Diet Quality in a Weight Gain Prevention Trial of Reproductive Aged Women: A Secondary Analysis of a Cluster Randomized Controlled Trial
Abstract
1. Introduction
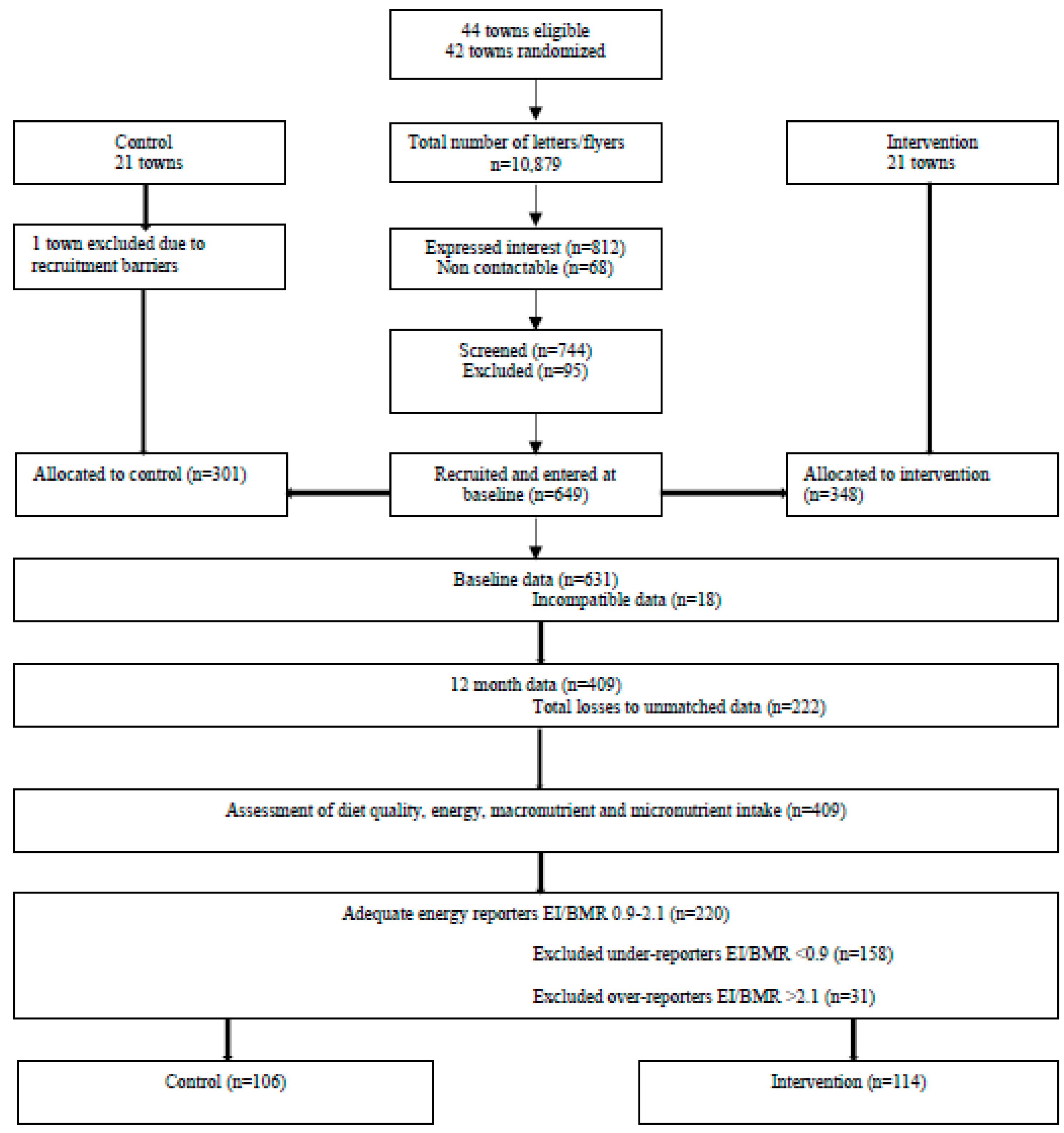
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Intervention
2.4. Control
2.5. Baseline Measures
2.6. Anthropometrics
2.7. Dietary Intake
2.8. Dietary Quality
2.9. Randomization
2.10. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HeLP-her | The Healthy Lifestyle Program |
| WHO | World Health Organization |
| BMI | Body Mass Index |
| CVD | Cardiovascular disease |
| RCT | Randomized controlled trial |
| FFQ | Food frequency questionnaire |
| DGI | Dietary Guideline Index |
| ADG | Australian dietary guideline |
| AGHE | Australian Guide to Healthy Eating |
| BMR | Basal metabolic rate |
| EI | Energy intake |
| AUD | Australian dollar |
| EI/BMR | Energy intake/ Basal metabolic rate |
| SD | Standard deviation |
| N | Number |
| CHO | Carbohydrate |
| %CHO | Carbohydrate density |
| %fat | Fat density |
| SFA | Saturated fat |
| %SFA | Saturated fat density |
| MUFA | Monounsaturated fat |
| %MUFA | Monounsaturated fat density |
| PUFA | Polyunsaturated fat |
| %PUFA | Polyunsaturated fat density |
| GI | Glycemic Index |
| GL | Glycemic load |
References
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- World Health Organization: Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 June 2016).
