Inula japonica Thunb. Flower Ethanol Extract Improves Obesity and Exercise Endurance in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Preparation of I. japonica Flower Ethanol Extract (IJE)
2.3. Differentiation and Oil Red O Staining in 3T3-L1 Cells
2.4. Cell Viability
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (PCR)
2.6. Immunoblotting
2.7. Animal Care and Diet Composition
2.8. Body Composition Analysis by Dual-Energy X-ray Absorptiometry (DEXA)
2.9. Triglyceride (TG) and Total Cholesterol (TC) Levels and Staining with Hematoxylin and Eosin (H&E)
2.10. Measurement of Exercise Capacity
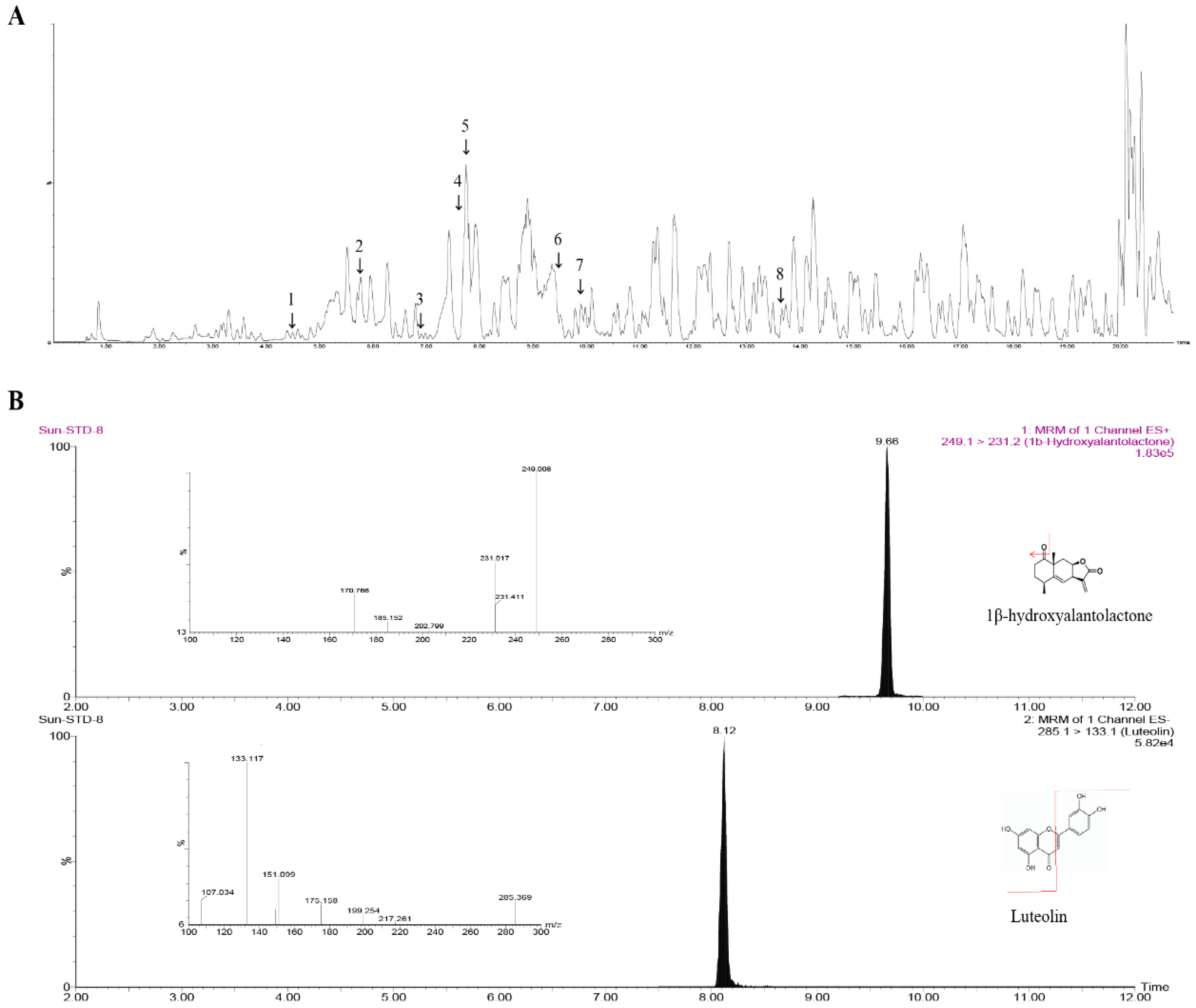
2.11. Liquid Chromatography–Mass Spectrometry (LC–MS/MS) Analysis
2.12. Immunofluorescence Study
2.13. Statistical Analysis
3. Results
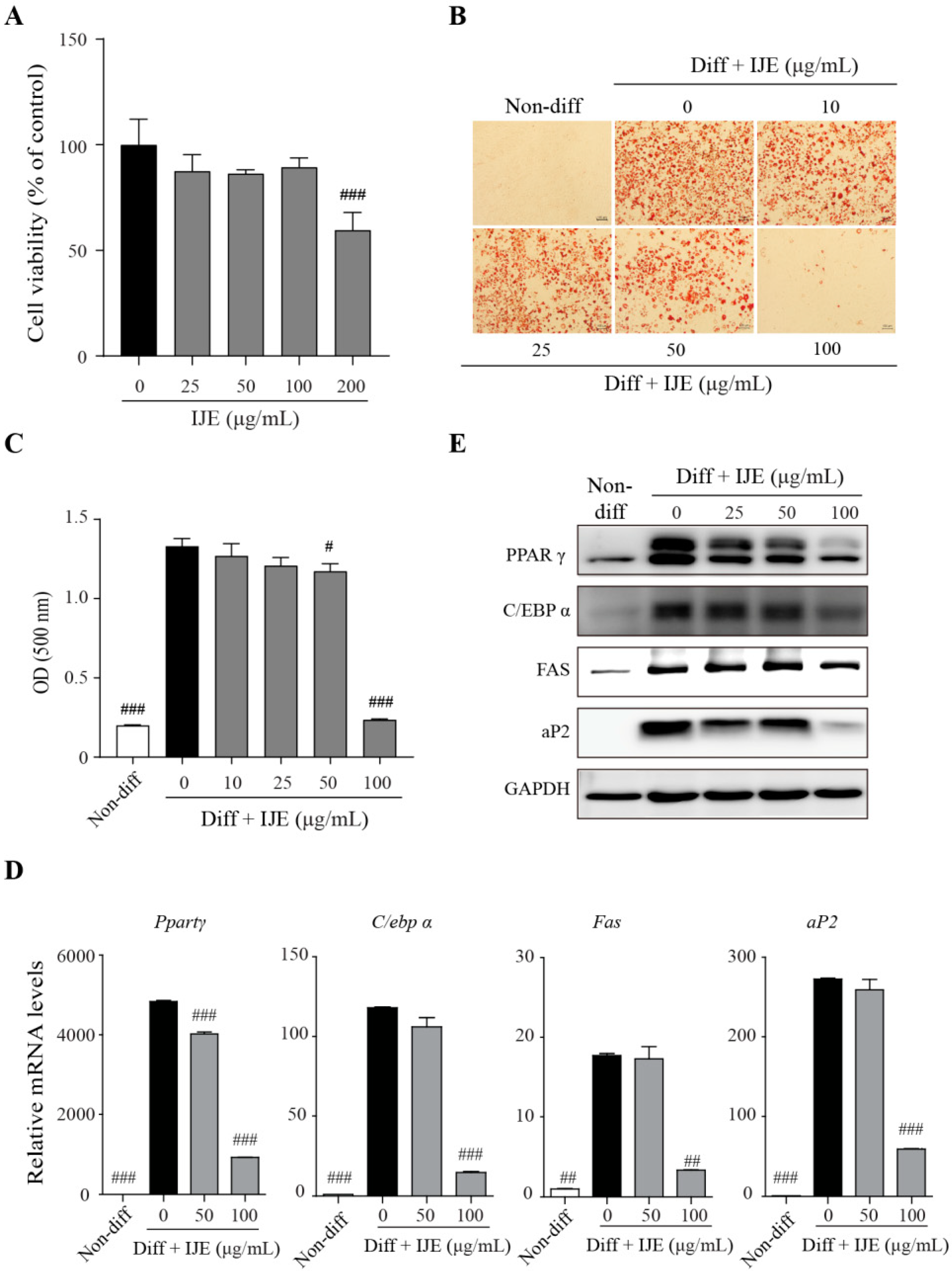
3.1. IJE Inhibits Adipogenic Differentiation of 3T3-L1 Cells
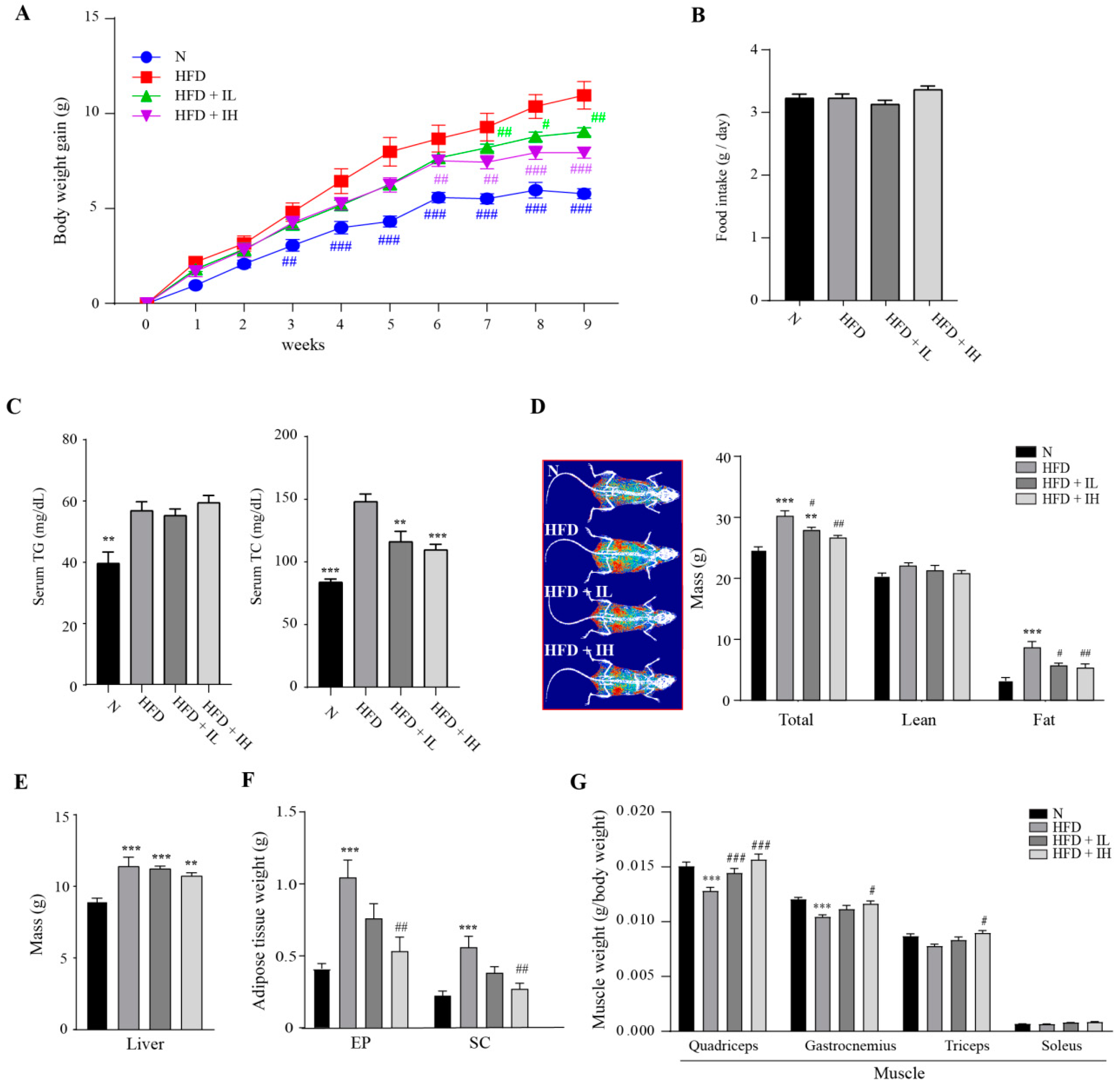
3.2. IJE Prevents Increase in Body Weight
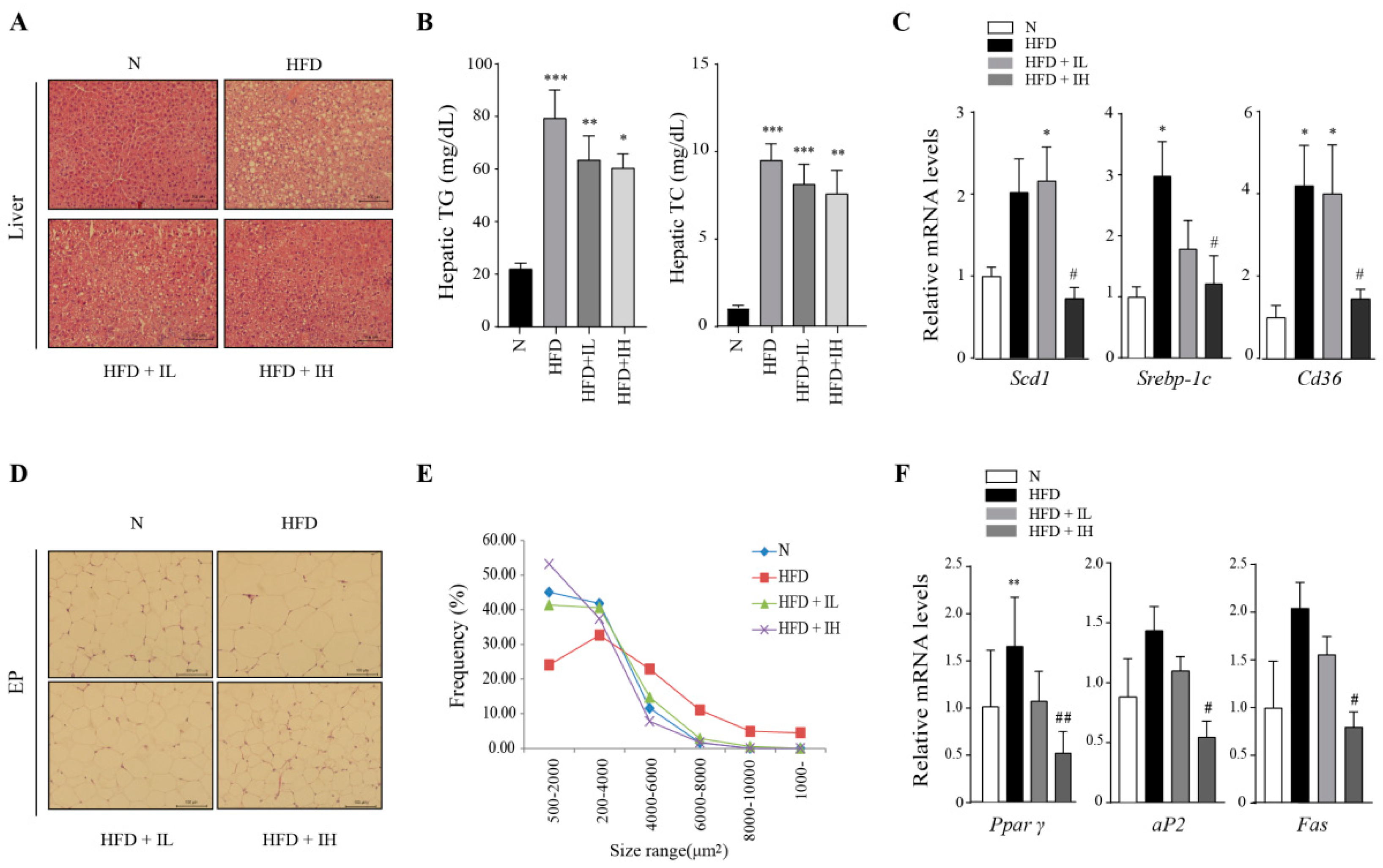
3.3. IJE Improves Abnormal Lipid Accumulation in the Liver and Adipose Tissue
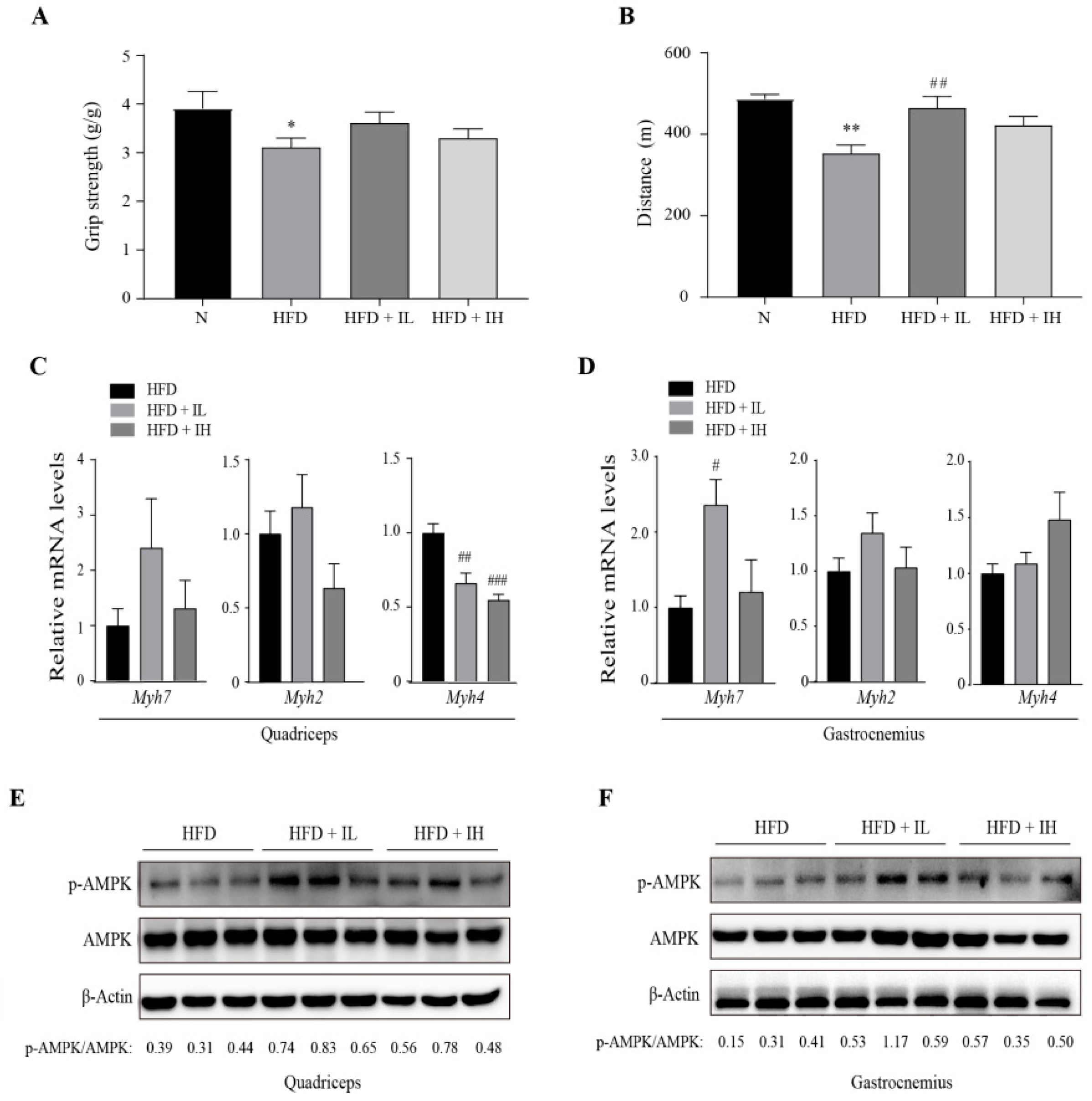
3.4. IJE Enhances Muscle Endurance Capacity
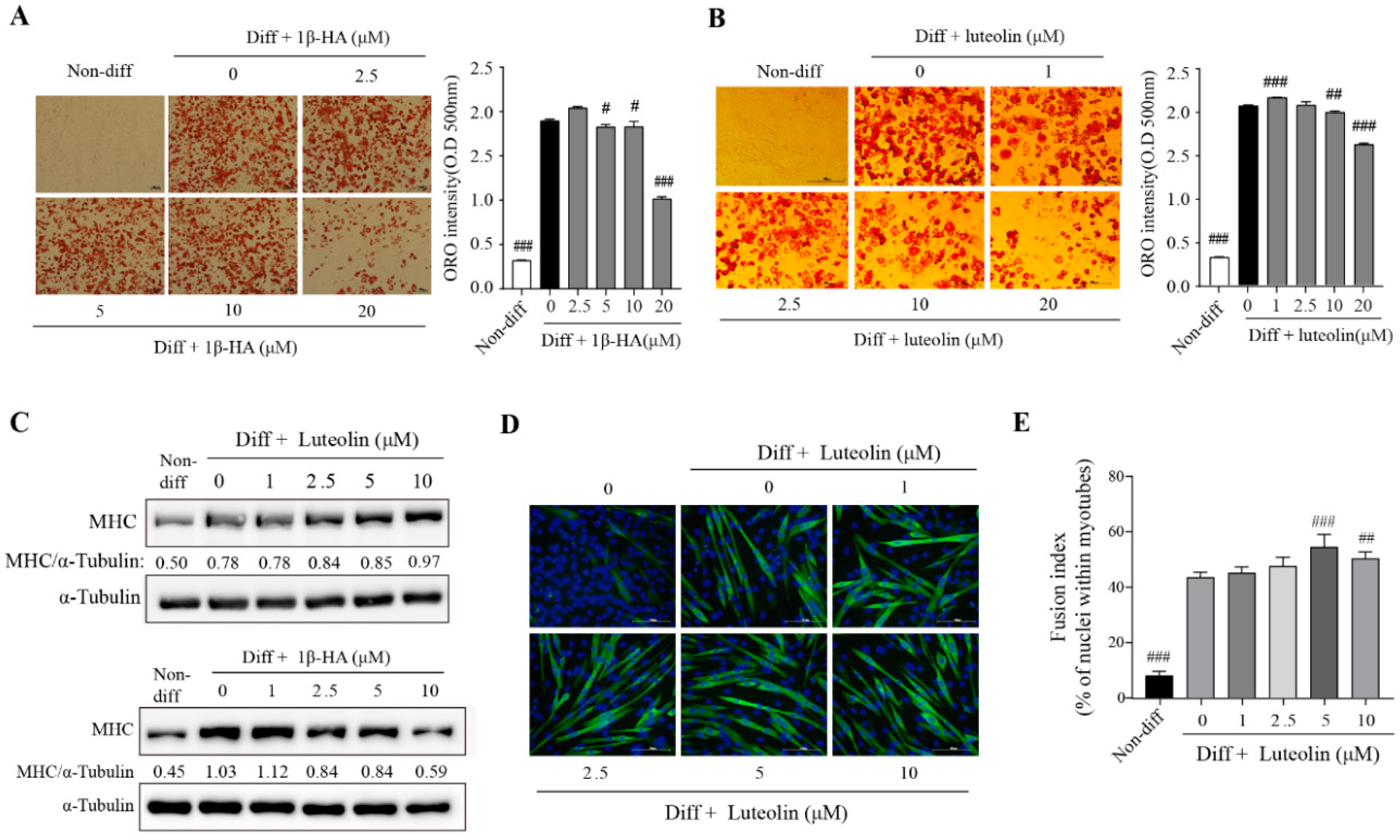
3.5. Effects of IJE Compounds, Luteolin and 1β-Hydroxyalantolactone (1β-HA), on Adipogenic Differentiation and Myogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seca, A.M.; Pinto, D.C.; Silva, A.M. Metabolomic Profile of the Genus Inula. Chem. Biodivers. 