1. Introduction
An epidemic of childhood obesity and the associated non-communicable diseases (NCD), such as diabetes and cardiovascular disease are of increasing international concern [
1]. Increasingly, data suggests the lifelong risk of NCD could be modified through early programming effects on obesity and adiposity [
2,
3]. It is evident that breastfed infants are at 15–20% reduced risk of obesity and obesity related disease later in life [
4,
5]. Whilst the protective mechanisms of breastfeeding are not fully understood [
6], the development of body composition (BC) in early life [
7], composition of human milk (HM) [
8,
9,
10], and infant breastfeeding patterns and behavior [
11,
12,
13] are all known to play an important role in the programming of these health outcomes.
An increased risk of obesity has been associated with both rapid weight gain [
14] and the elevated protein content in infant formulas [
9,
15]. Compared with 9 g/L in term HM (6–12 g/L, week 10/12) [
16], protein content ranged from 12 to 19 g/L in infant formulas and from 16 to 27 g/L in follow-up formulas [
15]. Thus, limiting protein intake from infant foods could be an effective strategy in reduction of childhood obesity, leading to the development of lower protein formulas to mimic growth rates of HM fed infants [
15]. HM is recognized as the best form of nutrition for optimal growth and development of the human infant and as such is species specific in composition. The stark compositional differences between HM and formula have been implicated in the differences in weight and BC between breastfed and formula-fed infants and decreased risk of later obesity for breastfed infants [
8,
9].
The protein content of HM, which is low yet highly bioavailable, appears to play a key role in infant growth [
9] and might provide a rationale for the reduced risk of being in rapid-growth trajectory [
17] and for the lower fat-free mass (FFM) and higher fat mass (FM) and percentage FM (%FM) of breastfed infants compared with formula-fed [
18] during early infancy. In term infants, the protein intakes from HM (three months, boys: 6.4 ± 1.2 g/day; girls: 5.8 ± 1.0 g/day) were found to be almost half that of formula (boys: 11.4 ± 2.0 g/day; girls: 10.5 ± 1.8 g/day), and at three and six months differences in protein intake and weight gain were associated with disproportionate gain in FM and FFM, resulting in %FM difference between the groups [
8]. These variations suggest a self-regulatory mechanism of milk intake (MI), which may be in part driven by HM components [
10] that are associated with feeding frequency (FFQ) [
19].
Despite MI and protein intakes being associated with breastfed infants’ weight and FFM gain [
8] very few comprehensive longitudinal HM protein intake studies are available through the exclusive breastfeeding and weaning periods. Also, there has been no investigation of the effect of either concentrations or daily intakes of specific HM fractions—such as casein and whey—on infant BC, yet these are highly variable between breastfeeding dyads. Daily intakes of HM components are the true reflection on what infant receives, unlike the concentrations, which can be misleading, especially if the milk intake is inadequate or if mixed feeding is present.
The protective role of HM and breastfeeding may be attributable to the effect of specific protein fractions on development of infant BC. Thus, the aim of this longitudinal study was to investigate relationships of concentrations and daily intakes of HM casein, whey and total protein with anthropometrics and BC of healthy term breastfed infants and their mothers during first 12 months postpartum. Further exploration of relationships of infant 24-h MI and FFQ with HM proteins was carried out.
2. Materials and Methods
2.1. Study Participants
Breastfed infants (
n = 20; 10 males, 10 females) of English-speaking, predominantly Caucasian, mothers of higher social-economic status from a developed country were recruited from the community, primarily from the West Australian branch of the Australian Breastfeeding Association. Inclusion criteria were: healthy singletons, gestational age ≥37 weeks, exclusively breastfed [
20] at 2 and 5 months, and maternal intention to breastfeed until 12 months. Exclusion criteria were: infant factors that could potentially influence growth and development of BC, maternal smoking, and low milk supply. All mothers provided written informed consent to participate in the study, which was approved by The University of Western Australia Human Research Ethics Committee (RA/1/4253, RA/4/1/2639) and registered with the Australian New Zealand Clinical Trials Registry (ACTRN12616000368437).
