Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly, High-Comorbidity Population. A Stepwise Multiple-Choice System Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting; Patient Selection and Inclusion Criteria
2.2. Diets and Controls
2.3. Biochemical Data, Compliance and Nutritional and Comorbidity Indexes
3. Statistical Analysis
3.1. Logistic Regression
3.2. Ethical Issues
4. Results
4.1. Baseline Data
4.2. Choice of Diet
4.3. Special Populations: Obese Patients
4.4. Special Populations: Diabetic Patients
4.5. Nutritional Parameters and Compliance
4.6. Characteristics of Patients Who Started Dialysis
4.7. Logistic Regression: Compliance
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Moss, A.H.; Davison, S.N. How the ESRD quality incentive program could potentially improve quality of life for patients on dialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Joly, D.; Anglicheau, D.; Alberti, C.; Nguyen, A.T.; Touam, M.; Grünfeld, J.P.; Jungers, P. Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J. Am. Soc. Nephrol. 2003, 14, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Kurella, M.; Covinsky, K.E.; Collins, A.J.; Chertow, G.M. Octogenarians and nonagenarians starting dialysis in the United States. Ann. Intern. Med. 2007, 146, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Susantitaphong, P.; Isaranuwatchai, S.; Chenbhanich, J.; Eiam-Ong, S.; Jaber, B.L. Dialysis Therapy and Conservative Management of Advanced Chronic Kidney Disease in the Elderly: A Systematic Review. Nephron 2017, 137, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Verberne, W.R.; Geers, A.B.; Jellema, W.T.; Vincent, H.H.; van Delden, J.J.; Bos, W.J. Comparative Survival among Older Adults with Advanced Kidney Disease Managed Conservatively Versus with Dialysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kovesdy, C.P.; Streja, E.; Rhee, C.M.; Soohoo, M.; Chen, J.L.T.; Molnar, M.Z.; Obi, Y.; Gillen, D.; Nguyen, D.V.; et al. Transition of care from pre-dialysis prelude to renal replacement therapy: The blueprints of emerging research in advanced chronic kidney disease. Nephrol. Dial. Transplant. 2017, 32, ii91–ii98. [Google Scholar] [CrossRef]
- Rivara, M.B.; Mehrotra, R. Timing of Dialysis Initiation: What Has Changed Since IDEAL? Semin. Nephrol. 2017, 37, 181–193. [Google Scholar] [CrossRef]
- Abra, G.; Kurella Tamura, M. Timing of initiation of dialysis: Time for a new direction? Curr. Opin. Nephrol. Hypertens. 2012, 21, 329–333. [Google Scholar] [CrossRef]
- Robinson, B.M.; Zhang, J.; Morgenstern, H.; Bradbury, B.D.; Ng, L.J.; McCullough, K.P.; Gillespie, B.W.; Hakim, R.; Rayner, H.; Fort, J.; et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014, 85, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Scalone, L.; Borghetti, F.; Brunori, G.; Viola, B.F.; Brancati, B.; Sottini, L.; Mantovani, L.G.; Cancarini, G. Cost-benefit analysis of supplemented very low-protein diet versus dialysis in elderly CKD5 patients. Nephrol. Dial. Transplant. 