- Brown, W. Australian Women and Their Weight: A Growing Problem. In a Meeting of the Commonwealth Department of Health and Ageing. Women’s Health Australia. Available online: https://www.alswh.org.au/images/content/pdf/achievement_reports/achievements-weight.pdf (accessed on 6 February 2016).
- Dennis, B.H.; Pajak, A.; Pardo, B.; Davis, C.E.; Williams, O.D.; Piotrowski, W. Weight gain and its correlates in Poland between 1983 and 1993. Int. J. Obes. 2000, 24, 1507. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare: Who Is Overweight? Available online: http://www.aihw.gov.au/who-is-overweight/ (accessed on 20 November 2016).
- Eberhardt, M.S.; Pamuk, E.R. The importance of place of residence: Examining health in rural and non rural areas. Am. J. Public Health 2004, 94, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Humphreys, J.S.; Wilson, M.G. Addressing the health disadvantage of rural populations: How does epidemiological evidence inform rural health policies and research? Aust. J. Rural Health 2008, 16, 56–66. [Google Scholar] [CrossRef]
- World Health Organization. Rural Poverty and Health Systems in the WHO European Region. Available online: http://www.euro.who.int/__data/assets/pdf_file/0019/130726/e94659.pdf?ua=1 (accessed on 15 November 2016).
- Palermo, C.; McCartan, J.; Kleve, S.; Sinha, K.; Shiell, A. A longitudinal study of the cost of food in Victoria influenced by geography and nutritional quality. Aust. N. Z. J. Public Health 2016, 40, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hillemeier, M.M.; Weisman, C.S.; Chuang, C.; Downs, D.S.; McCall-Hosenfeld, J.; Camacho, F. Transition to overweight or obesity among women of reproductive age. J. Womens Health 2011, 20, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.; Peterson, B.L.; Krause, K.M.; Swamy, G.K.; Lovelady, C.A. Predictors of postpartum weight change among overweight and obese women: Results from the Active Mothers Postpartum study. J. Womens Health 2012, 21, 215–222. [Google Scholar] [CrossRef]
- Agrawal, P.; Gupta, K.; Mishra, V.; Agrawal, S. Effects of sedentary lifestyle and dietary habits on body mass index change among adult women in India: Findings from a follow-up study. Ecol. Food Nutr. 2013, 52, 387–406. [Google Scholar] [CrossRef]
- Brown, W.J.; Kabir, E.; Clark, B.K.; Gomersall, S.R. Maintaining a Healthy BMI: Data From a 16-Year Study of Young Australian Women. Am. J. Prev. Med. 2016, 51, 165–178. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.K.; Patterson, A.J.; Smith, R.; Collins, C.E. Disparities exist between National food group recommendations and the dietary intakes of women. BMC Womens Health 2011, 11, 37. [Google Scholar] [CrossRef]
- Moran, L.J.; Sui, Z.; Cramp, C.S.; Dodd, J.M. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post-partum. Int. J. Obes. 2013, 37, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Hure, A.J.; Young, A.; Smith, R.; Collins, C.E. Diet and pregnancy status in Australian women. Public Health Nutr. 2009, 12, 853–861. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand; Commonwealth of Australia: Canberra, Australia, 2006.
- National Health and Medical Research Council. Australian Dietary Guidelines. Available online: www.nhmrc.gov.au/guidelines-publications/n55 (accessed on 16 July 2018).