2015, 12, 859–906. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zeng, Q.; Ren, J.; Qin, J.; Zhang, S.; Shen, Y.; Zhu, J.; Zhang, F.; Chang, R.; Zhu, Y.; et al. Sesquiterpene lactones from Inula falconeri, a plant endemic to the Himalayas, as potential anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 5408–5415. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Qin, J.J.; Cheng, X.R.; Shen, Y.H.; Shan, L.; Jin, H.Z.; Zhang, W.D. Inula sesquiterpenoids: Structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs 2014, 23, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Chan, B.C.; Kwok, H.F.; Wong, Y.L.; Leung, H.W.; Ji, C.J.; Fung, K.P.; Leung, P.C.; Tan, N.H.; Lau, C.B. Anti-angiogenesis and immunomodulatory activities of an anti-tumor sesquiterpene bigelovin isolated from Inula helianthus-aquatica. Eur. J. Med. Chem. 2013, 59, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Jin, M.; Yang, J.H.; Li, X.; Chao, G.H.; Park, H.H.; Park, Y.N.; Son, J.K.; Lee, E.; et al. Inula japonica extract inhibits mast cell-mediated allergic reaction and mast cell activation. J. Ethnopharmacol. 2012, 143, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.J.; Yang, M.; Ren, J.W. Anti-diabetic and hypolipidemic effects of aqueous-extract from the flower of Inula japonica in alloxan-induced diabetic mice. Biol. Pharm. Bull. 2006, 29, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.J.; Zhang, Y.; Diao, Y.L.; Qu, W.S.; Zhao, X.N. Effect