2010, 25, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Artaza-Artabe, I.; Sáez-López, P.; Sánchez-Hernández, N.; Fernández-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016, 93, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwaluw, N.; Mensink, M.; van Loon, L.J.; de Groot, L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.L.; Hayashi, A.P.; Jambassi-Filho, J.C.; de Capitani, M.D.; de Santana, D.A.; Gualano, B.; Roschel, H. Different protein and derivatives supplementation strategies combined with resistance training in pre-frail and frail elderly: Rationale and protocol for the “Pro-Elderly” Study. Nutr. Health. 2017, 23, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: A meta-analysis. PLoS ONE 2014, 9, e109141. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; Sarafidis, P.A.; Greenwood, S.A.; Campbell, K.L.; Hall, W.L.; Macdougall, I.C. Compliance with a structured weight loss program is associated with reduced systolic blood pressure in obese patients with chronic kidney disease. Am. J. Hypertens. 2012, 25, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Melby, C.L.; Paris, H.L.; Foright, R.M.; Peth, J. Attenuating the Biologic Drive for Weight Regain Following Weight Loss: Must What Goes Down Always Go Back Up? Nutrients 2017, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Pelletier, X.; Ross, A.B.; Thielecke, F. A High Rate of Non-Compliance Confounds the Study of Whole Grains and Weight Maintenance in a Randomised Intervention Trial-The Case for Greater Use of Dietary Biomarkers in Nutrition Intervention Studies. Nutrients 2017, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; de Gara, C.; Birch, D.W. Weight recidivism post-bariatric surgery: A systematic review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016, 17, 77. [Google Scholar] [CrossRef]
- Giordano, M.; Ciarambino, T.; Castellino, P.; Paolisso, G. Light and shadows of dietary protein restriction in elderly with chronic kidney disease. Nutrition 2013, 29, 1090–1093. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Moore, L.W.; Tortorici, A.R.; Chou, J.A.; St-Jules, D.E.; Aoun, A.; Rojas-Bautista, V.; Tschida, A.K.; Rhee, C.M.; Shah, A.A.; et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016, 17, 90. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef]
- Levey, A.S.; Greene, T.; Beck, G.J.; Caggiula, A.W.; Kusek, J.W.; Hunsicker, L.G.; Klahr, S. Dietary protein restriction and the progression of chronic renal disease: What have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J. Am. Soc. Nephrol. 1999, 10, 2426–2439. [Google Scholar]