- Manore, M.M.; Larson-Meyer, D.E.; Lindsay, A.R.; Hongu, N.; Houtkooper, L. Dynamic energy balance: An integrated framework for discussing diet and physical activity in obesity prevention—Is it more than eating less and exercising more? Nutrients 2017, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Aljadani, H.M.; Patterson, A.; Sibbritt, D.; Hutchesson, M.J.; Jensen, M.E.; Collins, C.E. Diet quality, measured by fruit and vegetable intake, predicts weight change in young women. J. Obes. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Pan, A.; Hou, T.; Chiuve, S.E.; Tobias, D.K.; Mozaffarian, D.; Willett, W.C.; Hu, F.B. Long-term change in diet quality is associated with body weight change in men and women. J. Nutr. 2015, 145, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Olsen, S.; Bao, W.; Bjerregaard, A.A.; Fung, T.T.; Manson, J.E.; Hu, F.B. Healthful dietary patterns and long-term weight change among women with a history of gestational diabetes mellitus. Int. J. Obes. 2016, 40, 1748–1753. [Google Scholar] [CrossRef]
- Zamora, D.; Gordon-Larsen, P.; He, K.; Jacobs, D.R.; Shikany, J.M.; Popkin, B.M. Are the 2005 Dietary Guidelines for Americans Associated With reduced risk of type 2 diabetes and cardiometabolic risk factors? Twenty-year findings from the CARDIA study. Diabetes Care 2011, 34, 1183–1185. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Tinker, L.; Manson, J.E.; Allison, M.; Rohan, T.; Zaslavsky, O.; Waring, M.E.; Asao, K.; Garcia, L.; Rosal; et al. Change in Dietary Patterns and Change in Waist Circumference and DXA Trunk Fat Among Postmenopausal Women. Obesity 2016, 24, 2176–2184. [Google Scholar] [CrossRef]
- Livingstone, K.M.; McNaughton, S.A. Diet quality is associated with obesity and hypertension in Australian adults: A cross sectional study. BMC Public Health 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Boggs, D.A.; Rosenberg, L.; Rodríguez-Bernal, C.L.; Palmer, J.R. Long-term diet quality is associated with lower obesity risk in young African American women with normal BMI at baseline. J. Nutr. 2013, 143, 1636–1641. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Dunstan, D.; Ball, K.; Shaw, J.; Crawford, D. Diet quality is associated with diabetes and cardio-metabolic risk factors. J. Nutr. 2009, 139, 734–742. [Google Scholar] [CrossRef]
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Aljadani, H.; Patterson, A.; Sibbritt, D.; Collins, C. The association between dietary patterns and weight change in adults over time: A systematic review of studies with follow up. JBI Database Syst. Rev. Implement Rep. 2013, 11, 272–316. [Google Scholar] [CrossRef]
- Harrison, C.L.; Teede, H.J.; Kozica, S.L.; Zoungas, S.; Lombard, C.B. Individual, social and environmental factors and their association with weight in rural dwelling women of reproductive age. Aust. N. Z. J. Public Health 2017, 41, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.; Harrison, C.; Kozica, S.; Zoungas, S.; Ranasinha, S.; Teede, H. Preventing Weight Gain in Women in Rural Communities: A Cluster Randomised Controlled Trial. PLoS Med. 2016, 13, e1001941. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.B.; Harrison, C.L.; Kozica, S.L.; Zoungas, S.; Keating, C.; Teede, H.J. Effectiveness and implementation of an obesity prevention intervention: The HeLP-her Rural cluster randomised controlled trial. BMC Public Health 2014, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.B.; Deeks, A.; Jolley, D.; Teede, H.J. Preventing weight gain: The baseline weight related behaviors and delivery of a randomized controlled intervention in community based women. BMC Public Health 2009, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Rural, Regional and Remote Health: A Guide to Remoteness Classifications; Rural Health Series No. 4. cat. No. PHE 53; Australian Institute of Health and Welfare: Canberra, Australia, 2004; pp. 2–4.
- Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA) 2011. Available online: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa2011?opendocument&navpos=260 (accessed on 5 December 2016).
- Australian Government Department of Health. Australia’s Physical Activity & Sedentary Behaviour Guidelines (18–64 Years). Available online: https://www.health.gov.au/internet/main/publishing.nsf/content/F01F92328EDADA5BCA257BF0001E720D/$File/brochure%20PA%20Guidelines_A5_18-64yrs.PDF (accessed on 16 July 2018).
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic Report of a WHO Consultation (WHO Technical Report Series 894); World Health Organization: Geneva, Switzerland, 2000; p. 9. ISBN 92-4-120894-5. [Google Scholar]
- Cancer Council Victoria. Dietary Questionnaire for Epidemiological Studies Version 2. Available online: http://www.cancervic.org.au/research/epidemiology/nutritional_assessment_services (accessed on 2 August 2016).
- Lewis, J.; Milligan, G.C.; Hunt, A. Nuttab 95: Nutrient Data Table for Use in Australia; Food Standards Australia New Zealand: Canberra, Australia, 1995. [Google Scholar]
- Foster-Powell, K.; Holt, S.; Brand-Miller, J. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Ball, K.; Crawford, D.; Mishra, G.D. An index of diet and eating patterns is a valid measure of diet quality in an Australian population. J. Nutr. 2008, 138, 86–93. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Guide to Healthy Eating. Available online: https://www.eatforhealth.gov.au/guidelines/australian-guide-healthy-eating (accessed on 22 October 2016).
- Jessri, M.; Lou, W.Y.; L’Abbé, M.R. Evaluation of different methods to handle misreporting in obesity research: Evidence from the Canadian national nutrition survey. Br. J. Nutr. 2016, 115, 147–159. [Google Scholar] [CrossRef]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1984, 39, 5–41. [Google Scholar]
- Australian Bureau of Statistics. 4363.0.55.001—Australian Health Survey: Users’ Guide, 2011-13 Under-Reporting in Nutrition Surveys. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4363.0.55.001Chapter651512011-13 (accessed on 6 April 2017).
