Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Antibodies
2.2. Animals
2.3. Treatment
2.4. Myocardial Tolerance to Ischemia-Reperfusion Injury
2.4.1. Experimental Protocol
2.4.2. Myocardial Function
2.4.3. Myocardial Energy Metabolism
31P Magnetic Resonance Spectroscopy (MRS)
Biochemical Analyses in Freeze-Clamped Heart
2.4.4. Expression of Proteins Involved in NO Pathway
2.4.5. Oxidative Stress
2.5. Statistical Analyses
3. Results
3.1. Effect of Resveratrol on Physiological Parameters
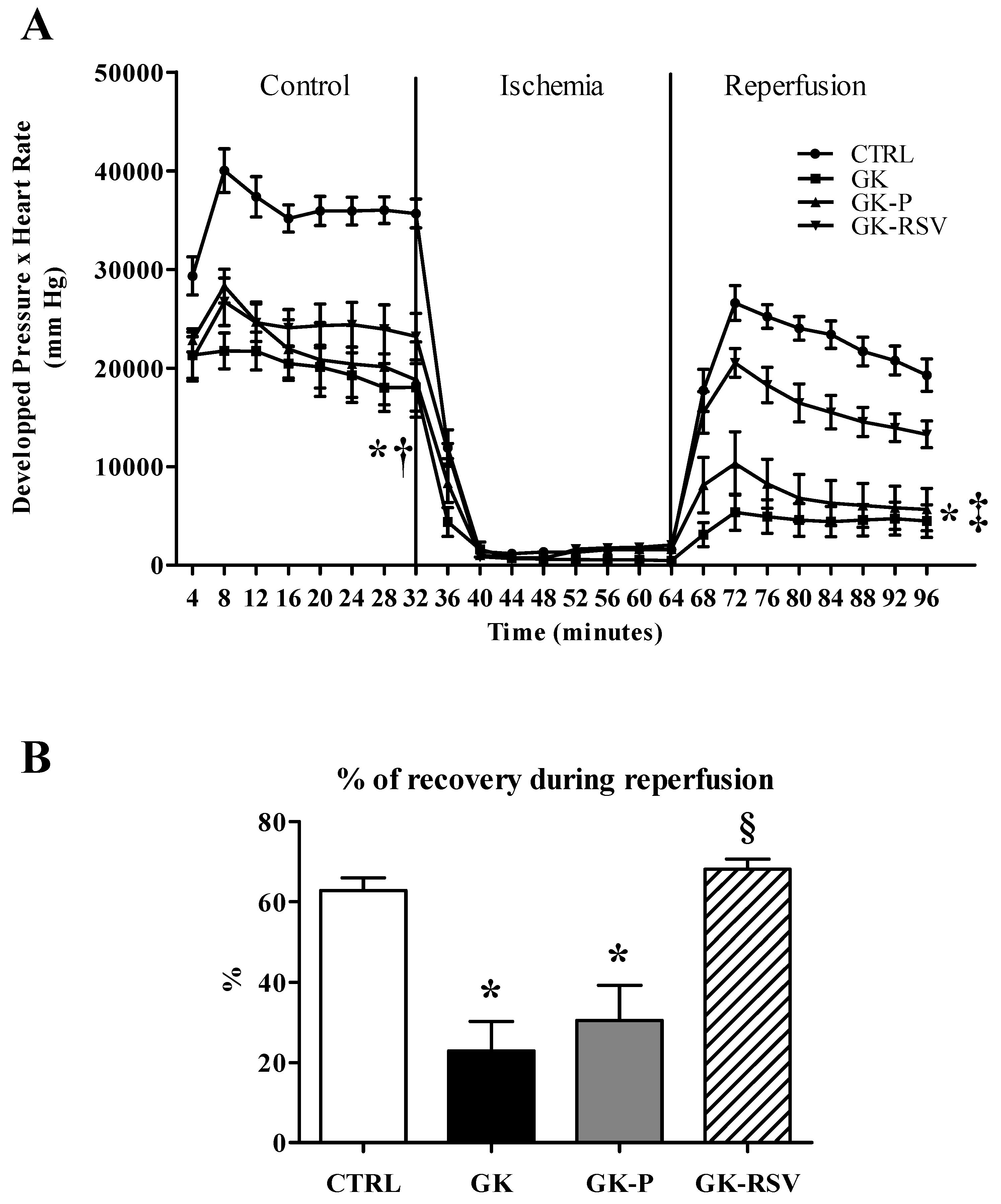
3.2. Effect of Resveratrol on Tolerance to Ischemia-Reperfusion Injury
3.2.1. Myocardial Function
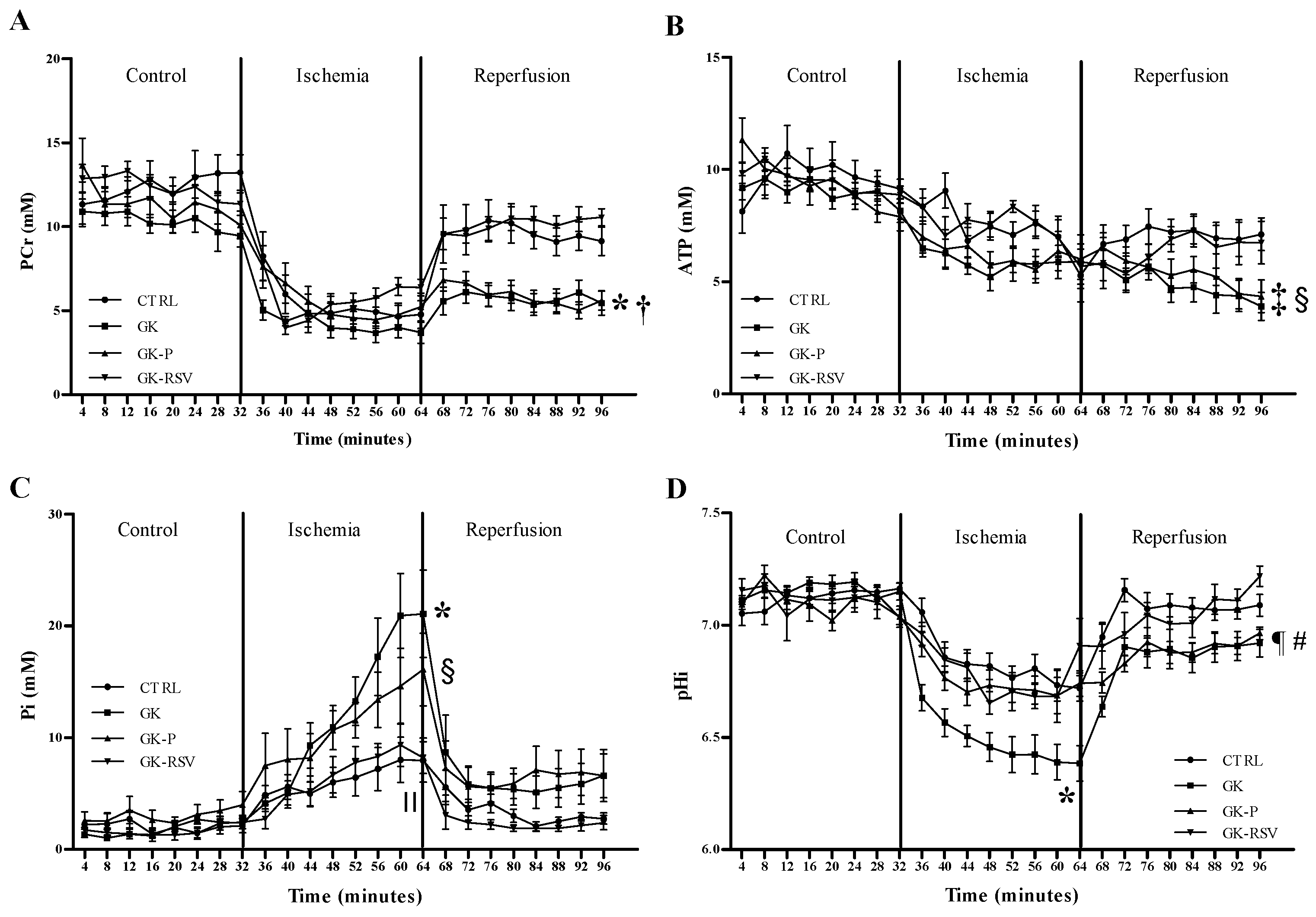
3.2.2. Myocardial Energy Metabolism
31P MRS
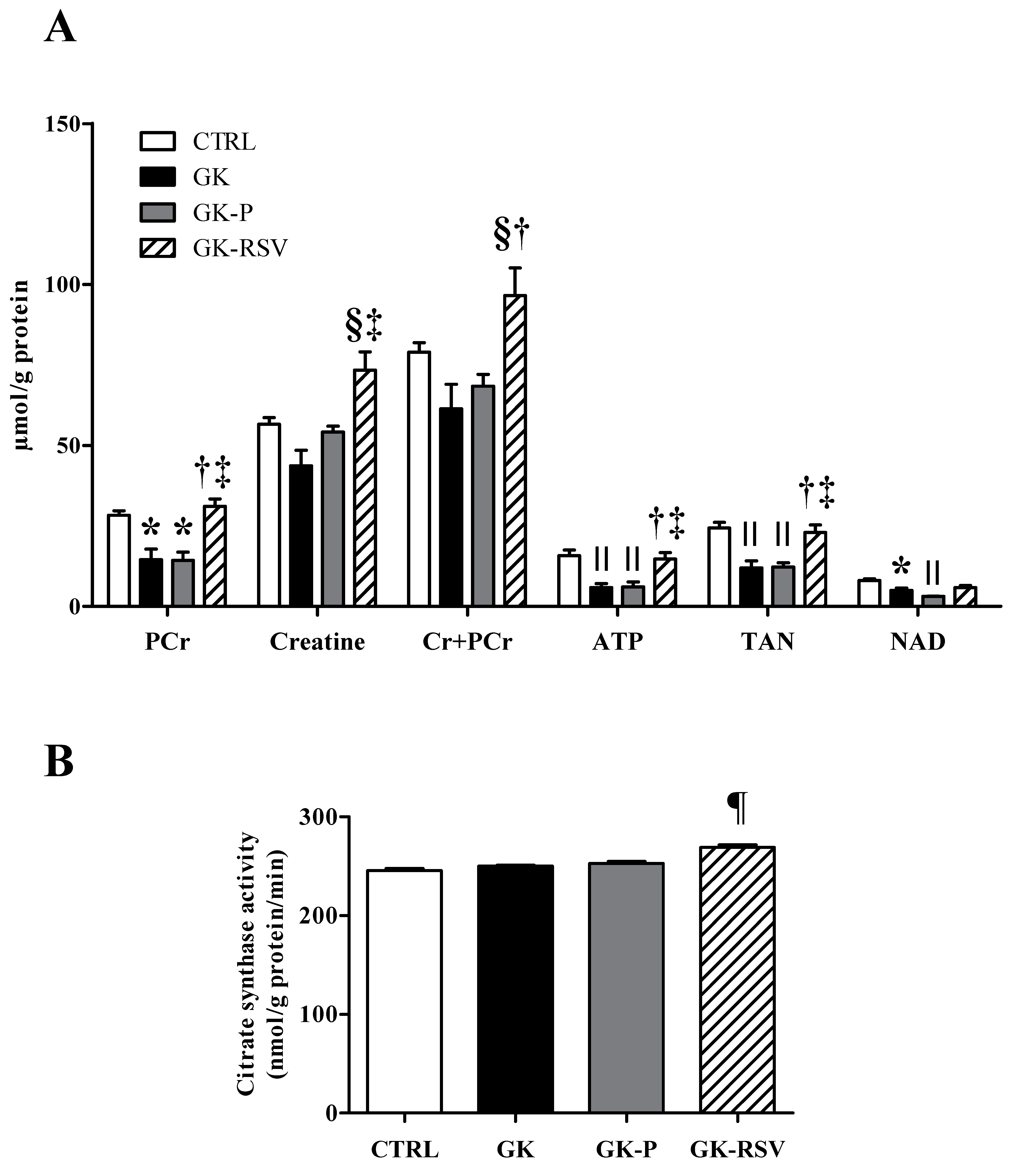
Biochemical Analysis in Freeze-Clamped Hearts
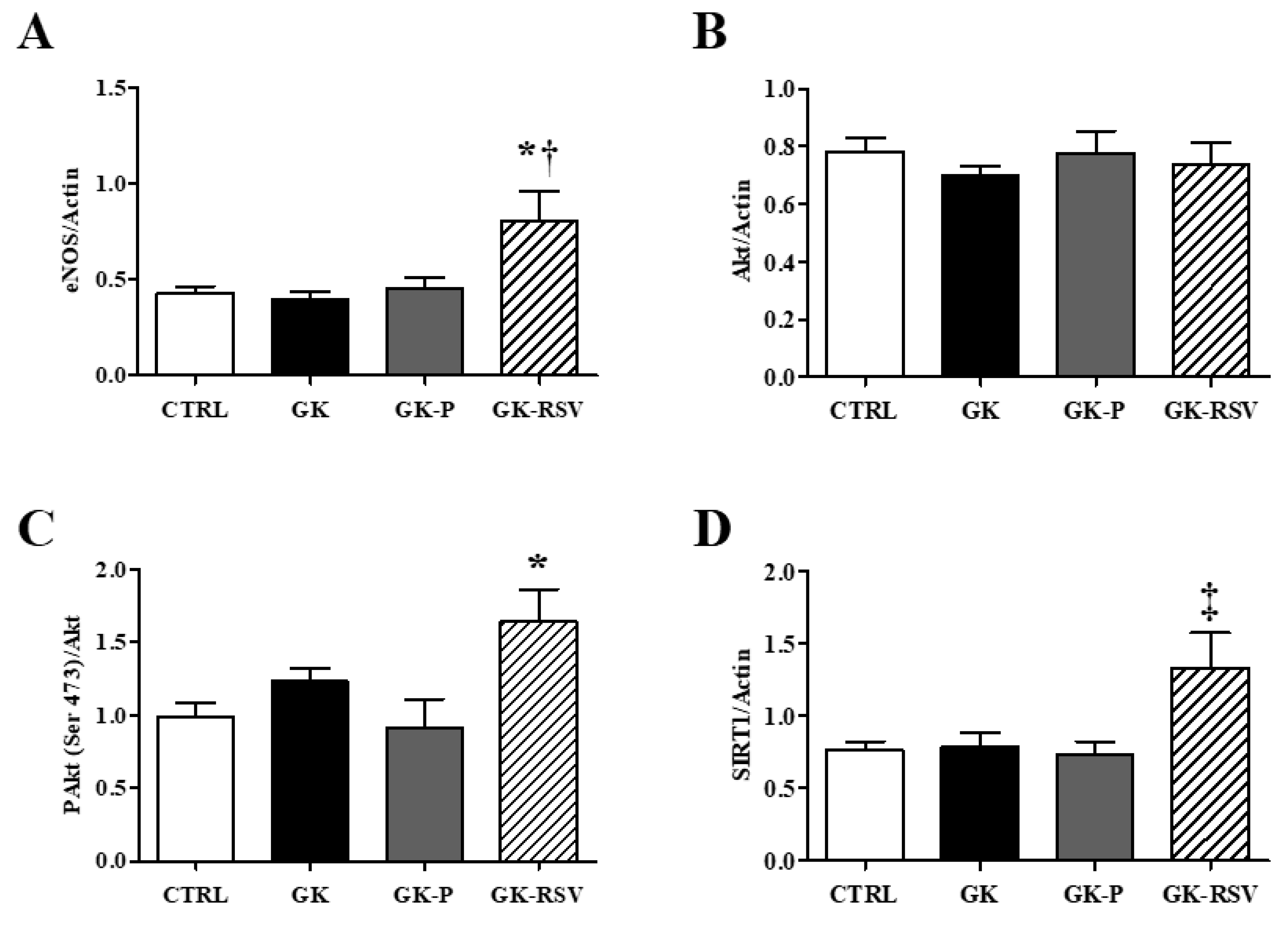
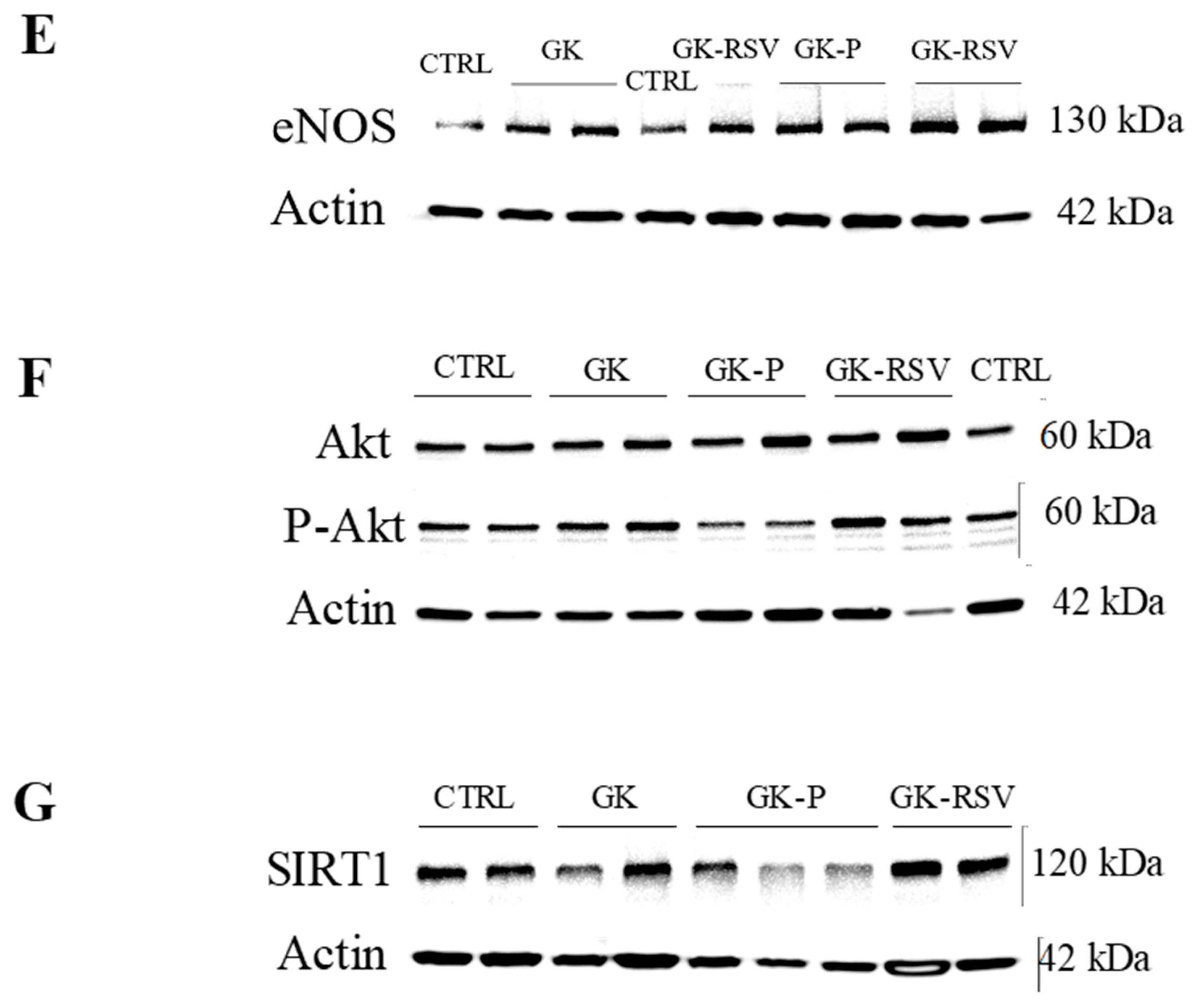
3.2.3. Coronary Flow and Expression of Proteins Involved in NO Pathway
Oxidative Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014, 57, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Regensteiner, J.G.; Golden, S.; Huebschmann, A.G.; Barrett-Connor, E.; Chang, A.Y.; Chyun, D.; Fox, C.S.; Kim, C.; Mehta, N.; Reckelhoff, J.F.; et al. Sex Differences in the Cardiovascular Consequences of Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 2424–2447. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Papacosta, O.; Lawlor, D.A.; Whincup, P.H.; Lowe, G.D.; Ebrahim, S.; Sattar, N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012, 55, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Jousilahti, P.; Qiao, Q.; Katoh, S.; Tuomilehto, J. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia 2005, 48, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Desrois, M.; Lan, C.; Movassat, J.; Bernard, M. Reduced up-regulation of the nitric oxide pathway and impaired endothelial and smooth muscle functions in the female type 2 diabetic goto-kakizaki rat heart. Nutr. Metab. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Kaul, S.; Gerstein, H.C.; Holman, R.R.; Zinman, B.; Skyler, J.S.; Green, J.B.; Buse, J.B.; Inzucchi, S.E.; Leiter, L.A.; et al. Cardiovascular Outcomes Trials in Type 2 Diabetes: Where Do We Go From Here? Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care 2018, 41, 14–31. [Google Scholar] [CrossRef]
- Selvaraju, V.; Joshi, M.; Kotha, S.R.; Parinandi, N.L.; Maulik, N. Resveratrol Emerges as a Miracle Cardioprotective Phytochemical Polyphenol and Nutraceutical. In Cardiovascular Diseases: Nutritional and Therapeutic Interventions; CRC Press: Boca Raton, FL, USA, 2013; pp. 401–420. [Google Scholar]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef]
- Luciano, T.F.; Marques, S.D.O.; Pieri, B.L.D.S.; Souza, D.R.D.; Lira, F.S.D.; Souza, C.T.D. Resveratrol reduces chronic inflammation and improves insulin action in the myocardium of high-fat diet-induced obese rats. Rev. Nutr. 2014, 27, 151–159. [Google Scholar] [CrossRef]
- Su, H.C.; Hung, L.M.; Chen, J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef]
