Postprandial Effects of Blueberry (Vaccinium angustifolium) Consumption on Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite in Healthy Adults: A Randomized, Placebo-Controlled Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Study Treatments
2.4. Study Day Visit Procedures
2.5. Biological Sample Collection and Analysis
2.6. Statistical Analysis
3. Results
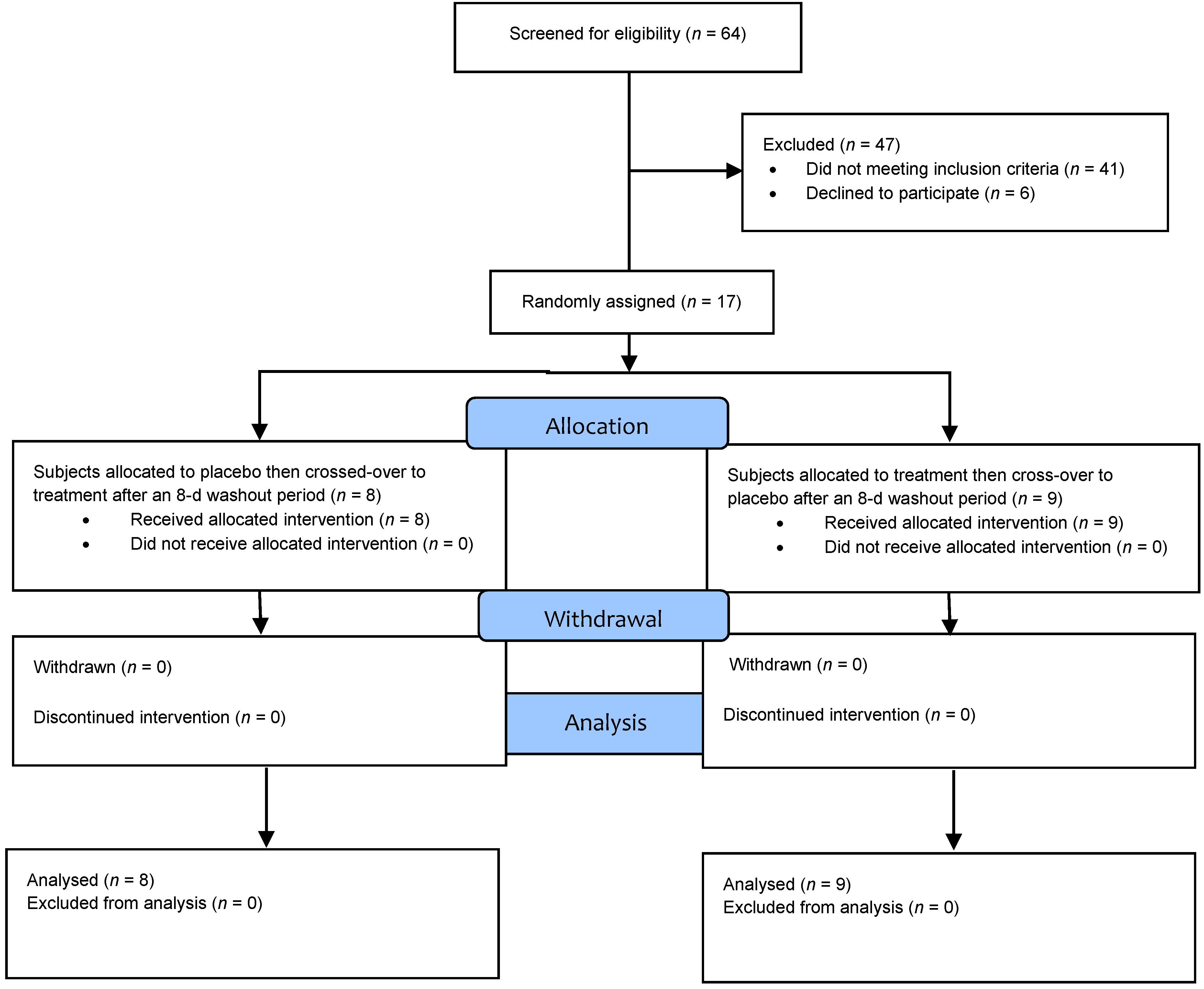
3.1. Baseline Subject Characteristics
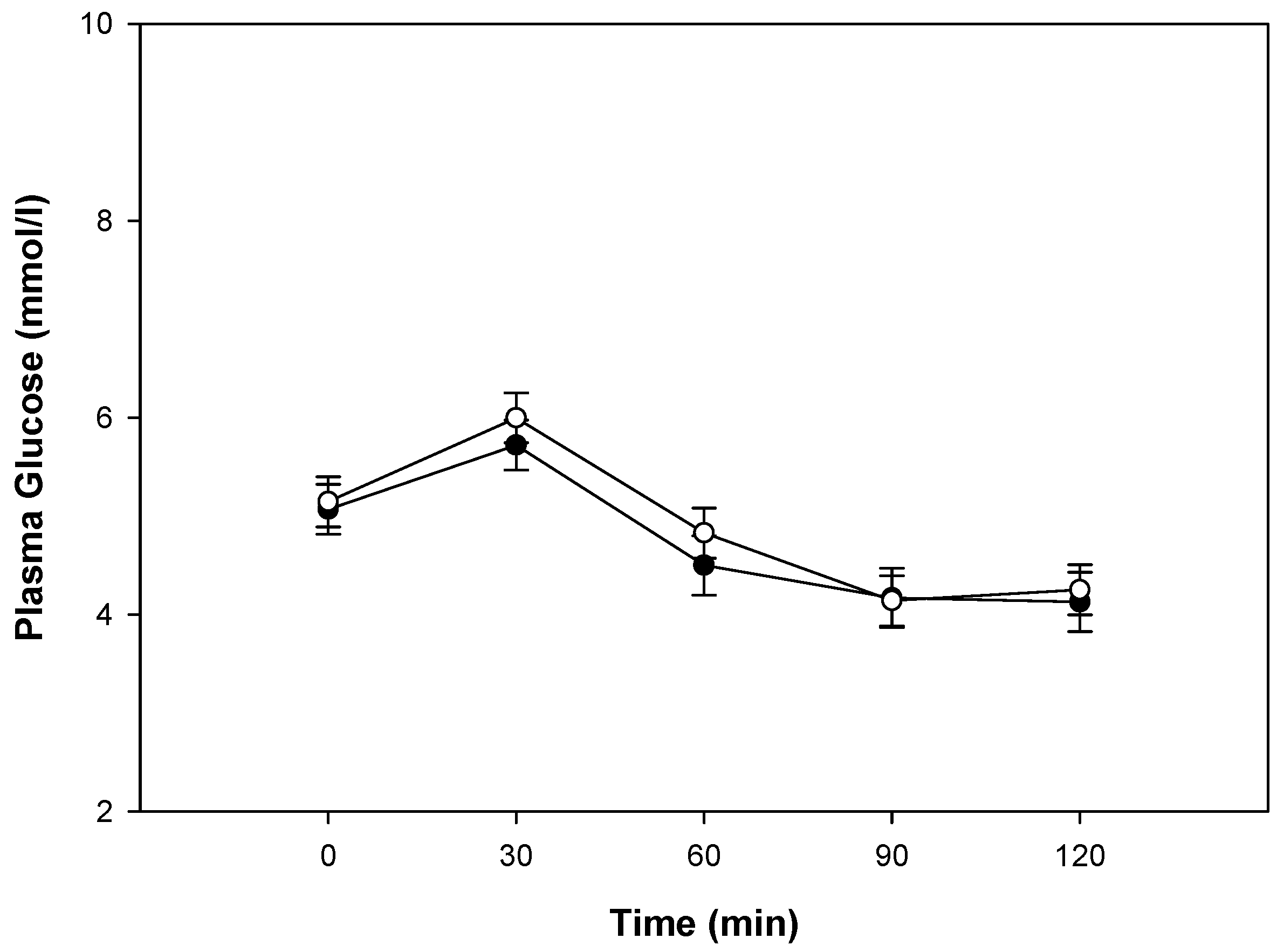
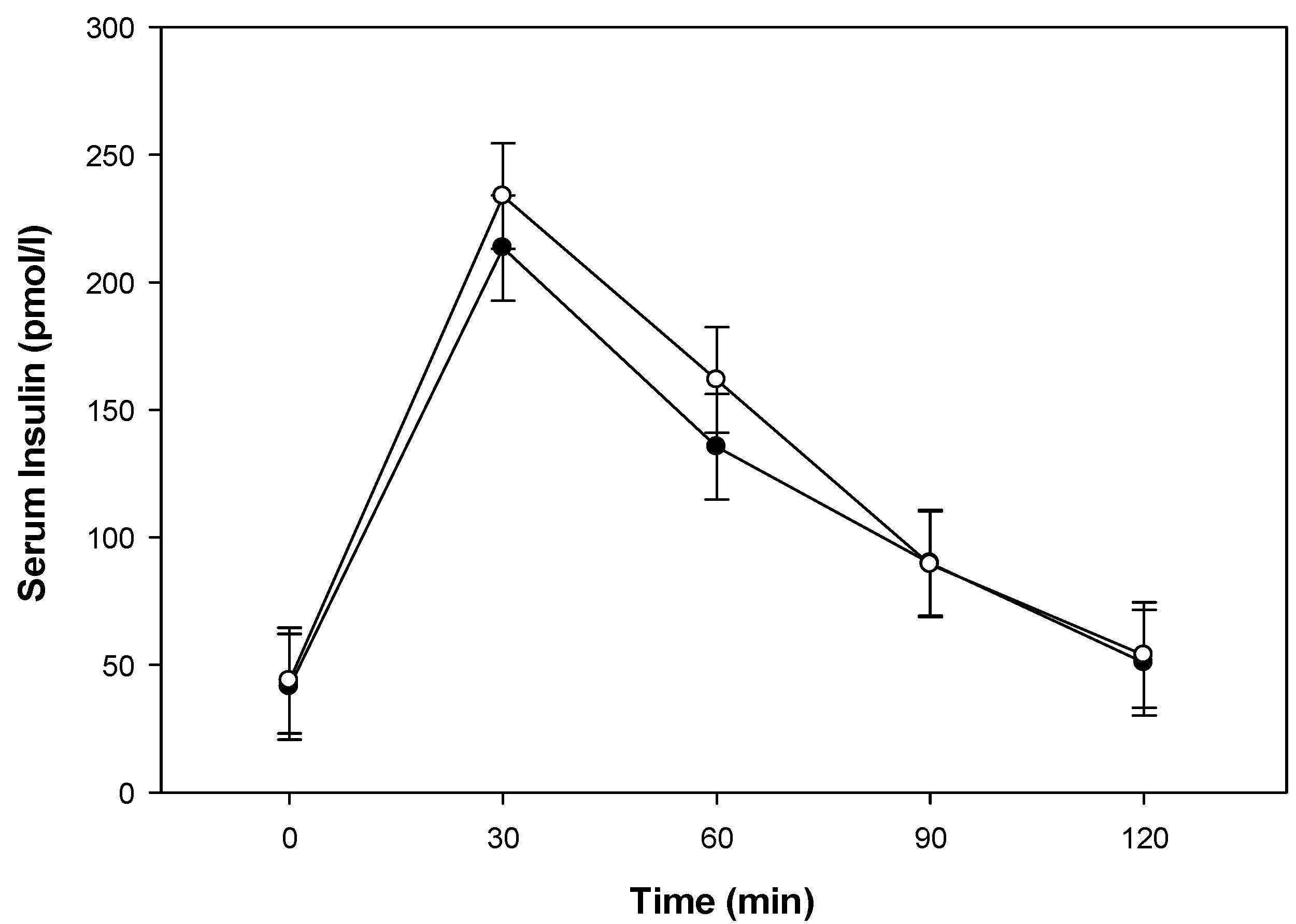
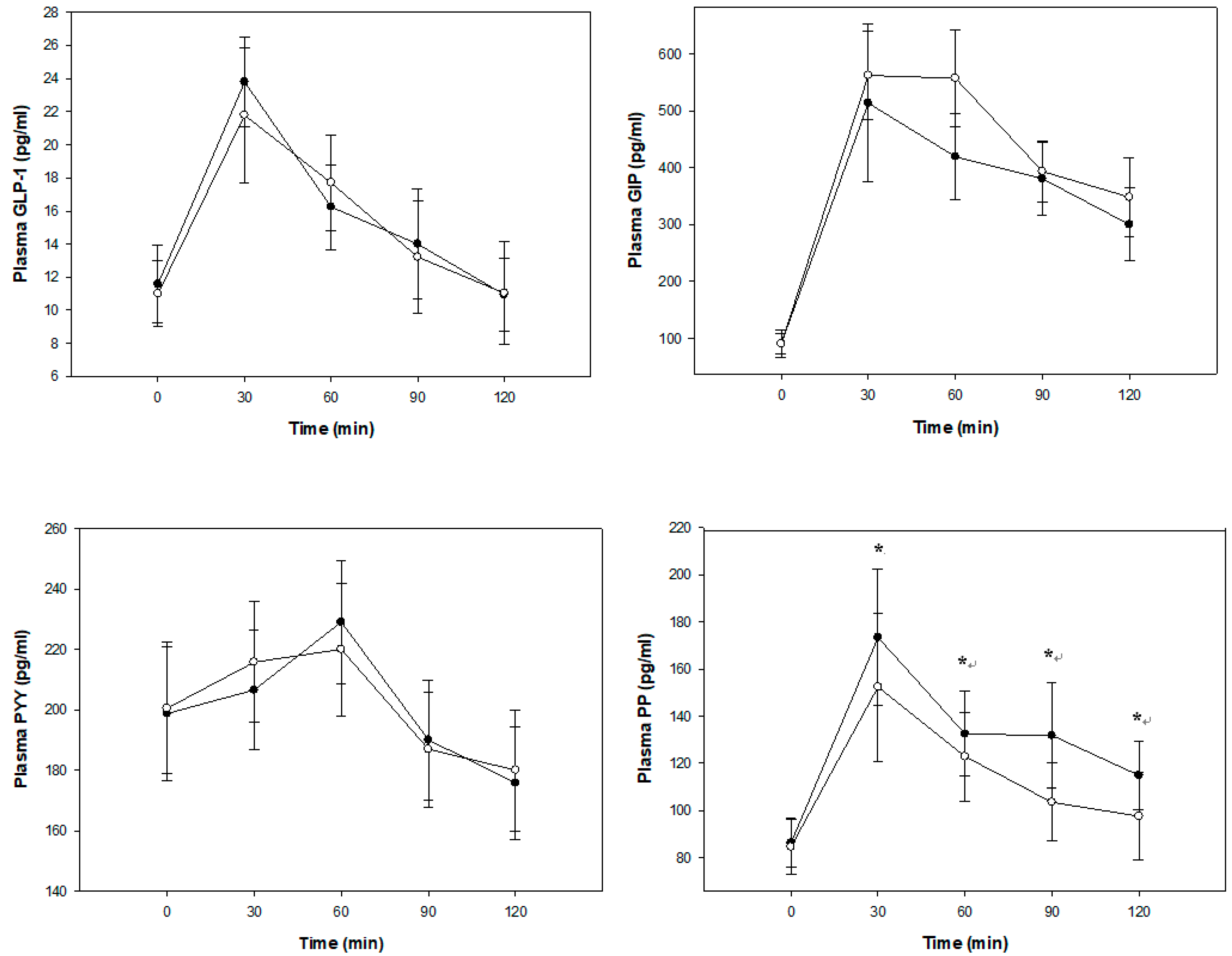
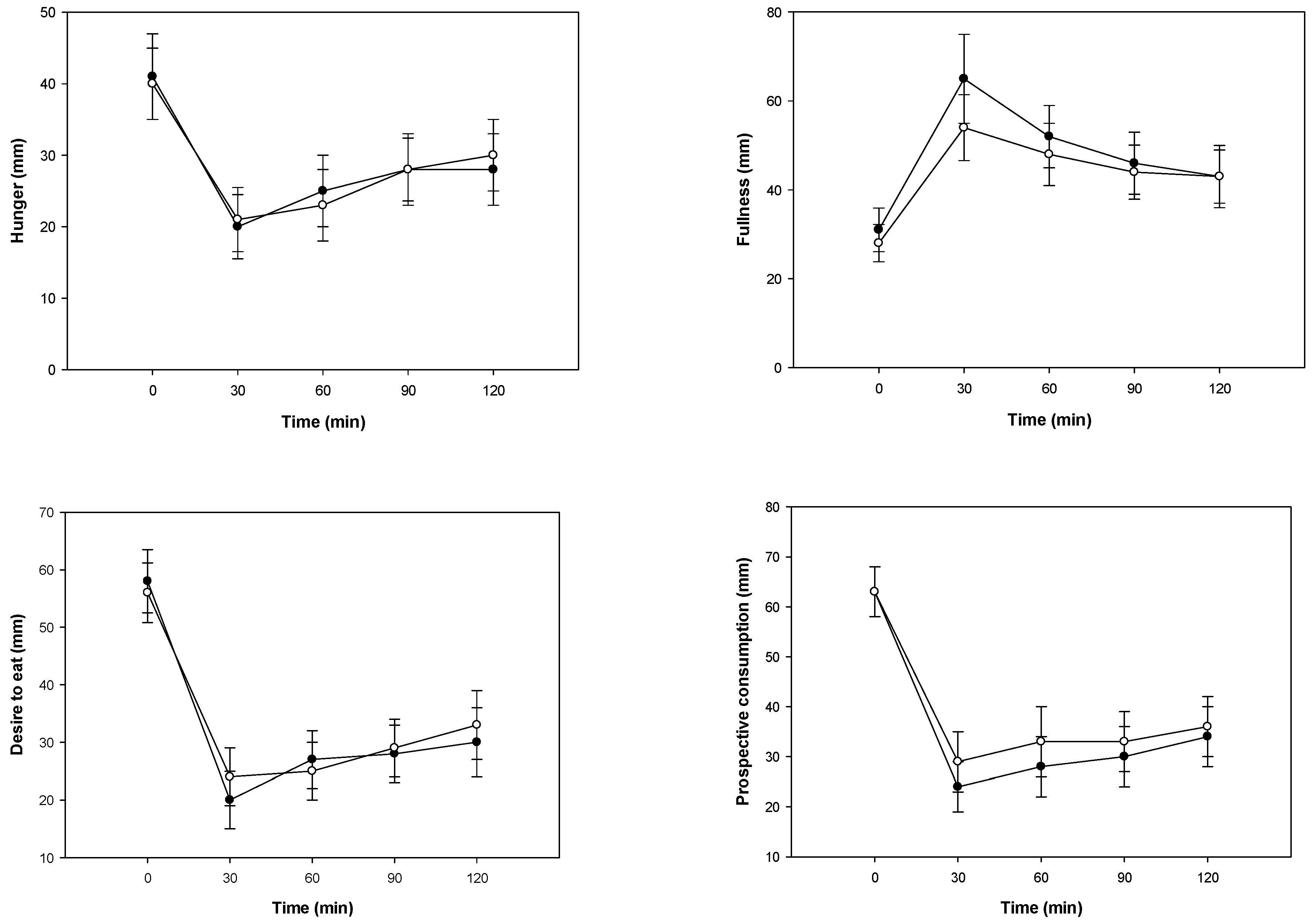
3.2. Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.A.; Steptoe, A. Psychosocial factors in diabetes and cardiovascular risk. Curr. Cardiol. Rep. 2016, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dubey, V.; Ghosh, A.R. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism 2016, 65, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Phillips-Eakley, A.K.; Smith, K.N. The effects of breakfast consumption and composition on metabolic wellness with a focus on carbohydrate metabolism. Adv. Nutr. 2016, 7, 613S–621S. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, S.P.; Wilding, J. GLP-1 as a target for therapeutic intervention. Curr. Opin. Pharmacol. 2016, 31, 44–49. [Google Scholar] [CrossRef]