of an antidiabetic polysaccharide from Inula japonica on constipation in normal and two models of experimental constipated mice. Phytother. Res. 2010, 24, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Diao, Y.; Wang, C.; Qu, W.; Zhao, X.; Ma, H.; Shan, J.; Sun, G. Structural characters and protecting beta-cells of a polysaccharide from flowers of Inula japonica. Int. J. Biol. Macromol. 2017, 101, 16–23. [Google Scholar] [CrossRef]
- Wu, M.; Li, T.; Chen, L.; Peng, S.; Liao, W.; Bai, R.; Zhao, X.; Yang, H.; Wu, C.; Zeng, H.; et al. Essential oils from Inula japonica and Angelicae dahuricae enhance sensitivity of MCF-7/ADR breast cancer cells to doxorubicin via multiple mechanisms. J. Ethnopharmacol. 2016, 180, 18–27. [Google Scholar] [CrossRef]
- Qin, J.J.; Jin, H.Z.; Fu, J.J.; Hu, X.J.; Wang, Y.; Yan, S.K.; Zhang, W.D. Japonicones A-D, bioactive dimeric sesquiterpenes from Inula japonica Thunb. Bioorg. Med. Chem. Lett. 2009, 19, 710–713. [Google Scholar] [CrossRef]
- Qin, J.J.; Wang, L.Y.; Zhu, J.X.; Jin, H.Z.; Fu, J.J.; Liu, X.F.; Li, H.L.; Zhang, W.D. Neojaponicone A, a bioactive sesquiterpene lactone dimer with an unprecedented carbon skeleton from Inula japonica. Chem. Commun. 2011, 47, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Jin, H.Z.; Zhu, J.X.; Fu, J.J.; Zeng, Q.; Cheng, X.R.; Zhu, Y.; Shan, L.; Zhang, S.D.; Pan, Y.Y.; et al. New sesquiterpenes from Inula japonica Thunb. with their inhibitory activities against LPS-induced No production in RAW 264.7 macrophages. Tetrahedron 2010, 66, 9379–9388. [Google Scholar] [CrossRef]