- Do, G.M.; Kwon, E.Y.; Kim, H.J.; Jeon, S.M.; Ha, T.Y.; Park, T.; Choi, M.S. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem. Biophys. Res. Commun. 2008, 374, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1alpha deacetylation. Acta Pharmacol. Sin. 2018, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Pharmacological prevention of eNOS uncoupling. Curr. Pharm. Des. 2014, 20, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Silan, C. The effects of chronic resveratrol treatment on vascular responsiveness of streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2008, 31, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kaga, S.; Zhan, L.; Bagchi, D.; Das, D.K.; Bertelli, A.; Maulik, N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell. Biochem. Biophys. 2006, 44, 43–49. [Google Scholar] [CrossRef]
- Lin, J.F.; Lin, S.M.; Chih, C.L.; Nien, M.W.; Su, H.H.; Hu, B.R.; Huang, S.S.; Tsai, S.K. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 2008, 83, 313–317. [Google Scholar] [CrossRef]
- Bagul, P.K.; Banerjee, S.K. Application of resveratrol in diabetes: Rationale, strategies and challenges. Curr. Mol. Med. 2015, 15, 312–330. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-beta/Smad3 pathway. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H424–H434. [Google Scholar] [CrossRef]
- Meng, Z.; Jing, H.; Gan, L.; Li, H.; Luo, B. Resveratrol attenuated estrogen-deficient-induced cardiac dysfunction: Role of AMPK, SIRT1, and mitochondrial function. Am. J. Transl. Res. 2016, 8, 2641–2649. [Google Scholar]