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 12; StataCorp: College Station, TX, USA, 2011. [Google Scholar]
- Thomson, J.L.; Tussing-Humphreys, L.M.; Onufrak, S.J.; Zoellner, J.M.; Connell, C.L.; Bogle, M.L.; Yadrick, K. A simulation study of the potential effects of healthy food and beverage substitutions on diet quality and total energy intake in Lower Mississippi Delta adults. J. Nutr. 2011, 141, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Kozica, S.L.; Lombard, C.B.; Ilic, D.; Ng, S.; Harrison, C.L.; Teede, H.J. Acceptability of delivery modes for lifestyle advice in a large scale randomised controlled obesity prevention trial. BMC Public Health 2015, 15, 699. [Google Scholar] [CrossRef] [PubMed]
- Kozica, S.L.; Lombard, C.B.; Teede, H.J.; Ilic, D.; Murphy, K.; Harrison, C.L. Initiating and continuing behaviour change within a weight gain prevention trial a qualitative investigation. PLoS ONE 2015, 10, e0119773. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; McGeechan, K.; Bauman, A.; Phongsavan, P.; Allman-Farinelli, M. Improved eating behaviours mediate weight gain prevention of young adults: Moderation and mediation results of a randomised controlled trial of TXT2BFiT, mHealth program. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.L.; Goodman, M.H.; Tussing-Humphreys, L. Diet quality and physical activity outcome improvements resulting from a church-based diet and supervised physical activity intervention for rural, Southern, African American adults: Delta Body and Soul III. Health Promot. Pract. 2015, 16, 677–688. [Google Scholar] [CrossRef]
- Tussing-Humphreys, L. A church-based diet and physical activity intervention for rural, lower Mississippi Delta African American adults: Delta Body and Soul effectiveness study, 2010–2011. Prev. Chronic Dis. 2013, 10, e92. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Hutchesson, M.J.; Jensen, M.; Morgan, P.; Callister, R.; Collins, C.E. Participants in an online weight loss program can improve diet quality during weight loss: A randomized controlled trial. Nutr. J. 2014, 13, 82. [Google Scholar] [CrossRef]
- Dietary Assessment Primer. National Institutes of Health, National Cancer Institute. Food Frequency Questionnaire at a Glance. Available online: http://dietassessmentprimer.cancer.gov/profiles/questionnaire/ (accessed on 27 January 2016).
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti-Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef]
- Ireland, P.; Jolley, D.; Giles, G.; O’Dea, K.; Powles, J.; Rutishauser, I.; Wahlqvist, M.L.; Williams, J. Development of the Melbourne FFQ: A food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac. J. Clin. Nutr. 1994, 3, 19–31. [Google Scholar] [PubMed]
- Bodnar, L.M.; Siega-Riz, A.M. A Diet Quality Index for Pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr. 2002, 5, 801–809. [Google Scholar] [CrossRef] [PubMed]
| 2013 Australian Dietary Guidelines | DGI Component and Description | Maximum Score (10) | Intermediate Score (5) | No (0) |
|---|---|---|---|---|
| Enjoy a wide variety of nutritious foods | Dietary variety: proportions of foods for each core food group that were consumed at least once per week | 100% | 50% | 0% |
| Eat plenty of vegetables, legumes and fruits | Vegetables: servings of vegetables and legumes per day | ≥5 | 2.5 | 0 |
| Fruit: servings of fruit per day | ≥2 | 1 | 0 | |