- Jin, Q.; Lee, J.W.; Jang, H.; Choi, J.E.; Lee, D.; Hong, J.T.; Kim, Y.; Lee, M.K.; Hwang, B.Y. Sesquiterpenes from Inula japonica with Inhibitory Effects on Nitric Oxide Production in Murine Macrophage RAW 264.7 Cells. J. Nat. Prod. 2016, 79, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Ding, L.F.; Tu, W.C.; Yang, H.; Su, J.; Peng, L.Y.; Li, Y.; Zhao, Q.S. Bioactive sesquiterpenoids from the flowers of Inula japonica. Phytochemistry 2016, 129, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Piao, D.; Kim, T.; Zhang, H.Y.; Choi, H.G.; Lee, C.S.; Choi, H.J.; Chang, H.W.; Woo, M.H.; Son, J.K. DNA Topoisomerase Inhibitory Activity of Constituents from the Flowers of Inula japonica. Chem. Pharm. Bull. 2016, 64, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, D.H.; Ahn, J.; Chung, W.J.; Jang, Y.J.; Seong, K.S.; Moon, J.H.; Ha, T.Y.; Jung, C.H. Pharmacokinetics, Tissue Distribution, and Anti-Lipogenic/Adipogenic Effects of Allyl-Isothiocyanate Metabolites. PLoS ONE 2015, 10, e0132151. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Nakamura, A. Lessons from Mouse Models of High-Fat Diet-Induced NAFLD. Int. J. Mol. Sci. 2013, 14. [Google Scholar] [CrossRef]