- Mitch, W.E. Dietary therapy in uremia: The impact on nutrition and progressive renal failure. Kidney Int. Suppl. 2000, 75, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Chan, M. Protein-controlled versus restricted protein versus low protein diets in managing patients with non-dialysis chronic kidney disease: A single centre experience in Australia. BMC Nephrol. 2016, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Scognamiglio, S.; Consiglio, V.; Mongilardi, E.; Bilocati, M.; Avagnina, P.; Versino, E. Diet as a system: An observational study investigating a multi-choice system of moderately restricted low-protein diets. BMC Nephrol. 2016, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Mongilardi, E.; Bilocati, M.; Avagnina, P.; Versino, E. Patient Survival and Costs on Moderately Restricted Low-Protein Diets in Advanced CKD: Equivalent Survival at Lower Costs? Nutrients 2016, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- Ikizler, T.A. Dietary protein restriction in CKD: The debate continues. Am. J. Kidney Dis. 2009, 53, 189–191. [Google Scholar] [CrossRef]
- Johnson, D.W. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: The case against. Nephrology (Carlton) 2006, 11, 58–62. [Google Scholar] [CrossRef]
- Food and Nutrition Board (FNB) of the Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fibre, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- World Health Organisation (WHO). Dietary Reference Intakes for Energy, Carbohydrate, Fibre, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients) (WHO Technical Report Series 935); World Health Organisation (WHO): Geneva, Switzerland, 2007. [Google Scholar]
- Kaja Kamal, R.M.; Farrington, K.; Busby, A.D.; Wellsted, D.; Chandna, H.; Mawer, L.J.; Sridharan, S.; Vilar, E. Initiating haemodialysis twice-weekly as part of an incremental programme may protect residual kidney function. Nephrol. Dial. Transplant. 2018. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Ventrella, F.; Capizzi, I.; Vigotti, F.N.; Mongilardi, E.; Grassi, G.; Loi, V.; Cabiddu, G.; Avagnina, P.; Versino, E. Low-Protein Diets in Diabetic Chronic Kidney Disease (CKD) Patients: Are They Feasible and Worth the Effort? Nutrients 2016, 8, 649. [Google Scholar] [CrossRef] [PubMed]
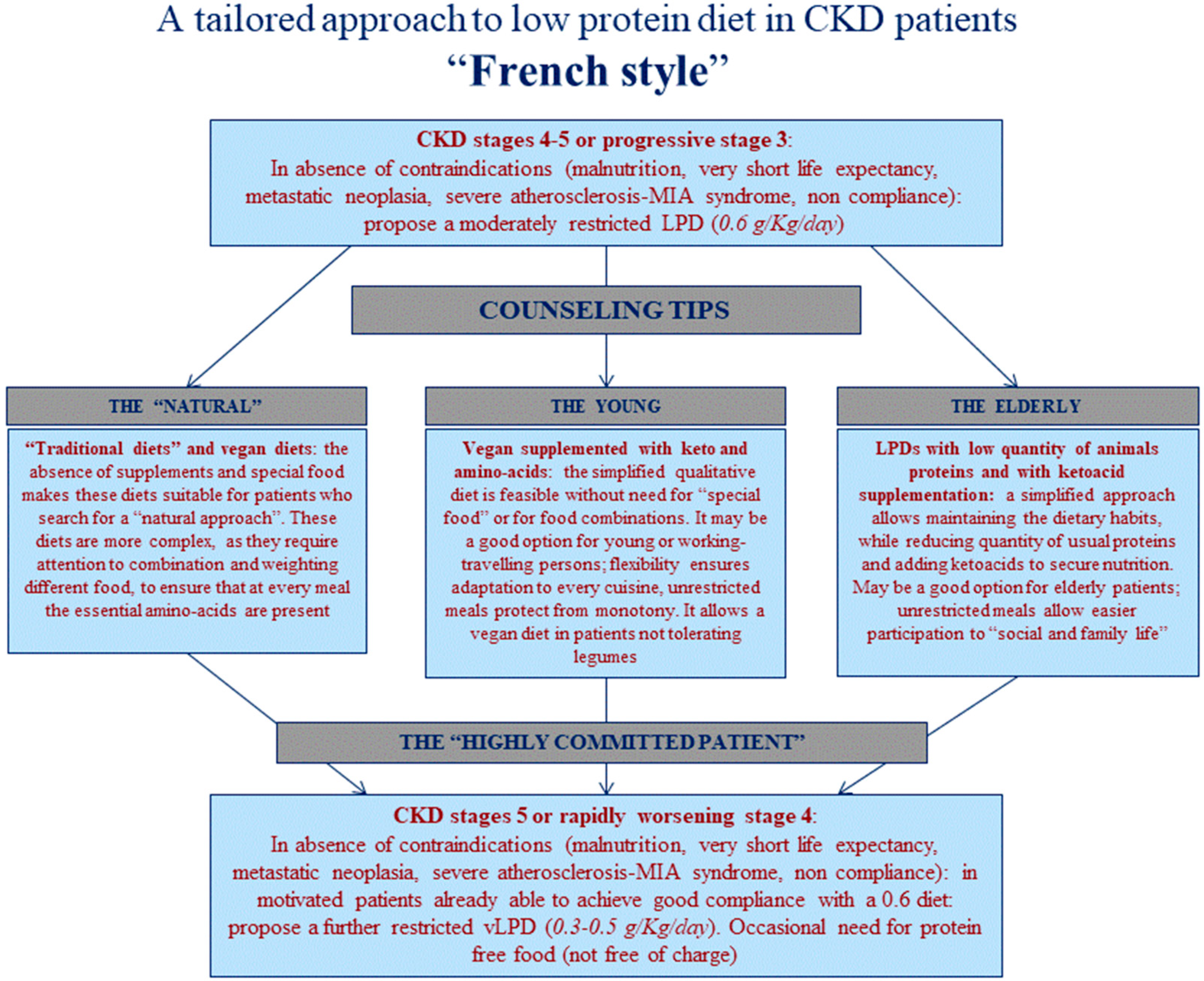
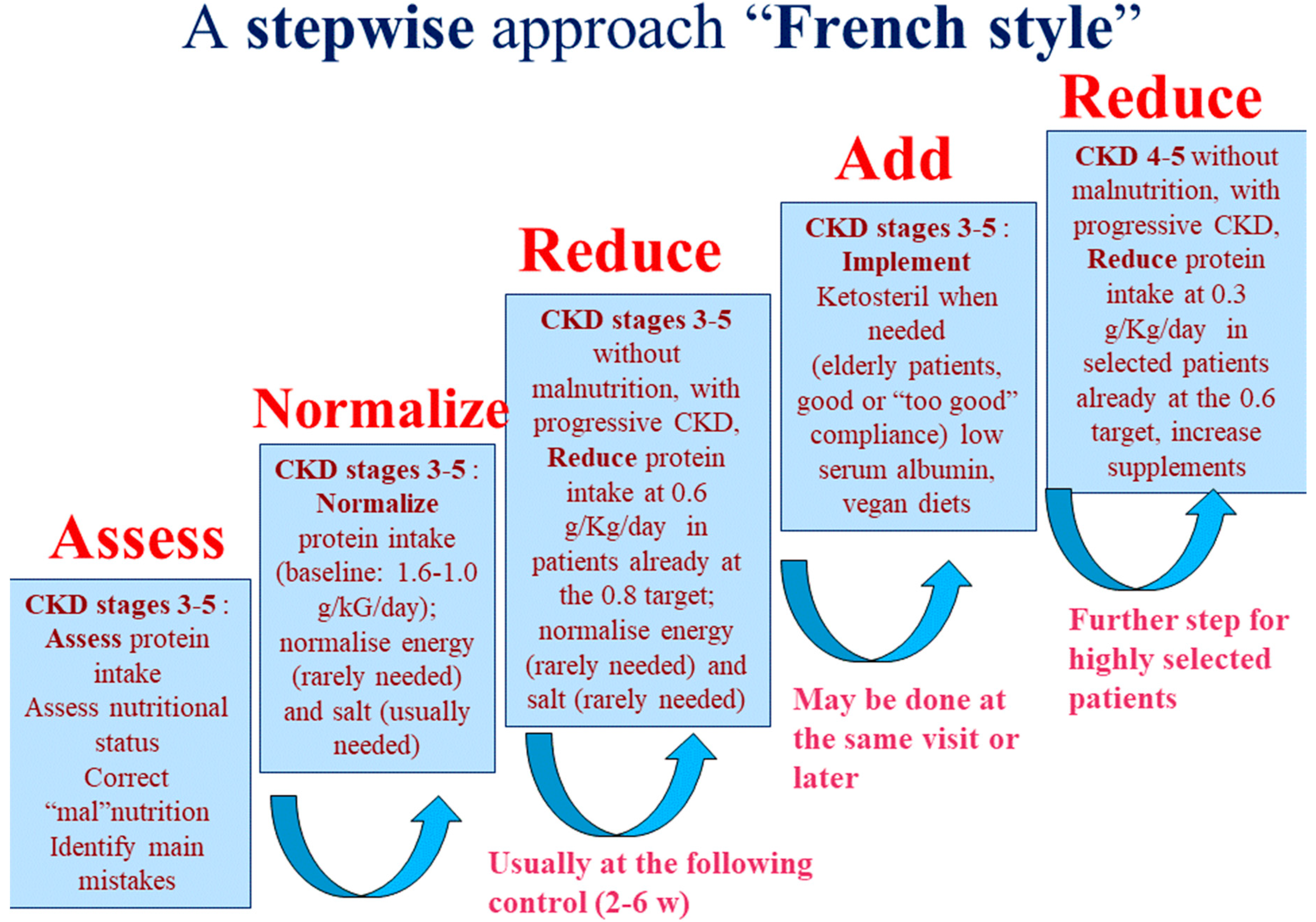
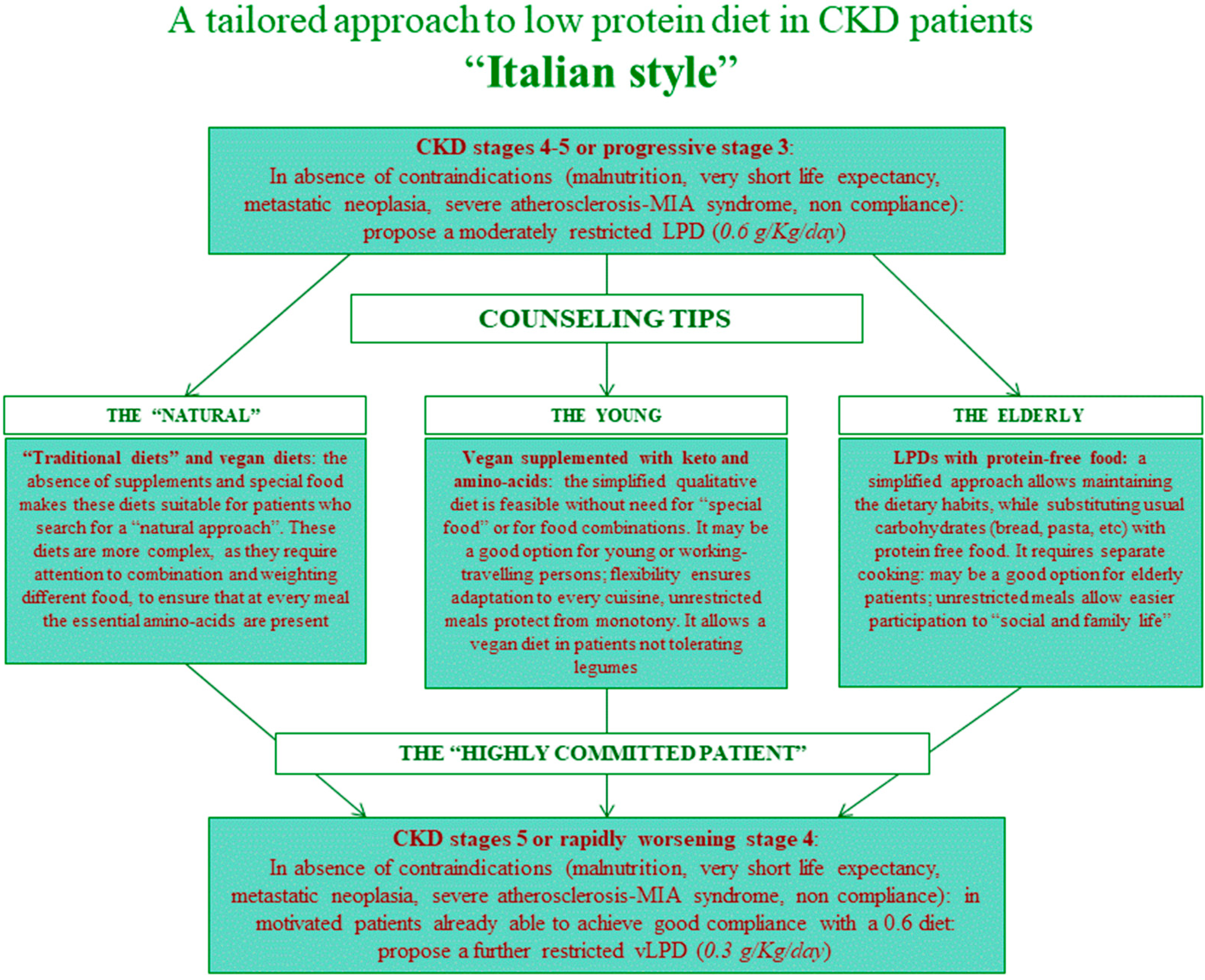
| First Diet | French Cohort (UIRAV) | Italian Reference Cohort | p |
|---|---|---|---|
| N | 131 | 457 | |
| Males, n (%) Females, n (%) | 82 (62.6%) 49 (37.4%) | 281 (68.5%) 176 (38.5%) | 0.818 * |
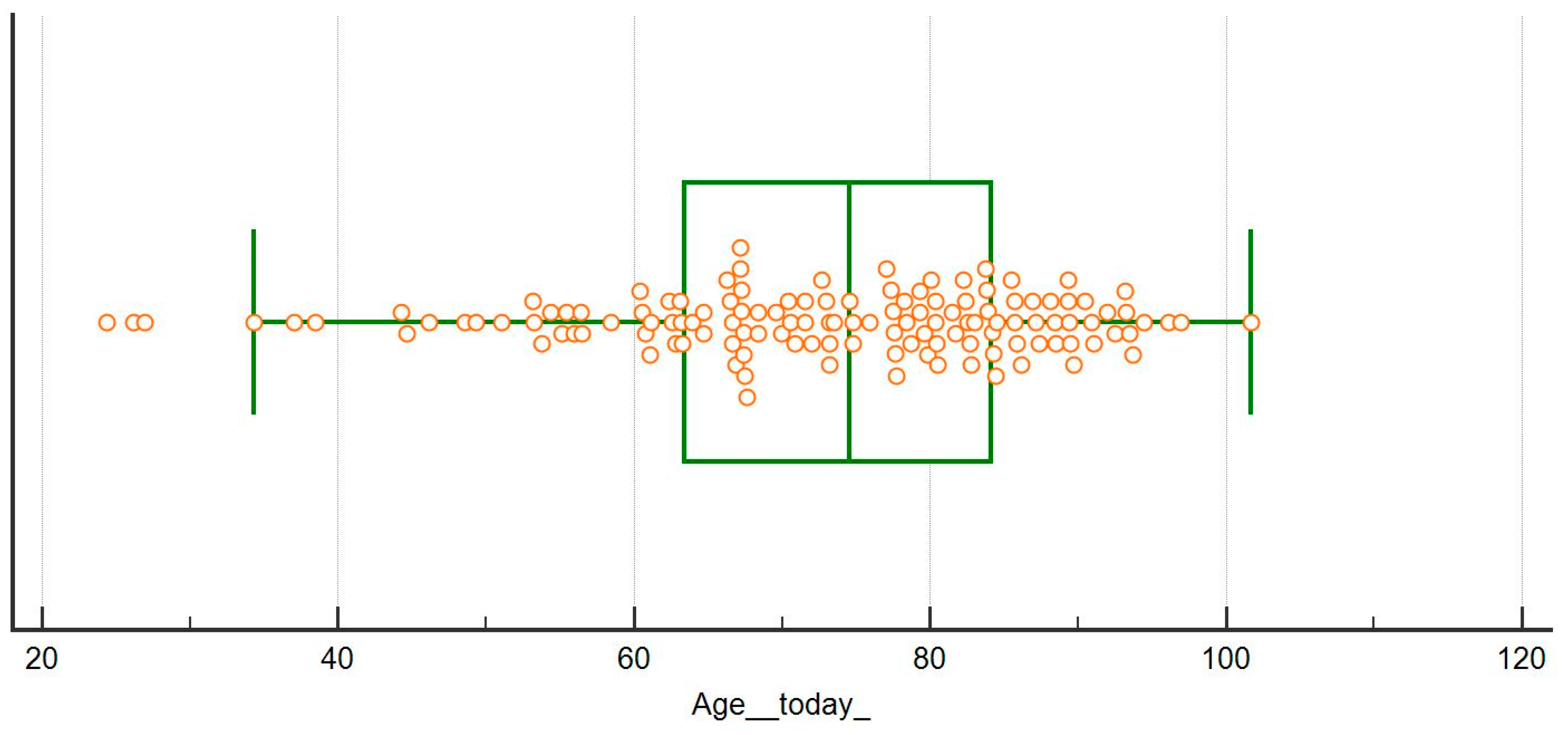
| Age (years), median (min-max) | 74 (24–101) | 70 (19–97) | 0.124 |
| Age over 65, n (%) | 95 (72.6%) | 274 (60%) | 0.010 * |
| Age over 80, n (%) | 49 (37.4%) | 73 (15.4%) | <0.001 * |
| CCI, median (min-max) | 8 (2–14) | 7 (2–13) | 0.018 |
| CCI ≥ 7, n (%) | 93 (71%) | 248 (54.8%) | 0.001 * |
| CCI ≥ 10, n (%) | 22 (16.8%) | 71 (15.5%) | 0.728 * |
| Diabetes, n (%) | 66 (50.4%) | 150 (32.8%) | <0.001 * |
| Cardiopathy, n (%) | 30 (22.9%) | 210 (46%) | <0.001 * |
| Neoplasia, n (%) | 17 (13%) | 99 (21.7%) | 0.028 * |
| BMI (kg/m2), median (min-max) | 28.3 (16.7–51.2) | 26.1 (13.3–51.4) | 0.115 |
| sCreatinine (mg/dL), median (min-max) | 2.6 (1.3–10.4) | 2.8 (0.5–74) | 0.225 |
| eGFR-EPI (mL/min), median (min-max) | 22 (4–68) | 20 (3–127) | 0.302 |