| Eat plenty of cereals (including breads, rice, pasta, and noodles), preferably whole-grain | Cereals: frequency of consumption of breads and cereals per day | ≥6 | 3 | 0 |
| Wholegrain cereals: proportion of whole-meal/wholegrain bread consumed relative to total bread | 100% | 50% | 0% | |
| Include lean meat, fish, poultry or alternatives | Meat and meat alternatives: frequency of consumption of lean meats and alternatives per day | ≥2.5 | 1.25 | 0 |
| Lean protein sources: proportion of lean meats and alternative relative to total meats and alternatives | 100% | 50% | 0% | |
| Include milks, yoghurts, cheeses, and/or alternatives Reduced fat varieties should be chosen, where possible | Dairy foods: frequency of consumption of dairy products per day | ≥2.5 | 1.25 | 0 |
| Low fat/reduced fat dairy: type of milk usually consumed | Low-fat milk | N/A | Whole milk | |
| Limit saturated fat intake and moderate total fat intake | Saturated fat intake: type of milk usually consumed | Low-fat milk | Whole milk | |
| Limit your alcohol intake if you choose to drink | Alcohol: frequency of consumption of all alcoholic beverages per day | ≤1 | 1.5 | ≥2 |
| Consume only moderate amounts of sugars and foods containing added sugars | Added sugars: frequency of consumption of soft drink, cordial, fruit juice, jam, chocolate, confectionary per day | F < 1.25 | 1.25 | F > 1.25 |
| Prevent weight gain: by being physically active and eating according to your energy needs | Extra foods: frequency of consumption of extra foods per day | F < 2.5 | 2.5 | F > 2.5 |
| Total DGI score | 0–130 |
| Characteristic | Control n = 106 | Intervention n = 114 | p-Value |
|---|---|---|---|
| Age (years) | 39.9 ± 6.2 | 40.9 ± 5.3 | 0.22 |
| Body mass index (BMI) (kg/m2) | 26.7 ± 5.1 | 27.4 ± 6.1 | 0.4 |
| Employment | 0.65 | ||
| Full time | 14 (13.2) | 20(17.7) | |
| Part time | 70 (66.0) | 68 (60.2) | |
| No paid work | 22 (20.8) | 25 (22.1) | |
| Marital status | 0.05 | ||
| Not married | 15 (14.2) | 7 (6.1) | |
| Married | 91 (85.9) | 107 (93.9) | |
| Education | 0.19 | ||
| No post school qualification | 18 (17.1) | 12 (10.5) | |
| Certificate/Diploma/Apprentice | 49 (46.7) | 49 (43.0) | |
| Bachelor degree and above | 38 (36.2) | 53 (46.5) | |
| Income | 0.37 | ||
| Australian dollar AUD 40,000 or less | 18 (17.5) | 17 (15.7) | |
| Australian dollar AUD 41–64,000 | 23 (22.3) | 19 (17.6) | |
| Australian dollar AUD 65–80,000 | 26 (25.2) | 20 (18.5) | |
| More than Australian dollar AUD 81,000 | 36 (35.0) | 52 (48.2) | |
| Smoking | 0.3 | ||
| No | 97 (91.5) | 105 (93.8) | |
| Yes | 2 (1.9) | 4 (3.6) | |
| Occasionally | 7 (6.6) | 3 (2.7) |
| Diet Quality | Control n = 106 | Percentage Change % | Intervention n = 114 | Percentage Change % | Unadjusted Difference β (95% Confidence Interval CI) p-Value | Adjusted Difference 1 β (95% Confidence Interval CI) p-Value |
|---|---|---|---|---|---|---|
| Total diet quality | ||||||
| Baseline | 84.2 (17.6) | 83.0 (16.6) | ||||
| Follow-up | 84.2 (17.2) | 88.5 (17.5) | ||||
| Mean change (95% CI) p-Value | −0.07 (−2.3, 2.2) 0.95 | 0 | 5.5 (3.3, 7.8) <0.001 | 6.2 | 5.6 (2.2, 9.0) 0.002 | 5.8 (2.5, 9.1) 0.001 |
| Dietary variety total | ||||||
| Baseline | 0.66 (0.09) | 0.66 (0.09) | ||||
| Follow-up | 0.67 (0.09) | 0.65 (0.09) | ||||
| Mean change (95% CI) p-Value | 0.005 (−0.006, 0.02) 0.37 | 1.5 | −0.01(−0.02, 0.0005) 0.04 | −1.5 | −0.02 (−0.04, 0.004) 0.10 | −0.02 (−0.04, −0.001) 0.04 |