- Shah, A.; Reyes, L.M.; Morton, J.S.; Fung, D.; Schneider, J.; Davidge, S.T. Effect of resveratrol on metabolic and cardiovascular function in male and female adult offspring exposed to prenatal hypoxia and a high-fat diet. J. Physiol. 2016, 594, 1465–1482. [Google Scholar] [CrossRef]
- Rocha, K.K.; Souza, G.A.; Ebaid, G.X.; Seiva, F.R.; Cataneo, A.C.; Novelli, E.L. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem. Toxicol. 2009, 47, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.I.; Lekli, I.; Mukherjee, S.; Das, M.; Bertelli, A.A.A.; Das, D.K. Does White Wine Qualify for French Paradox? Comparison of the Cardioprotective Effects of Red and White Wines and Their Constituents: Resveratrol, Tyrosol, and Hydroxytyrosol (Retraction of vol 56, pg 9362, 2008). J. Agric. Food Chem. 2012, 60, 2767. [Google Scholar] [CrossRef] [PubMed]
- Portha, B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab. Res. Rev. 2005, 21, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.; Willoughby, D. Alcohol consumption: The good, the bad, and the indifferent. Appl. Physiol. Nutr. Metab. 2008, 33, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Desrois, M.; Clarke, K.; Lan, C.; Dalmasso, C.; Cole, M.; Portha, B.; Cozzone, P.J.; Bernard, M. Upregulation of eNOS and unchanged energy metabolism in increased susceptibility of the aging type 2 diabetic GK rat heart to ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1679–H1686. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.F. Fatty acids and the risk of death during acute myocardial ischaemia. Clin. Sci. 2015, 128, 349–355. [Google Scholar] [CrossRef]