| GFR < 15 (mL/min) at enrollment, n (%) | 24 (18.3%) | 125 (27.4%) | 0.036 * |
| GFR < 10 (mL/min) at enrollment, n (%) | 11 (8.4%) | 44 (9.8%) | 0.670 * |
| Proteinuria (g/day), median (min-max) | 0.5 (0.1–8.3) | 0.80 (0.1–12) | 0.09 |
| Proteinuria ≥ 1 g/day, n (%) | 50 (38.2%) | 203 (44.4%) | 0.203 * |
| Proteinuria ≥ 3 g/day, n (%) | 22 (16.8%) | 79 (17.3%) | 0.895 * |
| Glomerulonephritis-systemic disease, n (%) | 3 (2.3%) | 95 (21.2%) | <0.001 * |
| First Diet | Normalization | Moderate Restriction Traditional | Moderate Restriction Plant-Based Supplemented | No Protein Restriction | p between Groups |
|---|---|---|---|---|---|
| N | 75 | 24 | 22 | 10 | |
| Males, n (%) Females, n (%) | 47 (62.7%) 28 (37.3%) | 18 (75%) 6 (25%) | 11 (50%) 11 (50%) | 6 (60%) 4 (40%) | 0.377 |
| Age (years), median (min-max) | 78 (24–101) | 74 (44–91) | 70 (34–89) | 67 (44–88) | 0.293 |
| Age over 65, n (%) | 54 (72%) | 19 (79.2%) | 15 (68.2%) | 7 (70%) | 0.853 * |
| Age over 80, n (%) | 36 (48%) | 6 (25%) | 4 (18.2%) | 3 (30%) | 0.031 * |
| CCI, median (min-max) | 8 (2–12) | 8 (4–14) | 6.5 (2–12) | 9 (5–12) | 0.046 |
| CCI ≥ 7, n (%) | 55 (73.3%) | 19 (79.2%) | 11 (50%) | 8 (80%) | 0.104 * |
| CCI ≥ 10, n (%) | 15 (20%) | 1 (4.2%) | 2 (9.2%) | 4 (40%) | 0.155 * |
| SGA A, n (%) | 65 (86.7%) | 23 (95.9%) | 19 (86.4%) | 4 (40%) | <0.001 * |
| SGA B, n (%) | 10 (13.3%) | 1 (4.2%) | 2 (9.1%) | 4 (40%) | |
| SGA C, n (%) | 0 (0%) | 0 (0%) | 1 (4.6%) | 2 (20%) | |
| MIS, median (min max) | 5 (1–14) | 5 (2–9) | 5 (2–16) | 9.5 (5–18) | <0.001 |
| MIS ≥ 9, n (%) | 8 (10.7%) | 2 (8.3%) | 5 (22%) | 7 (70%) | <0.001 * |
| MIS ≥ 14, n (%) | 1 (1.3%) | 0 (0%) | 1 (4.6%) | 2 (20%) | 0.009 * |
| Diabetes, n (%) | 37 (49.3%) | 17 (70.8%) | 10 (45.5%) | 2 (20%) | 0.047 * |
| Cardiopathy, n (%) | 23 (30.7%) | 1 (4.2%) | 5 (22.7%) | 1 (10%) | 0.041 * |
| Neoplasia, n (%) | 4 (5.3%) | 3 (12.5%) | 3 (13.6%) | 7 (70%) | <0.001 * |
| sCreatinine (mg/dL), median (min-max) | 2.3 (1.3–7.6) | 2.7 (1.5–9.6) | 3.3 (1.7–7.7) | 2.6 (1.3–10.4) | 0.004 |
| eGFR-EPI (mL/min), median (min-max) | 24 (8–68) | 22 (5–40) | 15 (5–46) | 23 (4–50) | 0.012 |
| eGFR < 15 (mL/min) at enrollment, n (%) | 7 (9.3%) | 3 (12.5%) | 12 (54.5%) | 2 (20%) | <0.001 * |
| GFR < 10 (mL/min) at enrollment, n (%) | 3 (4%) | 2 (8.3%) | 5 (22.7%) | 1 (10%) | 0.050 * |
| Proteinuria (g/day), median (min-max) | 0.3 (0.1–8.3) | 1.2 (0.1–4.5) | 1.8 (0.1–7) | 0.4 (0.1–3) | 0.003 |
| Proteinuria ≥ 1 (g/day), n (%) | 19 (25.3%) | 14 (58.3%) | 14 (63.6%) | 3 (30%) | 0.001 * |
| Proteinuria ≥ 3 (g/day), n (%) | 8 (10.7%) | 5 (20.8%) | 8 (36.4%) | 1 (10%) | 0.034 * |
| Glomerulonephritis-systemic disease, n (%) | 0 | 0 | 2 (9.1%) | 1 (10%) | 0.087 * |
| Ketoanalogues, n (%) | 10 (13%) | 0 | 22 (100%) | 1 (10%) | <0.001 * |
| Sex | Age | CCI | MIS | SGA | Kidney Disease | CKD Stage | Cause of Death | Protein Restriction | Dialysis |
|---|---|---|---|---|---|---|---|---|---|