| Vegetable total | ||||||
| Baseline | 2.4 (1.1) | 2.5 (1.1) | ||||
| Follow-up | 2.4 (1.1) | 2.4 (0.85) | ||||
| Mean change (95% CI) p-Value | 0.02 (−0.12, 0.15) 0.80 | 0 | −0.07 (−0.24, 0.10) 0.40 | −4.2 | −0.09 (−0.28, 0.10) 0.34 | −0.08(−0.26, 0.11) 0.41 |
| Fruit total | ||||||
| Baseline | 1.6 (0.90) | 1.7 (1.1) | ||||
| Follow-up | 1.6 (0.92) | 1.7 (1.0) | ||||
| Mean change (95% CI) p-Value | −0.004 (−0.14, 0.13) 0.95 | 0 | −0.009 (−0.17, 0.15) 0.91 | 0 | −0.005 (−0.20, 0.20) 0.96 | 0.04(−0.17, 0.25) 0.71 |
| Proportion grains | ||||||
| Baseline | 0.67 (0.58, 0.76) | 0.74 (0.44) | ||||
| Follow-up | 0.69 (0.46) | 0.83 (0.38) | ||||
| Mean change (95% CI) p-Value | 0.02 (−0.04, 0.09) 0.49 | 2.9 | 0.09 (0.02, 0.16) 0.02 | 10.8 | −0.0009 (−0.03, 0.03) 0.94 | −0.002 (−0.03, 0.03) 0.89 |
| Breads and cereals total | ||||||
| Baseline | 4.1 (1.6) | 4.4 (1.4) | ||||
| Follow-up | 3.9 (1.5) | 4.2 (1.5) | ||||
| Mean change (95% CI) p-Value | −0.22 (−0.47, 0.03) 0.08 | −5.1 | −0.28 (−0.59, 0.03) 0.08 | −4.8 | −0.06 (−0.40, 0.27) 0.70 | −0.06 (−0.40, 0.28) 0.72 |
| Lean meat total | ||||||
| Baseline | 2.3 (1.1) | 2.4 (0.95) | ||||
| Follow-up | 2.3 (1.3) | 2.5 (1.0) | ||||
| Mean change (95% CI) p-Value | −0.006 (−0.20, 0.19) 0.95 | 0 | 0.11 (−0.07, 0.29) 0.22 | 4 | 0.12 (−0.14, 0.38) 0.37 | 0.15 (−0.12, 0.42) 0.26 |
| Lean meat proportion | ||||||
| Baseline | 0.82 (0.11) | 0.82 (0.09) | ||||
| Follow-up | 0.84 (0.09) | 0.84 (0.08) | ||||
| Mean change (95% CI) p-Value | 0.02 (0.0002, 0.04) 0.05 | 2.4 | 0.02 (0.005, 0.04) 0.01 | 2.4 | 0.0009 (−0.03, 0.03) 0.94 | 0.002(−0.03, 0.03) 0.89 |
| Dairy total | ||||||
| Baseline | 1.9 (0.81) | 1.8 (0.67) | ||||
| Follow-up | 1.8 (0.68) | 1.8 (0.71) | ||||
| Change mean (95% CI) p-Value | −0.13 (−0.26, 0.005) 0.06 | −5.6 | 0.05 (−0.07, 0.18) 0.42 | 0 | 0.18 (0.02, 0.35) 0.03 | 0.14 (−0.06, 0.34) 0.16 |
| Extra foods total 2 | ||||||
| Baseline | 4.7 (2.0) | 5.0 (2.3) | ||||
| Follow-up | 4.3 (1.9) | 4.1 (2.0) | ||||
| Mean change (95% CI) p-Value | −0.40 (−0.74, −0.06) 0.02 | −9.3 | −0.84 (−1.2, −0.48) <0.001 | −22.0 | −0.44 (−0.97, 0.10) 0.11 | −0.36(−0.96, 0.24) 0.24 |
| Energy and Nutrients | Control n = 106 | Intervention n = 114 | Unadjusted Difference β (95% Confidence Interval CI) p-Value | Adjusted Difference 1 β (95% Confidence Interval CI) p-Value |
|---|---|---|---|---|
| Energy (kJ) | ||||
| Baseline | 8051.7 (1827.6) | 8286.6 (1990.5) | ||
| Follow-up | 7606.5 (1717.2) | 7760.3 (1621.8) | ||
| Mean change (95% CI) p-value | −445.3(−746.6, −143.9) 0.004 | −526.3(−848.9, −203.8) 0.002 | −81.1 (−443.2, 281.1) 0.65 | −42.9 (−460.0, 374.2) 0.84 |
| Protein (g) | ||||
| Baseline | 94.3 (26.1) | 93.0 (23.6) | ||
| Follow-up | 89.5 (26.2) | 91.8 (22.0) | ||
| Mean change (95% CI) p-value | −4.8 (−8.6, −0.94) 0.02 | −1.2 (−5.2, 2.7) 0.54 | 3.5 (−1.4, 8.5) 0.16 | 3.5 (−1.7, 8.8) 0.18 |
| % protein | ||||
| Baseline | 0.20 (0.03) | 0.19 (0.02) | ||
| Follow-up | 0.20 (0.03) | 0.20 (0.03) | ||
| Mean change (95% CI) p-value | 0.001 (−0.003, 0.005) 0.66 | 0.01 (0.006, 0.01) <0.001 | 0.009 (0.003, 0.01) 0.003 | 0.009 (0.002, 0.15) 0.01 |
| CHO (g) | ||||
| Baseline | 191.3 (51.2) | 198.4 (51.2) | ||
| Follow-up | 180.6 (45.2) | 182.5 (42.7) | ||
| Mean change (95% CI) p-value | −10.7 (−19.0, −2.4) 0.01 | −15.9 (−25.4, −6.4) 0.001 | −5.2 (−16.6, 6.1) 0.36 | −3.3 (−15.3, 8.7) 0.58 |
