counties; n = 116; Energy matched rice flour | Until 24 months of age | 24 | Development quotient, intelligent quotient |
| Zhifeng Fang, 2010 [28] | 6–24 | 3 counties; n = 146; YYB-A | 3 counties; n = 107; Blank control | 6 months | 12–30 | Prevalence of stunting, underweight, wasting and anemia |
| China Development and Research Foundation, 2011 [29] | 6–24 | 6 counties; n = 1034; YYB-A | 6 counties; n = 449; Blank control | 24 months | 24–36 | Prevalence of underweight, stunting and wasting, Hb concentration and anemia prevalence |
| Wenli Zhao, 2012 [30] | 0–60 | 2 counties; n = 676; YYB-A | 2 counties; n = 536; Blank control | 12 months | 12–72 | Height, weight, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Songli Fan, 2013 [31] | 6–24 | 27 counties; n = 113; YYB-A | 27 counties; n = 328; Blank control | 3 months | 9–27 | Hb concentration and prevalence of anemia |
| Wenhao Li, 2013 [32] | 4–30 | 3 counties; n = 146; YYB-A | 3 counties; n = 146; Blank control | 3 months | 7–33 | Height, weight, anemia prevalence, and Hb concentration |
| Lingyun Ren, 2013 [33] | 6–11 | One county; n = 76; YYB-A | One county; n = 78; Blank control | Until 24 months of age for each child | 24 | Height, weight, WAZ, HAZ, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Shangming Li, 2014 [34] | 0–36 | One county; n = 387; YYB-A | One county; n = 240; Blank control | Until 36 months of age for each child | 36 | Prevalence of stunting, underweight and anemia |
| Qin Hu, 2016 [35] | 6–36 | One county; n = 589; YYB-A | One county; n = 300; Blank control | 6 months | 12–42 | Height, weight, head circumference, Hb concentration and prevalence of anemia and rickets |
| Xiaoting Ding, 2016 [36] | 6–18 | 3 counties; n = 483; YYB-A | 3 counties; n = 248; Blank control | 6 months | 12–24 | Height, weight, WAZ, HAZ, WHZ, Hb concentration, prevalence of underweight, wasting, stunting and anemia |
| Yanfeng Zhang, 2016 [7] | 6–23 | One county; n = 2186; YYB-A | One county; n = 760; Blank control | Until 24 months of age for each child | 6–23 | Prevalence of stunting and anemia |
| Shuai Li, 2017 [37] | 6–24 | One county; n = 450; YYB-A | One county; n = 450; Blank control | 12 months | 18–36 months | Height, weight, WAZ, HAZ, WHZ, Hb concentration, prevalence of underweight, wasting, stunting and anemia |
| First Author, Year | Target Age (Months) | Intervention 1 | Intervention Duration | Age When Outcomes Measured (Months) | Outcomes Measured 2 |
|---|---|---|---|---|---|
| Hong Shen, 2011 [38] | 0–36 | One county; Pretest: n = 143 Posttest: n = 148; YYB-A | 8 months | 8–44 | Prevalence of anemia |
| Jing Sun, 2011 [39] | 6–24 | 2 counties; Pretest: n = 226 Posttest: n = 221; YYB-A | 20–24 months of age for each child | 6–24 | Prevalence of anemia |
| Lijuan Wang, 2011 [40] | 6–29 | One county; Pretest: n = 257 Posttest: n = 253; YYB-A | 15 months | 6–29 | Height, weight, WAZ, HAZ, prevalence of stunting and underweight, Hb concentration and prevalence of anemia |