- Desrois, M.; Sciaky, M.; Lan, C.; Cozzone, P.J.; Bernard, M. L-arginine during long-term ischemia: Effects on cardiac function, energetic metabolism and endothelial damage. J. Heart Lung Transpl. 2000, 19, 367–376. [Google Scholar] [CrossRef]
- Lazzarino, G.; Nuutinen, M.; Tavazzi, B.; Di Pierro, D.; Giardina, B. A method for preparing freeze-clamped tissue samples for metabolite analyses. Anal. Biochem. 1989, 181, 239–241. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Portha, B.; Giroix, M.H.; Tourrel-Cuzin, C.; Le-Stunff, H.; Movassat, J. The GK rat: A prototype for the study of non-overweight type 2 diabetes. Methods Mol. Biol. 2012, 933, 125–159. [Google Scholar] [CrossRef] [PubMed]
- Desrois, M.; Sidell, R.J.; Gauguier, D.; King, L.M.; Radda, G.K.; Clarke, K. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc. Res. 2004, 61, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.A.; Qureshi, M.A.; Sydorenko, V.; Parekh, K.; Jayaprakash, P.; Iqbal, T.; Singh, J.; Oz, M.; Adrian, T.E.; Howarth, F.C. Effects of exercise training on excitation-contraction coupling and related mRNA expression in hearts of Goto-Kakizaki type 2 diabetic rats. Mol. Cell. Biochem. 2013, 380, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Howarth, F.C.; Qureshi, M.A.; Jayaprakash, P.; Parekh, K.; Oz, M.; Dobrzynski, H.; Adrian, T.E. The Pattern of mRNA Expression Is Changed in Sinoatrial Node from Goto-Kakizaki Type 2 Diabetic Rat Heart. J. Diabetes Res. 2018, 2018, 8454078. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.P.; Chu, L.M.; Burgess, T.A.; Feng, J.; Han, Y.; Nezafat, R.P.; Leber, M.P.; Laham, R.J.; Manning, W.J.; Sellke, F.W. Resveratrol Preserves Myocardial Function and Perfusion in Remote Nonischemic Myocardium in a Swine Model of Metabolic Syndrome. J. Am. College Surg. 2012, 215, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ninh, V.K.; El Hajj, E.C.; Mouton, A.J.; El Hajj, M.C.; Gilpin, N.W.; Gardner, J.D. Chronic Ethanol Administration Prevents Compensatory Cardiac Hypertrophy in Pressure Overload. Alcohol. Clin. Exp. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, M.; Diamond, I.; Weiner, M.W.; Camacho, S.A.; Figueredo, V.M. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 1997, 94, 3235–3239. [Google Scholar] [CrossRef]
- Montaigne, D.; Marechal, X.; Coisne, A.; Debry, N.; Modine, T.; Fayad, G.; Potelle, C.; El Arid, J.M.; Mouton, S.; Sebti, Y.; et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 2014, 130, 554–564. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Banuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Billimoria, F.R.; Katyare, S.S.; Patel, S.P. Insulin status differentially affects energy transduction in cardiac mitochondria from male and female rats. Diabetes Obes. Metab. 2006, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; Geny, B.; Laakso, M.; Puigserver, P.; Auwerx, J. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Thor, D.; Han, X.; Anderson, L.; Rahimian, R. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: A shift in the relative importance of EDRFs. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1183–H1198. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.P.; Hsu, S.C.; Li, D.E.; Chen, K.H.; Kuo, C.Y.; Hung, L.M. Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Morgan, B.; Potter, B.J.; Ma, L.; Dellsperger, K.C.; Ungvari, Z.; Zhang, C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: In vivo demonstration with magnetic resonance imaging. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H985–H994. [Google Scholar] [CrossRef]
- Brunner, F.; Maier, R.; Andrew, P.; Wolkart, G.; Zechner, R.; Mayer, B. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Cardiovasc. Res. 2003, 57, 55–62. [Google Scholar] [CrossRef]
- Dao, T.M.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthelemy, S.; Barra, Y.; et al. Resveratrol increases glucose induced GLP-1 secretion in mice: A mechanism which contributes to the glycemic control. PLoS ONE 2011, 6, e20700. [Google Scholar] [CrossRef]
- Kong, W.; Chen, L.L.; Zheng, J.; Zhang, H.H.; Hu, X.; Zeng, T.S.; Hu, D. Resveratrol supplementation restores high-fat diet-induced insulin secretion dysfunction by increasing mitochondrial function in islet. Exp. Biol. Med. 2015, 240, 220–229. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Tuncay, E.; Vassort, G. Resveratrol and diabetic cardiac function: Focus on recent in vitro and in vivo studies. J. Bioenerg. Biomembr. 2012, 44, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, N.K.; Thungapathra, M. Resveratrol regulates body weight in healthy and ovariectomized rats. Nutr. Metab. 2017, 14, 30. [Google Scholar] [CrossRef] [PubMed]