- Klitgaard, H.; Bergman, O.; Betto, R.; Salviati, G.; Schiaffino, S.; Clausen, T.; Saltin, B. Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflug. Arch. 1990, 416, 470–472. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Yin, L.; Jensen-Urstad, A.P.; Funai, K.; Coleman, T.; Baird, J.H.; El Ramahi, M.K.; Razani, B.; Song, H.; Fu-Hsu, F. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metab. 2012, 16, 189–201. [Google Scholar] [CrossRef]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef] [PubMed]
- Sishi, B.; Loos, B.; Ellis, B.; Smith, W.; du Toit, E.F.; Engelbrecht, A.M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 2011, 96, 179–193. [Google Scholar] [CrossRef]
- Cappel, D.A.; Lantier, L.; Palmisano, B.T.; Wasserman, D.H.; Stafford, J.M. CETP Expression Protects Female Mice from Obesity-Induced Decline in Exercise Capacity. PLoS ONE 2015, 10, e0136915. [Google Scholar] [CrossRef]
- Ferns, S.J.; Wehrmacher, W.H.; Serratto, M. Effects of obesity and gender on exercise capacity in urban children. Gend. Med. 2011, 8, 224–230. [Google Scholar] [CrossRef]
- Farooqui, A.A. Phytochemicals, Signal Transduction, and Neurological Disorders; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Cosan, D.; Soyocak, A.; Basaran, A.; Degirmenci, I.; Gunes, H.V. The effects of resveratrol and tannic acid on apoptosis in colon adenocarcinoma cell line. Saudi Med. J. 2009, 30, 191–195. [Google Scholar]
- López-Nicolás, J.M.; García-Carmona, F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV—Visible absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C. Effects of physical activity and inactivity on muscle fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef]