| Caixia Dong, 2012 [41] | 6–18 | One county; Pretest: n = 314 Posttest: n = 242; YYB-A | 18 months until 24 months of age for each child | 6–24 | WAZ, HAZ, WHZ, prevalence of underweight, stunting and wasting, Hb concentration and anemia prevalence |
| Linjiang Wang, 2012 [42] | 6–24 | One county; Pretest: n = 327 Posttest: n = 300; YYB-A | 18 months until 24 months of age for each child | 6–24 | WAZ, HAZ, Hb concentration |
| Lixiang Li, 2012 [43] | 6–24 | One county; Pretest: n = 327 Posttest: n = 307; YYB-A | 6 months | 6–24 | Height, weight, WAZ, HAZ, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Zengkang Xu, 2012 [44] | 6–24 | One county; Pretest: n = 327 Posttest: n-300; YYB-A | 18 months | 6–24 | Heigh, weight, WAZ, HAZ, WHZ, Hb concentration, and prevalence of underweight, wasting, stunting and anemia |
| Zuyang Liu, 2013 [45] | 6–24 | 5 counties; Pretest: n = 659 Posttest: n = 506; YYB-A | 18 months until 24 months of age | 6–24 | Prevalence of stunting, underweight, wasting and anemia |
| Jianhong Qin, 2014 [46] | 6–24 | One county; Pretest: n = 159 Posttest: n = 206; YYB-A | 12 months until 24 months of age for each child | 6–24 | Prevalence of underweight and stunting, Hb concentration and anemia prevalence, prevalence of diarrhea and respiratory infection |
| Junsheng Huo, 2015 [47]47 | 6–23 | 8 counties; Pretest: n = 1290 Posttest: n = 1040; YYB-A | 18 months until 24 months of age for each child | 6–23 | Hb concentration and prevalence of anemia |
| Qiannan Zhang, 2015 [9]9 | 6–23 | 2 counties; Pretest: n = 596 Posttest: n = 589; YYB-A | 12 months until 24 months of age for each child | 6–23 | Height, weight, WAZ, HAZ, prevalence of stunting, underweight and wasting, Hb concentration and prevalence of anemia |
| Qiujing Jiang, 2016 [48] | 6–24 | 3 counties; Pretest: n = 596 Posttest: n = 589; YYB-A | 12 months until 24 months of age for each child | 6–24 | Height, weight, prevalence of stunting and underweight, Hb concentration and prevalence of anemia |
| Jie Wang, 2017 [6] | 6–23 | 3 counties; Pretest: n = 823 Posttest: n = 693; YYB-A | 18 months until 24 months of age for each child | 6–23 | Prevalence of underweight, stunting, wasting and anemia, micronutrient status |
| Ping Wu, 2017 [49] | 6–24 | One county; Pretest: n = 156 Posttest: n = 156; YYB-A | 24 months until 24 months of age | 6–24 | Prevalence of stunting, underweight, wasting and anemia |
| Post-Only Studies with Concurrent-Control | Number of Studies | Total Intervention Participants | Total Control Participants | Mean Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Hemoglobin concentration (g/L) | 7 | 2810 | 1714 | 4.43 (1.55, 7.30) | 0.003 | 96 |
| Height (cm) | 6 | 2061 | 1689 | 2.46 (0.96, 3.97) | 0.001 | 94 |
| Weight (kg) | 6 | 2061 | 1689 | 0.79 (0.25, 1.32) | 0.004 | 97 |
| Height-for-age Z score (SD) | 3 | 1009 | 776 | −0.03 (−0.68, 0.62) | 0.94 | 95 |
| Weight-for-height Z score (SD) | 3 | 1009 | 776 | 0.27 (0.09, 0.45) | 0.003 | 66 |
| Weight-for-age Z score (SD) | 3 | 1009 | 776 | 0.20 (−0.11, 0.51) | 0.21 | 88 |