| % CHO | ||||
| Baseline | 0.40 (0.06) | 0.41 (0.06) | ||
| Follow-up | 0.40 (0.05) | 0.40 (0.06) | ||
| Mean change (95% CI) p-value | 0.0009 (−0.008, 0.01) 0.85 | −0.006 (−0.02, 0.003) 0.20 | −0.007(−0.02, 0.007) 0.31 | −0.005(−0.02, 0.009) 0.46 |
| Fat (g) | ||||
| Baseline | 80.6 (21.8) | 82.6 (24.7) | ||
| Follow-up | 76.6 (21.2) | 77.1 (21.5) | ||
| Mean change (95% CI) p-value | −3.9 (−7.7, −0.18) 0.04 | −5.5 (−9.3, −1.8) 0.004 | −1.6 (−6.3, 3.1) 0.49 | −1.6 (−7.5, 4.3) 0.59 |
| % Fat | ||||
| Baseline | 0.37 (0.04) | 0.37 (0.05) | ||
| Follow-up | 0.37 (0.04) | 0.36 (0.05) | ||
| Mean change (95% CI) p-value | 0.002 (−0.005, 0.009) 0.59 | −0.002 (−0.01, 0.006) 0.64 | −0.004 (−0.01, 0.006) 0.45 | −0.005 (−0.02, 0.008) 0.42 |
| SFA (g) | ||||
| Baseline | 34.4 (11.0) | 34.5 (11.9) | ||
| Follow-up | 32.3 (10.3) | 31.6 (10.2) | ||
| Mean change (95% CI) p-value | −2.1 (−3.8, −0.34) 0.02 | −2.8 (−4.5, −1.1) 0.001 | −0.78 (−3.0, 1.4) 0.48 | −0.83 (−3.4, 1.8) 0.52 |
| % SFA | ||||
| Baseline | 0.16 (0.03) | 0.15 (0.03) | ||
| Follow-up | 0.16 (0.03) | 0.15 (0.03) | ||
| Mean change (95% CI) p-value | −0.0004 (−0.004, 0.004) 0.83 | −0.003 (−0.008, 0.002) 0.24 | −0.002 (−0.008, 0.003) 0.40 | −0.003(−0.01, 0.004) 0.41 |
| MUFA (g) | ||||
| Baseline | 28.6 (7.7) | 29.6 (9.2) | ||
| Follow-up | 27.2 (7.7) | 28.0 (8.2) | ||
| Mean change (95% CI) p-value | −1.4 (−2.8, −0.03) 0.04 | −1.7 (−3.1, −0.26) 0.02 | −0.28 (−2.1, 1.6) 0.76 | −0.17(−2.5, 2.1) 0.88 |
| % MUFA | ||||
| Baseline | 0.13 (0.02) | 0.13 (0.02) | ||
| Follow-up | 0.13 (0.02) | 0.13 (0.02) | ||
| Mean change (95% CI) p-value | 0.0005 (−0.003, 0.004) 0.76 | 0.0007 (−0.003, 0.004) 0.66 | 0.0003 (−0.005, 0.005) 0.91 | 0.00002(−0.006, 0.006) 0.10 |
| PUFA (g) | ||||
| Baseline | 10.6 (3.9) | 11.4 (4.0) | ||
| Follow-up | 10.4 (3.8) | 10.8 (3.9) | ||
| Mean change (95% CI) p-value | −0.16 (−0.80, 0.48) 0.62 | −0.60 (−1.3, 0.13) 0.11 | −0.44 (−1.4, 0.56) 0.38 | −0.46 (−1.6, 0.67) 0.41 |
| % PUFA | ||||
| Baseline | 0.05 (0.02) | 0.05 (0.01) | ||
| Follow-up | 0.05 (0.01) | 0.05 (0.01) | ||
| Mean change (95% CI) p-value | 0.002 (−0.0006, 0.004) 0.16 | 0.0001 (−0.002, 0.003) 0.92 | −0.001 (−0.006, 0.003) 0.47 | −0.002 (−0.006, 0.002) 0.31 |
| Fiber (g) | ||||
| Baseline | 21.5 (5.5) | 22.9 (6.7) | ||
| Follow-up | 21.1 (5.6) | 22.6 (6.0) | ||
| Mean change (95% CI) p-value | −0.35 (−1.2, 0.51) 0.42 | −0.29 (−1.5, 0.90) 0.63 | 0.07 (−1.2, 1.3) 0.91 | 0.32 (−1.1, 1.7) 0.65 |
| Cholesterol (mg) | ||||
| Baseline | 314.8 (98.8) | 315.0 (111.2) | ||
| Follow-up | 301.0 (102.7) | 310.0 (113.1) | ||
| Mean change (95% CI) p-value | −13.8 (−30.7, 3.1) 0.11 | −5.0 (−23.0, 13.0) 0.58 | 8.8 (−14.3, 31.9) 0.45 | 8.4 (−16.7, 33.6) 0.50 |
| GI | ||||
| Baseline | 50.7 (3.6) | 51.2 (4.0) | ||
| Follow-up | 50.4 (3.5) | 49.5 (3.7) | ||
| Mean change (95% CI) p-value | −0.38 (−0.94, 0.19) 0.19 | −1.7 (−2.3, −1.1) <0.001 | −1.3 (−2.1, −0.56) 0.001 | −1.2 (−2.1, −0.24) 0.02 |
| GL | ||||
| Baseline | 96.8 (29.8) | 101.2 (29.6) | ||
| Follow-up | 90.6 (25.7) | 90.2 (25.2) | ||
| Mean change (95% CI) p-value | −6.2 (−11.0, −1.3) 0.01 | −10.9 (−16.3, −5.5) <0.001 | −4.8 (−11.3, 1.7) 0.15 | −3.4 (−10.4, 3.6) 0.33 |
| Calcium (mg) | ||||
| Baseline | 970.0 (294.6) | 929.3 (248.0) | ||
| Follow-up | 919.4 (259.2) | 936.4 (245.1) | ||
| Mean change (95% CI) p-value | −50.6 (−96.3, −4.8) 0.03 | 7.1 (−34.7, 48.9) 0.74 | 57.6 (8.0, 107.3) 0.02 | 48.3 (−11.6, 108.1) 0.11 |