2.2. Study Session
Measurements were made when the infants were 2 and/or 5, 9, and 12 months of age. Participants visited our laboratory at King Edward Memorial Hospital for Women (Subiaco, Perth, WA, Australia) for up to four monitored breastfeeding sessions between March 2013 and September 2015. At each study session, the infant was weighed pre-feed, and then the mother breastfed her infant. Infant bioelectrical impedance spectroscopy (BIS) measurements were made pre-feed, unless impractical, then they were taken post-feed [
21]. Ultrasound skinfold and anthropometric measurements were made post-feed. Clothing was removed for the measurements except for a dry diaper and a sleeveless shirt.
Maternal weight, height, and BIS measurements were recorded. Small (1−2 mL) pre- and post-feed milk samples were collected into 5 mL polypropylene vials (Disposable Products, Adelaide, SA, Australia) from the breast/s that infant was fed from and samples were frozen at −20 °C for biochemical analysis. Current feeding frequency (FFQ) of the infants was self-reported by mothers.
2.3. Anthropometric Measurements
Infants weight was determined before breastfeeding using Medela Electronic Baby Weigh Scales (±2.0 g; Medela Inc., McHenry, IL, USA). Infant crown-heel length was measured once to the nearest 0.1 cm using non-stretch tape and a headpiece and a footpiece, both applied perpendicularly to the hard surface. Infant head circumference was measured with a non-stretch tape to the nearest 0.1 cm.
Maternal weight was measured using Seca electronic scales (±0.1 kg; Seca, Chino, CA, USA). Height was self-reported by participants or measured against a calibrated marked wall (accuracy ±0.1 cm).
Infant and maternal body mass index (BMI) were calculated as kg/m2.
2.4. Body Composition with Bioelectrical Impedance Spectroscopy
The methods for measuring maternal and infant BC with BIS using Impedimed SFB7 bioelectrical impedance analyser (ImpediMed, Brisbane, QLD, Australia) as well as age-specific equations used for calculations of infant BC parameters during this study have been published previously [
22]. The within participant coefficient of variation (CV) for maternal %FM was 0.21% [
23]. Within participant CV for infant resistance measurements at 50 kHz (R
50) was 1.5% [
21].
2.5. Ultrasound Skinfold Measurements
The method for measuring infant skinfolds using the Aplio XG ultrasound machine (Toshiba, Tokyo, Japan) with a 14-8 MHz transducer (PLT-1204BX) and sterile water-based ultrasonic gel (Parker Laboratories Inc., Fairfield, NJ, USA) as well as the equations for calculations of infant BC parameters during this study have been published previously [
22,
24]. Briefly, single ultrasound scans of four anatomical sites (biceps, subscapular, suprailiac and triceps) were performed on the left side of the body with minimal compression. At all tome points, infant BC was calculated with both, 2-skinfolds (US 2SF: triceps, subscapular) and 4-skinfolds (US 4SF: biceps, subscapular, suprailiac and triceps).
2.6. Body Composition Indices
The indices of height-normalized BC were calculated for mothers and infants: FM index (FMI) was calculated as FM/length
2, and FFM index (FFMI) was calculated as FFM/length
2; both expressed as kg/m
2 [
25].
2.7. 24-h Milk Intake and Feeding Frequency
Infant 24-h MI was measured by mothers using the 24-h milk production (MP) protocol, weighing infants at home with the Medela Electronic Baby Weigh Scales pre- and post each breastfeed during a 24-h period plus one breastfeeding, and recording amounts of HM (g) consumed by the infant (including expressed HM if any) [
26]. 24-h MI was determined as previously described with potential underestimation of 3–10% [
26] and FFQ (meals per 24-h) was recorded [
27]. 24-h MI was measured at three time points: between 2 and 5 (4.0 ± 1.3) months, when MI is shown to be stable [
27], and within 2 weeks of 9 (9.4 ± 0.3) and 12 (12.2 ± 0.4) months. Given that measuring 24-h MI is not always practical, particularly at the later stages of lactation, mothers were also asked to estimate how frequently the infant fed, and self-reported (SR) the typical time between the meals (e.g., each 2 h) during the week prior to the study session as a proxy measure of FFQ.
2.8. Calculated Daily Intakes of Human Milk Proteins
24-h MI values from the 24-h MP, and casein, whey and total protein concentrations (pooled pre-/post-feed) from samples taken at the study sessions were used for determination of calculated daily intakes (CDI). These CDI were considered representative of a typical daily intake due to the absence of significant short-term (weekly) [
28] and circadian [
29,
30] variations in HM protein concentrations during the established lactation.