| Pre-Post Studies | Number of Studies | Total Posttest Participants | Total Pretest Participants | Mean Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Hemoglobin concentration (g/L) | 7 | 3370 | 3817 | 6.58 (2.71, 10.45) | <0.001 | 99 |
| Height (cm) | 5 | 2088 | 2213 | 2.46 (1.35, 3.58) | <0.001 | 90 |
| Weight (kg) | 5 | 1800 | 2212 | 0.72 (0.33, 1.10) | <0.001 | 94 |
| Height-for-age Z score (SD) | 5 | 1691 | 1821 | 0.24 (0.10, 0.39) | <0.001 | 70 |
| Weight-for-height Z score (SD) | 4 | 1438 | 1564 | 0.28 (−0.05, 0.61) | 0.10 | 95 |
| Weight-for-age Z score (SD) | 5 | 1691 | 1821 | 0.25 (−0.05, 0.56) | 0.11 | 95 |
| Post-Only Studies with Concurrent-Control | Number of Studies | Total Intervention Participants | Total Control Participants | Risk Ratio (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) | Risk Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Anemia | 11 | 5869 | 3518 | 0.55 (0.45, 0.67) | <0.001 | 84 | −0.13 (−0.20, −0.07) | <0.001 | 95 |
| Stunting | 7 | 4368 | 2405 | 0.60 (0.44, 0.81) | <0.001 | 71 | −0.05 (−0.08, −0.03) | <0.001 | 52 |
| Underweight | 6 | 2218 | 1659 | 0.51 (0.39, 0.65) | <0.001 | 22 | −0.04 (−0.06, −0.03) | <0.001 | 0 |
| Wasting | 5 | 1831 | 1419 | 0.48 (0.32, 0.70) | <0.001 | 21 | −0.02 (−0.04, −0.01) | <0.001 | 50 |
| Pre-Post Studies | Number of Studies | Total Posttest Participants | Total Pretest Participants | Risk Ratio (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) | Risk Difference (95% CI) | p-Value for Summary Effects | I2 for Heterogeneity (%) |
| Anemia | 13 | 5300 | 6019 | 0.58 (0.50, 0.68) | <0.001 | 89 | −0.20 (−0.26, −0.13) | <0.001 | 93 |
| Stunting | 10 | 3873 | 4345 | 0.75 (0.60, 0.95) | 0.02 | 67 | −0.03 (−0.05, −0.01) | 0.01 | 68 |
| Underweight | 10 | 3873 | 4345 | 0.59 (0.42, 0.83) | 0.002 | 63 | −0.03 (−0.05, −0.01) | 0.003 | 72 |
| Wasting | 7 | 2793 | 3202 | 0.72 (0.45, 1.15) | 0.17 | 60 | −0.01 (−0.03, 0.00) | 0.14 | 64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, X.; Sudfeld, C.R.; Liu, Y.; Tang, K.; Huang, Y.; Fawzi, W. The Effect of the Yingyangbao Complementary Food Supplement on the Nutritional Status of Infants and Children: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2404. https://doi.org/10.3390/nu11102404
Li Z, Li X, Sudfeld CR, Liu Y, Tang K, Huang Y, Fawzi W. The Effect of the Yingyangbao Complementary Food Supplement on the Nutritional Status of Infants and Children: A Systematic Review and Meta-Analysis. Nutrients. 2019; 11(10):2404. https://doi.org/10.3390/nu11102404
Chicago/Turabian StyleLi, Zhihui, Xinyi Li, Christopher R. Sudfeld, Yuning Liu, Kun Tang, Yangmu Huang, and Wafaie Fawzi. 2019. "The Effect of the Yingyangbao Complementary Food Supplement on the Nutritional Status of Infants and Children: A Systematic Review and Meta-Analysis" Nutrients 11, no. 10: 2404. https://doi.org/10.3390/nu11102404
APA StyleLi, Z., Li, X., Sudfeld, C. R., Liu, Y., Tang, K., Huang, Y., & Fawzi, W. (2019). The Effect of the Yingyangbao Complementary Food Supplement on the Nutritional Status of Infants and Children: A Systematic Review and Meta-Analysis. Nutrients, 11(10), 2404. https://doi.org/10.3390/nu11102404