| M | 67 | 12 | 11 | B | NAS | 4 | Neoplasia (liver) | None (short life expectancy) | no |
| F | 44 | 9 | 10 | B | Interstitial nephropathy | 4-5 | Neoplasia (lung) | None (short life expectancy, PEW) | no |
| F | 49 | 8 | 7 | A | FSGS | 5 | Heart failure (primary pulmonary hypertension) | None (short life expectancy, non compliance) | PD |
| M | 84 | 7 | 9 | B | NAS | 4 | Cardiac death | Normalisation | no |
| M | 88 | 8 | 10 | A | NAS | 4 | Popliteal artery rupture | Normalisation | no |
| M | 88 | 10 | 9 | C | NAS | 5 | Cardiac death | None (short life expectancy, patient’s choice) | no |
| M | 81 | 7 | 3 | A | NAS | 5 | Cardiac death | Normalisation | no |
| F | 60 | 6 | 14 | C | Diabetic nephropathy | 5 | Hemorrhage due to (voluntary) section of the dialysis catheter | None (PEW, non compliance) | HD |
| M | 65 | 11 | 18 | C | Interstitial nephropathy | 3B | Neoplasia (lung) | None (short life expectancy, PEW) | no |
| First Diet | BMI ≥ 30 kg/m2 | BMI < 30 kg/m2 | p |
|---|---|---|---|
| N | 53 | 78 | |
| Males, n (%) Females, n (%) | 29 (54.7%) 24 (45.3%) | 53 (68%) 25 (32%) | 0.126 * |
| Age (years) median (min-max) | 73 (24–93) | 76 (26–101) | 0.221 |
| Age over 65, n (%) | 35 (66%) | 60 (76.9%) | 0.172 * |
| Age over 80, n (%) | 17 (32%) | 32 (41%) | 0.301 * |
| CCI, median (min-max) | 8 (2–14) | 8 (2–12) | 0.341 |
| CCI ≥ 7, n (%) | 40 (75.5%) | 53 (68%) | 0.354 * |
| CCI ≥ 10, n (%) | 9 (17%) | 13 (16.7%) | 0.963 * |
| Diabetes, n (%) | 37 (69.9%) | 29 (37.2%) | <0.001 * |
| Cardiopathy, n (%) | 15 (28.3%) | 15 (19.2%) | 0.227 * |
| Neoplasia, n (%) | 3 (5.7%) | 14 (18%) | 0.041 * |
| sCreatinine (mg/dL), median (min-max) | 2.5 (1.3–9.6) | 2.6 (1.3–10.4) | 0.683 |
| eGFR-EPI (mL/min), median (min-max) | 22 (5–57) | 23 (4–68) | 0.957 |
| Proteinuria (g/day), median (min-max) | 0.8 (0.1–8.3) | 0.4 (0.1–6.5) | 0.090 |
| Proteinuria ≥ 1 (g/day), n (%) | 24 (45.3%) | 26 (33.3%) | 0.169 * |
| Proteinuria ≥ 3 (g/day), n (%) | 12 (22.7%) | 10 (12.8%) | 0.142 * |
| SGA A, n (%) | 49 (92.5%) | 62 (79.5%) | 0.096 * |
| SGA B, n (%) | 4 (7.6%) | 13 (16.7%) | |
| SGA C, n (%) | 0 (0%) | 3 (3.9%) | |
| MIS, median (min-max) | 5 (1–11) | 5 (1–18) | 0.974 |
| MIS ≥ 9, n (%) | 6 (11.3%) | 16 (20.5%) | 0.168 * |
| MIS ≥ 14, n (%) | 0 (0%) | 4 (5.1%) | 0.095 * |
| Normalization of protein intake, n (%) | 33 (62.3%) | 42 (53.9%) | 0.120 * |
| Moderate restriction traditional, n (%) | 12 (22.6%) | 12 (15.4%) | |
| Plant-based supplemented, (0.6) n (%) | 7 (13.2%) | 15 (19.2%) | |
| No restriction, n (%) | 1 (1.9%) | 9 (11.5%) | |
| BMI (kg/m2) median (min-max) | 34.8 (51.2–30.3) | 25.9 (29.8–16.7) | <0.001 |
| BMI ≥ 35, n (%) | 25 (47.2%) | 0 (0%) | <0.001 * |
| BMI ≥ 40, n (%) | 10 (18.9%) | 0 (0.00%) | <0.001 * |
| First Diet | Diabetes | No Diabetes | p |
|---|---|---|---|
| N | 66 | 65 | |
| Males, n (%) Females, n (%) | 42 (63.6%) 24 (36.4%) | 40 (61.5%) 25 (38.5%) | 0.804 * |
| Age (years), median (min-max) | 72 (26–101) | 77 (24–96) | 0.175 |