- Niederberger, E.; King, T.S.; Russe, O.Q.; Geisslinger, G. Activation of AMPK and its Impact on Exercise Capacity. Sports Med. 2015, 45, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Gao, S.; Cheng, J.; Li, Y.; Shan, L.; Hu, Z. 1beta-Hydroxyalantolactone, a sesquiterpene lactone from Inula japonica, attenuates atopic dermatitis-like skin lesions induced by 2,4-dinitrochlorobenzene in the mouse. Pharm. Biol. 2016, 54, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, S.H.; Kim, Y.S.; Ryu, S.Y.; Hwang, J.T.; Yang, H.J.; Kim, G.H.; Kwon, D.Y.; Kim, M.S. Luteolin inhibits adipogenic differentiation by regulating PPARgamma activation. BioFactors 2009, 35, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, B.; Xu, Y.; Meng, S.; Wang, Y.; Jiang, Y. Luteolin reduces cancer-induced skeletal and cardiac muscle atrophy in a Lewis lung cancer mouse model. Oncol. Rep. 2018, 40, 1129–1137. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| C/ebpα | CAAGAACAGCAACGAGTACCG | GTCACTGGTCAACTCCAGCAC |
| Pparγ | TCGCTGATGCACTGCCTATG | GAGAGGTCCACAGAGCTGATT |
| Fas | GGAGGTGGTGATAGCCGGTAT | TGGGTAATCCATAGAGCCCAG |
| aP2 | CCGCAGACGACAGGA | CTCATGCCCTTTCATAAACT |
| Cd36 | ATGGGCTGTGATCGGAACTG | GTCTTCCCAATAAGCATGTCTCC |
| Scd1 | TTCTTGCGATACACTCTGGTGC | CGGGATTGAATGTTCTTGTCGT |
| Srebp-1c | TGGATTGCACATTTGAAGACAT | GCCAGAGAAGCAGAAGAG |
| Myh7 | CTCAAGCTGCTCAGCAATCTATTT | GGAGCGCAAGTTTGTCATAAGT |