2.9. Sample Preparation
Prior to further analysis, HM samples were thawed for 2 h at room temperature, mixed on Intelli-Mixer RM-2M (ELMI, Riga, Latvia) at 50 revolutions per min in “UU” mode for 15 s, then, after gentle inversion (three times), aliquoted into 1.5 mL tubes (Sarstedt, Numbrecht, Germany). Pre- and post-feed samples were pooled for measuring casein and whey and total protein. Milk samples were defatted by centrifugation at room temperature in a Beckman Microfuge 11 (Aberdon Enterprise Inc., Elk Grove Village, IL, USA) at 10,000×
g for 10 min and removing the fat layer by clipping it off together with the top of the tube [
31]. Skim HM was used for measuring protein concentrations. Standard assays were adapted for and carried out using a JANUS workstation (PerkinElmer, Inc., Waltham, MA, USA) and measured on EnSpire (PerkinElmer, Inc., Waltham, MA, USA).
2.10. Human Milk Fractions
Casein and whey proteins were separated by the method described by Kunz and Lonnerdal [
32] and Khan et al. [
30]. Protein concentrations (total protein, casein, and whey proteins) were measured using the Bradford Protein Assay adapted from Mitoulas et al. [
29]. Recovery of protein was 100.6 ± 5.2% (
n = 5) with a detection limit of 0.031 g/L and an inter-assay CV of 7.8% (
n = 18). Casein-whey ratio was calculated as follows:
2.11. Statistical Analyses
Data for this analysis came from the longitudinal study, the details of which have been described previously [
22]. Descriptive statistics are reported as mean ± standard deviation (SD) (range); model parameters as estimate ± SE (standard error).
During this longitudinal study infants were measured at four time points (2 and/or 5, 9 and 12 months). An approximate sample size was calculated using the ‘F tests–Linear multiple regression: Fixed model:
R2 increase’ option in G*Power [
33] as if this was a cross-sectional study with equal numbers at each time. Allowing four predictors (three for age comparisons), α = 0.05 and 14 participants (56 sample points = 14 participants × 4 time points) would give the study power of 0.80 to detect an effect size of 0.15. This approach was selected, as there is no closed form expression suitable for the calculation of sample sizes for this research design [
34], with the consideration that longitudinal study design is more powerful. Recruitment of participants at the 5-months point was introduced, as many mothers would not commit to a study that required breastfeeding to 12 months, when approached at 2 months. As a result, required number of participants was increased to 20 in order to maintain predicted power; this also addressed issues relating to missed visits. Missing data was dealt with using available case analysis.
The analyses for systematic differences in concentrations and CDI of adipokines at different months after birth used linear mixed model with age as effect factor and participant as a random factor. Differences between each month were analysed using general linear hypothesis tests (Tukey’s all pair comparisons).
Relationships between: (a) maternal BC and protein concentrations/CDI, and (b) protein concentrations/CDI and infant BC, (c) protein concentrations and breastfeeding parameters (24-h MI/FFQ), and (d) FFQ and CDI of proteins were analysed using linear mixed effects models. Each protein concentration/CDI or infant BC measure/index was considered separately as the response variable, and each model contained fixed effects of infant age (months), a predictor (maternal BC measure/index, protein concentration/CDI and breastfeeding parameters (24-h MI/FFQ)) and an interaction between infant age and predictor, as well as a random intercept per participant. If the interaction was not significant results were reported for the same model fitted without the interaction to assist in understanding the nature of the relationship between the predictor and outcome.
To investigate if there were differences by sex, relationships between infant characteristics and protein concentrations were also analysed using linear mixed effects models accounting for age and sex and an interaction between infant age and predictor, as well as a random intercept per participant.
Relationships between CDI of proteins measured between 2 and 5, and at 9 and 12 months after birth and changes (Δ) in infant BC and anthropometric parameters between the time points were analysed using linear regression models.
Owing to the large number of comparisons, a false discovery rate adjustment [
35] was performed on associated subgroupings of results to the interaction
p-value if it was less than 0.05 or to the main effect
p-value. The adjusted significance levels are reported in Results and Tables and set at the 5% level otherwise. Statistical analysis was performed in R 3.1.2 [
36]. Additional packages were used for linear mixed effects models (nlme, lme4, and car) [
37,
38,
39], intra-class correlations (irr) [
40], Tukey’s all pair comparisons (multcomp) [
41] and graphics (ggplot2) [
42].