| Age over 65, n (%) | 46 (67.9%) | 49 (75.4%) | 0.468 * |
| Age over 80, n (%) | 22 (33.3%) | 27 (41.5%) | 0.334 * |
| CCI, median (min-max) | 8 (5–14) | 7 (2–12) | <0.001 |
| CCI ≥ 7, n (%) | 57 (86.4%) | 36 (55.4%) | <0.001 * |
| CCI ≥ 10, n (%) | 13 (19.7%) | 9 (13.8%) | 0.372 * |
| Cardiopathy, n (%) | 19 (28.8%) | 11 (16.9%) | 0.108 * |
| Neoplasia, n (%) | 3 (4.5%) | 14 (21.5%) | 0.004 * |
| sCreatinine (mg/dL), median (min-max) | 2.6 (1.3–7.7) | 2.58 (1.3–10.4) | 0.945 |
| eGFR-EPI (mL/min), median (min-max) | 22 (7–57) | 23 (4–7) | 0.872 |
| Proteinuria (g/day), median (min-max) | 0.7 (0.1–8.7) | 0.4 (0.1–7) | 0.364 |
| Proteinuria ≥ 1 (g/day), n (%) | 28 (42.4%) | 22 (33.9%) | 0.088 * |
| Proteinuria ≥ 3 (g/day), n (%) | 11 (16.7%) | 11 (16.9%) | 0.969 * |
| SGA A, n (%) | 59 (89.39%) | 52 (80%) | |
| SGA B, n (%) | 5 (7.6%) | 12 (18.5%) | |
| SGA C, n (%) | 2 (3%) | 1 (1.5%) | 0.161 * |
| MIS, median (min-max) | 5 (1–17) | 5 (1–18) | 0.589 |
| MIS ≥ 9, n (%) | 8 (12.1%) | 14 (21.5%) | 0.151 * |
| MIS ≥ 14, n (%) | 3 (4.6%) | 1 (1.5%) | 0.319 * |
| Normalization of protein intake, n (%) | 37 (56.1%) | 38 (58.5%) | 0.047 |
| Traditional (0.6), n (%) | 17 (25.6%) | 7 (10.8%) | |
| Plant-based supplemented (0.6), n (%) | 10 (15.2%) | 12 (18.5%) | |
| No restriction, n (%) | 2 (3%) | 8 (12.3%) | |
| BMI (kg/m2), median (min-max) | 31 (16.7–51.2) | 27 (18.3–50) | 0.001 |
| BMI ≥ 35, n (%) | 17 (26%) | 8 (12%) | 0.051 * |
| BMI ≥ 40, n (%) | 6 (9.1%) | 4 (6.2%) | 0.528 * |
| HbA1c at enrollment (Pre) (%), median (min-max) At last update (Post) (%), median (min-max) | Pre 7.1 (5.1–11.2) Post 6.7 (4.9–9.4) | 0.104 |
| Normalization (0.8) N = 32 | Traditional (0.6) N = 17 | Plant Based Supplemented (0.6) N = 16 | All Cases N = 65 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base Line | Last Update | p | Base Line | Last Update | p | Base Line | Last Update | p | Base Line | Last Update | p | |
| “Good adherence” (%) | 72% | 76% | 75% | 74% | 0.934 ≠ | |||||||
| Protein intake, g/kg/day median (min-max) | 1.2 (0.7–1.5) | 0.8 (0.6–1.4) | <0.001 * | 0.9 (0.6–1.3) | 0.7 (0.5–0.9) | <0.001 * | 1.1 (0.5–1.5) | 0.7 (0.4–1) | <0.001 * | 1.1 (0.5–1.5) | 0.7 (0.4–1.4) | <0.001 * |
| Creatinine, mg/dL mean (± SD) | 2.4 (± 0.6) | 2.6 (± 0.8) | 0.088 | 2.9 (± 1) | 3.1 (± 1.7) | 0.369 | 3.6 (± 1.5) | 3.9 (± 1.8) | 0.550 | 2.84 (± 1.10) | 3.05 (± 1.46) | 0.013 |
| Proteinuria g/24 h median (min-max) | 0.4 (0.1–6.2) | 0.5 (0.1–6.2) | 0.149 * | 1.2 (0.1–4.5) | 1.4 (0.1–2) | 0.999 * | 1.4 (0.1–5.7) | 2.5 (0.1–5.8) | 0.893 * | 0.6 (0.1–6.16) | 0.7 (0.1–6.16) | 0.566 * |
| Proteinuria ≥ 1 g/24 h n (%) | 9 (28%) | 7 (22%) | 0.625 † | 10 (59%) | 10 (59%) | 0.999 † | 9 (56%) | 8 (50%) | 0.999 † | 28 (43%) | 25 (38%) | 0.453 † |
| Albumin, g/dL median (min-max) | 3.5 (3.1–4) | 3.6 (2.9–4.2) | 0.037 * | 3.5 (2.9–3.9) | 3.7 (2.9–4) | 0.034 * | 3.2 (2.8–3.9) | 3.3 (2.7–3.9) | 0.216 * | 3.5 (2.8–4) | 3.6 (2.7–4.2) | 0.001 * |