- Johnson, M.H.; De Mejia, E.G. Phenolic compounds from fermented berry beverages modulated gene and protein expression to increase insulin secretion from pancreatic beta-cells in vitro. J. Agric. Food Chem. 2016, 64, 2569–2581. [Google Scholar] [CrossRef]
- Wootten, D.; Simms, J.; Koole, C.; Woodman, O.L.; Summers, R.J.; Christopoulos, A.; Sexton, P.M. Modulation of the glucagon-like peptide-1 receptor signaling by naturally occurring and synthetic flavonoids. J. Pharmacol. Exp. Ther. 2011, 336, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nayak, B.P.; Kumar, A. Obesity: Single house for many evils. Minerva Endocrinol. 2016, 41, 499–508. [Google Scholar] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol. Nutr. Food Res. 2013, 57, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.randomization.com/ (accessed on 18 May 2017).
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Kirkpatrick, S.I.; Subar, A.F.; Douglass, D.; Zimmerman, T.P.; Thompson, F.E.; Kahle, L.L.; George, S.M.; Dodd, K.W.; Potischman, N. Performance of the automated self-administered 24-hour recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am. J. Clin. Nutr. 2014, 100, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Finlayson, G.; Dalton, M.; Caudwell, P.; Blundell, J.E. Metabolic phenotyping guidelines: studying eating behaviour in humans. J. Endocrinol. 2014, 222, G1–G12. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.E.; Pratt, M.; Meade, C.M.; Henry, C.J.K. The addition of raspberries and blueberries to a starch-based food does not alter the glycaemic response. Br. J. Nutr. 2011, 106, 335–338. [Google Scholar] [CrossRef]
- Torronen, R.; Kolehmainen, M.; Sarkkinen, E.; Mykkänen, H.; Niskanen, L. Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am. J. Clin. Nutr. 2012, 96, 527–533. [Google Scholar] [CrossRef]
- Torronen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkänen, H.; Niskanen, L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Torronen, R.; Sarkkinen, E.; Niskanen, T.; Tapola, N.; Kilpi, K.; Niskanen, L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2012, 107, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Torronen, R.; Sarkkinen, E.; Tapola, N.; Hautaniemi, E.; Kilpi, K.; Niskanen, L. Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br. J. Nutr. 2010, 103, 1094–1097. [Google Scholar] [PubMed]