| Myh2 | AAGCGAAGAGTAAGGCTGTC | GTGATTGCTTGCAAAGGAAC |
| Myh4 | CACCTGGACGATGCTCTCAGA | GCTCTTGCTCGGCCACTCT |
| Rn18s | CTCAACACGGGAAACCTCAC | CGCTCCACCAACTAAGAACG |
| Actb | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC |
| Ingredient (g/kg) | N | HFD | HFD+IL | HFD+IH |
|---|---|---|---|---|
| Casein | 200 | 200 | 200 | 200 |
| Corn oil | 50 | 50 | 50 | 50 |
| IJE | 0 | 0 | 2.5 | 5 |
| Lard | 0 | 200 | 200 | 200 |
| Cholesterol | 0 | 5 | 5 | 5 |
| Corn starch | 350 | 145 | 145 | 145 |
| Sucrose | 300 | 300 | 300 | 300 |
| Cellulose | 50 | 50 | 50 | 50 |
| Mineral | 35 | 35 | 35 | 35 |
| Vitamin | 10 | 10 | 10 | 10 |
| Methionine | 3 | 3 | 3 | 3 |
| Choline bitartrate | 2 | 2 | 2 | 2 |
| No. | Molecular Weight | Molecular Formula | Molecular Weight [M+H]+ | Actual Mass | Mass Error | Retention Time (min) | Fragment (By. HMDB) |
|---|---|---|---|---|---|---|---|
| 1 | 265.1459 | C15H20O4 | 265.144 | 265.1427 | −1.3 | 4.5 | 249, 231, 163 |
| 2 | 250.3 | C15H22O3 | 251.1647 | 251.164 | −0.7 | 5.8 | 251, 235, 215, 205, 147 |
| 3 | 264.1362 | C15H24O3 | 265.1427 | 265.1427 | −1.3 | 6.9 | 247, 233, 201, 187, 173 |
| 4 | 252.173 | C15H24O3 | 253.1735 | 253.1735 | −6.9 | 7.7 | 235, 223, 211, 195, 183 |
| 5 | 286.0477 | C15H10O6 | 287.0572 | 287.0572 | 1.6 | 7.75 | 287, 255, 153 |
| 6 | 248.3 | C15H20O3 | 249.1487 | 249.1487 | −0.4 | 9.3 | 249, 231, 213, 203, 195 |
| 7 | 248.322 | C15H20O3 | 249.1487 | 249.1487 | −0.4 | 9.9 | 233, 231, 215, 213, 143 |