| Albumin < 3 g/dL n (%) | 0 (0%) | 1 (3%) | 0.999 † | 2 (12%) | 0 (0%) | 0.999 † | 4 (25%) | 2 (13%) | 0.625 † | 6 (9%) | 4 (6%) | 0.687 † |
| PTH, ng/L median (min-max) | 92.5 (27–252) | 84 (18–614) | 0.423 * | 115 (33–328) | 105 (44–490) | 0.720 * | 145.5 (13–188) | 113 (24–251) | 0.229 * | 101 (13–328) | 91 (18–614) | 0.941 * |
| BUN, mg/dL median (min-max) | 47.5 (26–79) | 46.4 (26.3–99.4) | 0.135 * | 48 (26–121) | 43.1 (30.5–74.2) | 0.329 * | 57.5 (25–81) | 57.1 (25–124.1) | 0.900 * | 48 (25–121) | 47.1 (25–124.1) | 0.811 * |
| HCO3 mmol/L median (min-max) | 24.5 (18–34) | 23 (16–33) | 0.150 * | 24 (16–34) | 24 (16–32) | 0.599 * | 21 (17–31) | 22 (17–31) | 0.770 * | 24 (16–34) | 23 (16–33) | 0.193 * |
| Hemoglobin, g/dL mean (± SD) | 12.6 (± 1.4) | 12.3 (± 1.4) | 0.251 | 12.4 (± 1.5) | 12.1 (± 1.1) | 0.332 | 11.3 (± 1.6) | 11.1 (± 1.1) | 0.570 | 12.2 (± 1.6) | 12.9 (± 1.3) | 0.108 |
| GFR CKD-EPI mL/min/1.73 m2 median (min-max) | 23 (15–57) | 24 (12–52) | 0.140 * | 22 (10–35) | 21 (7–44) | 0.854 * | 15 (7–38) | 14.5 (6–36) | 0.110 * | 22 (7–57) | 21 (6–52) | 0.053 * |
| GFR MDRD, mL/min/1.73 m2 median (min-max) | 24 (15–55) | 25 (12–50) | 0.153 * | 23 (10–34) | 23 (8–45) | 0.934 * | 15 (7–42) | 15.5 (6–32) | 0.110 * | 22 (7–57) | 21 (6–50) | 0.071 * |
| Sex | Age | CCI | MIS | SGA | Kidney Disease | Type of Dialysis | Diet | Days in UIRAV to Dialysis Start |
|---|---|---|---|---|---|---|---|---|
| M | 69 | 10 | 7 | A | Cardio renal syndrome | HD–urgent (cardio-renal) | Normalization | 79 § |
| F | 49 | 8 | 7 | A | FSGS and cardio renal syndrome | PD incremental * | None | § |
| M | 82 | 8 | 7 | A | Cardio renal syndrome | HD–urgent (cardio-renal) | Normalization | 88 |
| M | 79 | 7 | 6 | A | NAS | HD incremental ** | 0.6 plant-based | 26 §§ |
| F | 53 | 4 | 5 | A | APL syndrome | HD incremental | 0.6 traditional | § |
| F | 77 | 8 | 8 | A | Diabetic nephropathy | HD incremental | 0.6 traditional | 98 |
| F | 60 | 6 | 14 | C | Diabetic nephropathy | HD incremental * | None (low irregular intake) | § |
| Model including Charlson Comorbidity Index, Age | ||||
|---|---|---|---|---|
| Crude OR (95% CIs) | p | Adjusted OR (95% CIs) | p | |
| Sex: Female | 0.98 (0.67–1.45) | 0.951 | 0.60 (0.12–2.99) | 0.535 |
| CCI < 7 | 2.02 (0.88–4.64) | 0.100 | 2.29 (0.47–1.16) | 0.302 |
| eGFR-EPI (mL/min) ≥ 20 | 1.07 (0.33–3.46) | 0.908 | 1.13 (0.27–4.65) | 0.863 |
| Age (years) < 65 | 1.64 (0.46–5.82) | 0.444 | 1.43 (0.28–7.32) | 0.668 |
| BMI (kg/m2) ≥ 30 | 5.16 (1.29–20.57) | 0.013 | 3.89 (0.89–16.79) | 0.070 |
| Model including Diabetes, Age | ||||
| Sex: Female | 0.98 (0.67–1.45) | 0.951 | 1.98 (0.45–8.77) | 0.794 |
| eGFR-EPI (mL/min) ≥ 20 | 1.07 (0.33–3.46) | 0.908 | 1.32 (0.36–4.84) | 0.675 |
| Age (years) < 65 | 1.64 (0.46–5.82) | 0.444 | 1.98 (0.45–8.78) | 0.365 |
| BMI (kg/m2) ≥ 30 | 5.16 (1.29–20.57) | 0.013 | 5.70 (1.32–24.73) | 0.020 |
| Diabetes: no | 1.30 (0.42–4.07) | 0.652 | 0.89 (0.24–3.29) | 0.868 |
| Model including Age only | ||||
| Sex: Female | 0.98 (0.67–1.45) | 0.951 | 0.83 (0.20–3-36) | 0.791 |