- Dougkas, A.; Ostman, E. Protein-enriched liquid preloads varying in macronutrient content modulate appetite and appetite-regulating hormones in healthy adults. J. Nutr. 2016, 146, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kreidler, S.M.; Muller, K.E.; Grunwald, G.K.; Ringham, B.M.; Coker-Dukowitz, Z.T.; Sakhadeo, U.R.; Glueck, D.H. GLIMMPSE: Online power computation for linear models with and without a baseline covariate. J. Stat. Softw. 2013, 54, i10. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Kuhn, P.; Rojo, L.E.; Lila, M.A.; Raskin, I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol. Res. 2013, 68, 59–67. [Google Scholar] [CrossRef]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Le, P.M.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A study of glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Riso, P.; Klimis-Zacas, D.; Del Bo, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Enteroendocrine Cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Le Roux, C.W.; Cohen, M.A.; Park, A.J.; Ellis, S.M.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 2003, 88, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries and anthocyanins: Promising functional food ingredients with postprandial glycaemia-lowering effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef]
- Steinert, R.E.; Beglinger, C.; Langhans, W. Intestinal GLP-1 and satiation: From man to rodents and back. Int. J. Obes. 2016, 40, 198–205. [Google Scholar] [CrossRef]
- Lafond, D.W.; Greaves, K.A.; Maki, K.C.; Leidy, H.J.; Romsos, D.R. Effects of two dietary fibers as part of ready-to-eat cereal (RTEC) breakfasts on perceived appetite and gut hormones in overweight women. Nutrients 2015, 7, 1245–1266. [Google Scholar] [CrossRef]
- James, L.J.; Funnell, M.P.; Milner, S. An afternoon snack of berries reduces subsequent energy intake compared to an isoenergetic confectionary snack. Appetite 2015, 95, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Freudenheim, J.L. A review of study designs and methods of dietary assessment in nutritional epidemiology of chronic disease. J. Nutr. 1993, 123 (Suppl. 2), 401–405. [Google Scholar] [CrossRef] [PubMed]
| Treatment Beverage | ||
|---|---|---|
| Whole Blueberries | Placebo Gel | |
| Energy, kcal | 80 | 78 |
| Water, g | 120 | 117 |