| CTRL | GK | GK-P | GK-RSV | |
|---|---|---|---|---|
| Glycemia (g/L) | 1.64 ± 0.07 | 2.46 ± 0.09 * | 2.52 ± 0.15 * | 2.49 ± 0.07 * |
| Free Fatty Acids (mM) | 0.21 ± 0.05 | 0.18 ± 0.02 | 0.17 ± 0.04 | 0.16 ± 0.04 |
| Body Weight (g) | 289.4 ± 6.6 | 284.6 ± 4.4 | 267.5 ± 5.7 | 269.7 ± 6.4 |
| Heart Weight (g) | 0.85 ± 0.02 | 1.05 ± 0.03 * † ‡ | 0.89 ± 0.02 | 0.91 ± 0.02 |
| (Ratio Heart/Body Weight) × 1000 | 2.95 ± 0.09 | 3.69 ± 0.06 * II ¶ | 3.32 ± 0.06 § | 3.38 ± 0.09 § |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fourny, N.; Lan, C.; Sérée, E.; Bernard, M.; Desrois, M. Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart. Nutrients 2019, 11, 105. https://doi.org/10.3390/nu11010105
Fourny N, Lan C, Sérée E, Bernard M, Desrois M. Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart. Nutrients. 2019; 11(1):105. https://doi.org/10.3390/nu11010105
Chicago/Turabian StyleFourny, Natacha, Carole Lan, Eric Sérée, Monique Bernard, and Martine Desrois. 2019. "Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart" Nutrients 11, no. 1: 105. https://doi.org/10.3390/nu11010105
APA StyleFourny, N., Lan, C., Sérée, E., Bernard, M., & Desrois, M. (2019). Protective Effect of Resveratrol against Ischemia-Reperfusion Injury via Enhanced High Energy Compounds and eNOS-SIRT1 Expression in Type 2 Diabetic Female Rat Heart. Nutrients, 11(1), 105. https://doi.org/10.3390/nu11010105