| eGFR-EPI (mL/min) ≥ 20 | 1.07 (0.33–3.46) | 0.908 | 1.31 (0.36–4.76) | 0.685 |
| Age (years) < 65 | 1.64 (0.46–5.82) | 0.444 | 2.04 (0.48–8.74) | 0.337 |
| BMI (kg/m2) ≥ 30 | 5.16 (1.29–20.57) | 0.013 | 5.52 (1.35–22.60) | 0.017 |
| Model including Charlson Comorbidity Index, Age | ||||
|---|---|---|---|---|
| Crude OR (95% CIs) | p | Adjusted OR (95% CIs) | p | |
| Sex: Female | 0.67 (0.22–2.04) | 0.479 | 1.19 (0.28–5.07) | 0.749 |
| Charlson Index < 7 | 0.67 (0.20–2.24) | 0.511 | 0.43 (0.09–2.09) | 0.295 |
| eGFR-EPI (mL/min) ≥ 20 | 1.50 (0.53–4.26) | 0.445 | 2.08 (0.55–7.86) | 0.283 |
| Age (years) < 65 | 0.83 (0.25–2.69) | 0.750 | 0.87 (0.19–4.09) | 0.865 |
| BMI (kg/m2) ≥ 30 | 4.29 (1.43–12.81) | 0.007 | 5.97 (1.59–22.44) | 0.008 |
| Model including Diabetes, Age | ||||
| Sex: Female | 0.67 (0.22–2.04) | 0.479 | 0.64 (0.18–2.24) | 0.486 |
| eGFR-EPI (mL/min) ≥ 20 | 1.50 (0.53–4.26) | 0.445 | 1.62 (0.51–5.14) | 0.410 |
| Age (years) < 65 | 0.83 (0.25–2.69) | 0.750 | 1.05 (0.27–4.09) | 0.940 |
| BMI (kg/m2) ≥ 30 | 4.29 (1.43–12.81) | 0.007 | 4.61 (1.42–14.97) | 0.011 |
| Diabetes: no | 1.61 (0.59–4.45) | 0.353 | 1.02 (0.32–3.27) | 0.971 |
| Model including Age only | ||||
| Sex: Female | 0.67 (0.22–2.04) | 0.479 | 0.64 (0.18–2.24) | 0.487 |
| eGFR-EPI (mL/min) ≥ 20 | 1.50 (0.53–4.26) | 0.445 | 1.63 (0.51–5.14) | 0.408 |
| Age (years) < 65 | 0.83 (0.25–2.69) | 0.750 | 1.055 (0.28–3.94) | 0.945 |
| BMI (kg/m2) ≥ 30 | 4.29 (1.43–12.81) | 0.007 | 4.64 (1.51–14.29) | 0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fois, A.; Chatrenet, A.; Cataldo, E.; Lippi, F.; Kaniassi, A.; Vigreux, J.; Froger, L.; Mongilardi, E.; Capizzi, I.; Biolcati, M.; et al. Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly, High-Comorbidity Population. A Stepwise Multiple-Choice System Approach. Nutrients 2019, 11, 36. https://doi.org/10.3390/nu11010036
Fois A, Chatrenet A, Cataldo E, Lippi F, Kaniassi A, Vigreux J, Froger L, Mongilardi E, Capizzi I, Biolcati M, et al. Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly, High-Comorbidity Population. A Stepwise Multiple-Choice System Approach. Nutrients. 2019; 11(1):36. https://doi.org/10.3390/nu11010036
Chicago/Turabian StyleFois, Antioco, Antoine Chatrenet, Emanuela Cataldo, Francoise Lippi, Ana Kaniassi, Jerome Vigreux, Ludivine Froger, Elena Mongilardi, Irene Capizzi, Marilisa Biolcati, and et al. 2019. "Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly, High-Comorbidity Population. A Stepwise Multiple-Choice System Approach" Nutrients 11, no. 1: 36. https://doi.org/10.3390/nu11010036
APA StyleFois, A., Chatrenet, A., Cataldo, E., Lippi, F., Kaniassi, A., Vigreux, J., Froger, L., Mongilardi, E., Capizzi, I., Biolcati, M., Versino, E., & Piccoli, G. B. (2019). Moderate Protein Restriction in Advanced CKD: A Feasible Option in An Elderly, High-Comorbidity Population. A Stepwise Multiple-Choice System Approach. Nutrients, 11(1), 36. https://doi.org/10.3390/nu11010036