| Fat, g | 0.24 | 0 |
| Protein, g | 0 | 0 |
| Carbohydrate, g | 18 | 18 |
| Sugars, g | 10.3 | 11.1 |
| Sucrose, g | 0 | 0 |
| Glucose, g | 4.8 | 5.3 |
| Fructose, g | 5.5 | 5.8 |
| Fiber, g | 6.2 | 6.2 |
| Phenolics, g | 720 | 0 |
| Anthocyanins, mg | 401 | 0 |
| Total 2 | Range | |
|---|---|---|
| Age (years) | 47 ± 15 | 21–63 |
| Height (cm) | 165.3 ± 12.3 | 143.0–188.0 |
| Weight (kg) | 64.3 ± 13.1 | 48.0–96.2 |
| Body mass index (kg/m2) | 23.4 ± 3.0 | 19.5–30.2 |
| Body fat (%) | 23.2 ± 7.0 | 13.1–34.8 |
| Systolic blood pressure (mm Hg) | 115.9 ± 6.3 | 102–124 |
| Diastolic blood pressure (mm Hg) | 75.1 ± 7.3 | 55–86 |
| Glucose (mmol/L) | 4.9 ± 0.3 | 4.1–5.4 |
| Insulin (pmol/L) | 38.5 ± 14.2 | 14.0–66.0 |
| Subjective physical activity level (%) | ||
| Mild exercise | 12 | |
| Occasional vigorous exercise | 29 | |
| Regular vigorous exercise | 59 | |
| Ethnicity (%) | ||
| White, not Hispanic | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stote, K.; Corkum, A.; Sweeney, M.; Shakerley, N.; Kean, T.; Gottschall-Pass, K. Postprandial Effects of Blueberry (Vaccinium angustifolium) Consumption on Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite in Healthy Adults: A Randomized, Placebo-Controlled Crossover Trial. Nutrients 2019, 11, 202. https://doi.org/10.3390/nu11010202
Stote K, Corkum A, Sweeney M, Shakerley N, Kean T, Gottschall-Pass K. Postprandial Effects of Blueberry (Vaccinium angustifolium) Consumption on Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite in Healthy Adults: A Randomized, Placebo-Controlled Crossover Trial. Nutrients. 2019; 11(1):202. https://doi.org/10.3390/nu11010202
Chicago/Turabian StyleStote, Kim, Adele Corkum, Marva Sweeney, Nicole Shakerley, Terri Kean, and Katherine Gottschall-Pass. 2019. "Postprandial Effects of Blueberry (Vaccinium angustifolium) Consumption on Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite in Healthy Adults: A Randomized, Placebo-Controlled Crossover Trial" Nutrients 11, no. 1: 202. https://doi.org/10.3390/nu11010202
APA StyleStote, K., Corkum, A., Sweeney, M., Shakerley, N., Kean, T., & Gottschall-Pass, K. (2019). Postprandial Effects of Blueberry (Vaccinium angustifolium) Consumption on Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite in Healthy Adults: A Randomized, Placebo-Controlled Crossover Trial. Nutrients, 11(1), 202. https://doi.org/10.3390/nu11010202