| 8 | 308.16 | C17H24O5 | 309.1702 | 309.1738 | 3.6 | 13.6 | 291, 275, 185, 145 |
| Compounds | IJE (mg/g) | R2 | Linear Rang (μg/mL) | LOQ (μg/mL) | LOD (μg/mL) |
|---|---|---|---|---|---|
| Luteolin | 2.71 ± 0.35 | 0.9969 | 0.5–10 | 0.28 | 0.08 |
| 1β-hydroxyalantolactone | 1.04 ± 0.01 | 0.999 | 0.5–10 | 1.08 | 0.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Lee, D.-H.; Kim, M.J.; Ahn, J.; Jang, Y.-J.; Ha, T.-Y.; Jung, C.H. Inula japonica Thunb. Flower Ethanol Extract Improves Obesity and Exercise Endurance in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 17. https://doi.org/10.3390/nu11010017
Park S-H, Lee D-H, Kim MJ, Ahn J, Jang Y-J, Ha T-Y, Jung CH. Inula japonica Thunb. Flower Ethanol Extract Improves Obesity and Exercise Endurance in Mice Fed a High-Fat Diet. Nutrients. 2019; 11(1):17. https://doi.org/10.3390/nu11010017
Chicago/Turabian StylePark, So-Hyun, Da-Hye Lee, Min Jung Kim, Jiyun Ahn, Young-Jin Jang, Tae-Youl Ha, and Chang Hwa Jung. 2019. "Inula japonica Thunb. Flower Ethanol Extract Improves Obesity and Exercise Endurance in Mice Fed a High-Fat Diet" Nutrients 11, no. 1: 17. https://doi.org/10.3390/nu11010017
APA StylePark, S.-H., Lee, D.-H., Kim, M. J., Ahn, J., Jang, Y.-J., Ha, T.-Y., & Jung, C. H. (2019). Inula japonica Thunb. Flower Ethanol Extract Improves Obesity and Exercise Endurance in Mice Fed a High-Fat Diet. Nutrients, 11(1), 17. https://doi.org/10.3390/nu11010017