| Iron (mg) | ||||
| Baseline | 13.5 (3.6) | 14.0 (4.4) | ||
| Follow-up | 12.8 (3.4) | 13.7 (3.8) | ||
| Mean change (95% CI) p-value | −0.69 (−1.3, −0.11) 0.02 | −0.35 (−1.2, 0.50) 0.42 | 0.33 (−0.56, 1.2) 0.46 | 0.49 (−0.46, 1.4) 0.31 |
| Folate (µg) | ||||
| Baseline | 265.9 (67.4) | 276.5 (91.1) | ||
| Follow-up | 258.2 (60.4) | 268.5 (69.9) | ||
| Mean change (95% CI) p-value | −7.7 (−18.3, 2.9) 0.15 | −8.0 (−25.0, 9.1) 0.36 | −0.28 (−16.3, 15.7) 0.97 | 4.8 (−12.1, 21.8) 0.57 |
| Sodium (mg) | ||||
| Baseline | 2557.1 (713.1) | 2605.9 (755.4) | ||
| Follow-up | 2379.6 (604.8) | 2421.7 (638.7) | ||
| Mean change (95% CI) p-value | −177.5 (−287.0, −68.0) 0.002 | −184.2 (-300.8, −67.7) 0.002 | −6.7 (−140.7, 127.2) 0.92 | −4.9 (−159.2, 149.3) 0.95 |
| Characteristics | Unadjusted β (95% Confidence Interval CI) p-Value | Adjusted 1 β (95%ConfidenceInterval CI) p-Value |
|---|---|---|
| Age (years) | 0.14 (−0.09, 0.37) 0.23 | 0.18 (−0.14, 0.50) 0.25 |
| BMI (kg/m2) | 0.12 (−0.24, 0.47) 0.51 | 0.12 (−0.28, 0.53) 0.55 |
| Smoking | ||
| No | Ref | Ref |
| Yes | −0.15(−4.8, 4.5) 0.95 | −0.72(−6.4, 4.9) 0.80 |
| Occasionally | 4.0 (−2.2, 10.2) 0.20 | 4.7 (−0.85, 10.2) 0.10 |
| Employment | ||
| Full time | Ref | Ref |
| Part time | 0.99 (−3.9, 5.9) 0.69 | 4.0 (−1.0, 9.0) 0.12 |
| No paid work | 0.94 (−4.1, 6.0) 0.71 | 3.7 (−3.0, 10.4) 0.27 |
| Marital status | ||
| Not married | Ref | Ref |
| Married | 2.1 (−2.2, 6.3) 0.33 | 1.8 (−2.5, 6.1) 0.40 |
| Education | ||
| No post school qualification | Ref | |
| Certificate/diploma/apprentice | 4.1 (−0.41, 8.5) 0.07 | 3.8 (−1.4, 9.0) 0.15 |
| Bachelor degree and above | 2.5 (−2.5, 7.5) 0.32 | 2.3 (−3.5, 8.0) 0.43 |
| Income | ||
| Australian dollar AUD 40,000 or less | Ref | Ref |
| Australian dollar AUD 1–64,000 | 0.61 (−4.5, 5.7) 0.81 | 0.62(−4.5, 5.7) 0.81 |
| Australian dollar AUD 65–80,000 | −0.32 (−5.7, 5.1) 0.91 | −0.72 (−7.1, 5.7) 0.82 |
| More than Australian dollar AUD 81,000 | 0.31 (−5.0, 5.6) 0.91 | 0.20 (−5.8, 6.2) 0.95 |
| Group | ||
| Control | Ref | Ref |
| Intervention | 5.6 (2.2, 9.0) 0.002 | 5.8 (2.5, 9.1) 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, J.C.; Moran, L.J.; Teede, H.J.; Ranasinha, S.; Lombard, C.B.; Harrison, C.L. Diet Quality in a Weight Gain Prevention Trial of Reproductive Aged Women: A Secondary Analysis of a Cluster Randomized Controlled Trial. Nutrients 2019, 11, 49. https://doi.org/10.3390/nu11010049
Martin JC, Moran LJ, Teede HJ, Ranasinha S, Lombard CB, Harrison CL. Diet Quality in a Weight Gain Prevention Trial of Reproductive Aged Women: A Secondary Analysis of a Cluster Randomized Controlled Trial. Nutrients. 2019; 11(1):49. https://doi.org/10.3390/nu11010049
Chicago/Turabian StyleMartin, Julie C., Lisa J. Moran, Helena J. Teede, Sanjeeva Ranasinha, Catherine B. Lombard, and Cheryce L. Harrison. 2019. "Diet Quality in a Weight Gain Prevention Trial of Reproductive Aged Women: A Secondary Analysis of a Cluster Randomized Controlled Trial" Nutrients 11, no. 1: 49. https://doi.org/10.3390/nu11010049
APA StyleMartin, J. C., Moran, L. J., Teede, H. J., Ranasinha, S., Lombard, C. B., & Harrison, C. L. (2019). Diet Quality in a Weight Gain Prevention Trial of Reproductive Aged Women: A Secondary Analysis of a Cluster Randomized Controlled Trial. Nutrients, 11(1), 49. https://doi.org/10.3390/nu11010049