4. Discussion
A dose–response relationship between breastfeeding duration and childhood obesity has been previously confirmed [
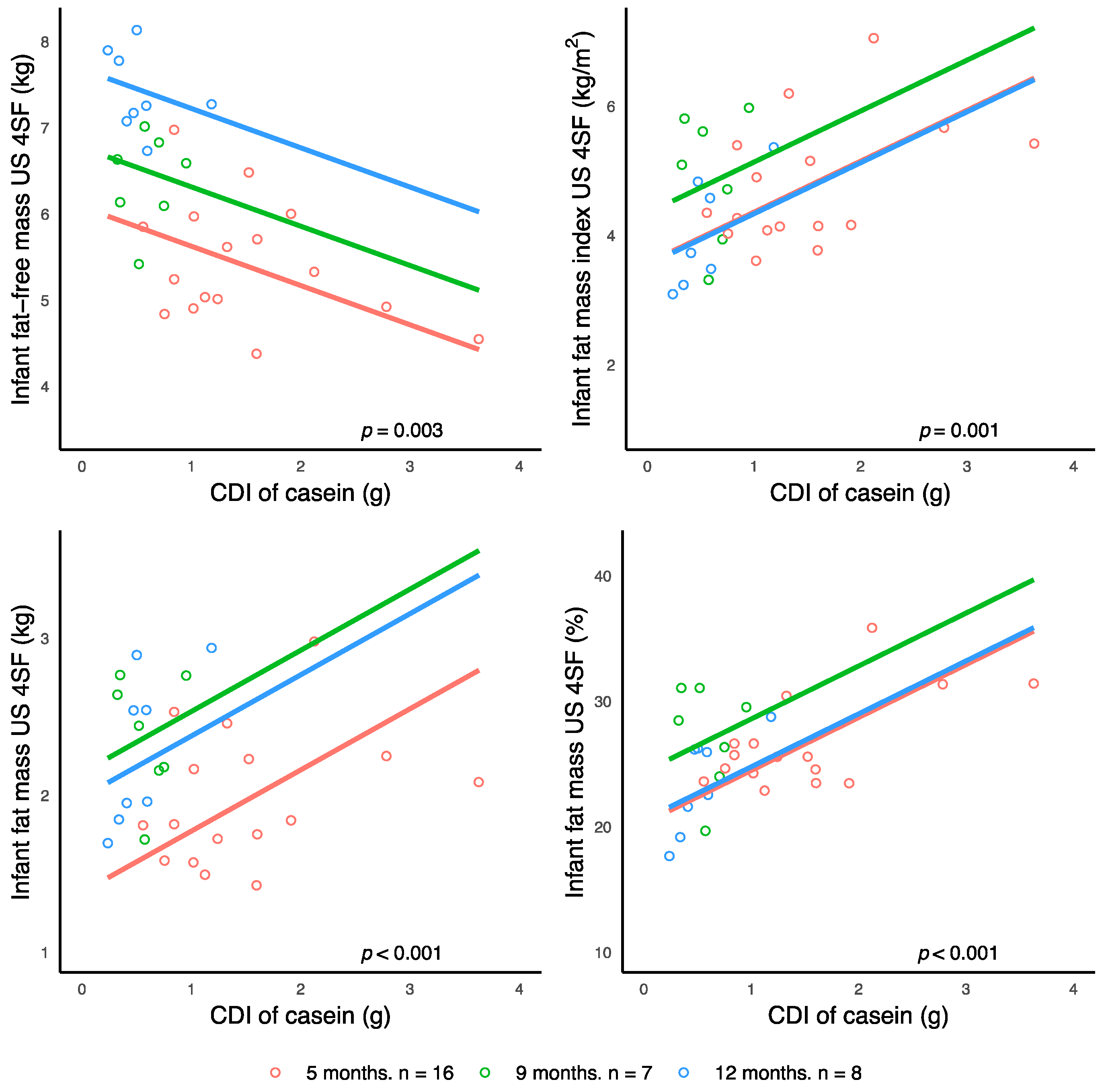
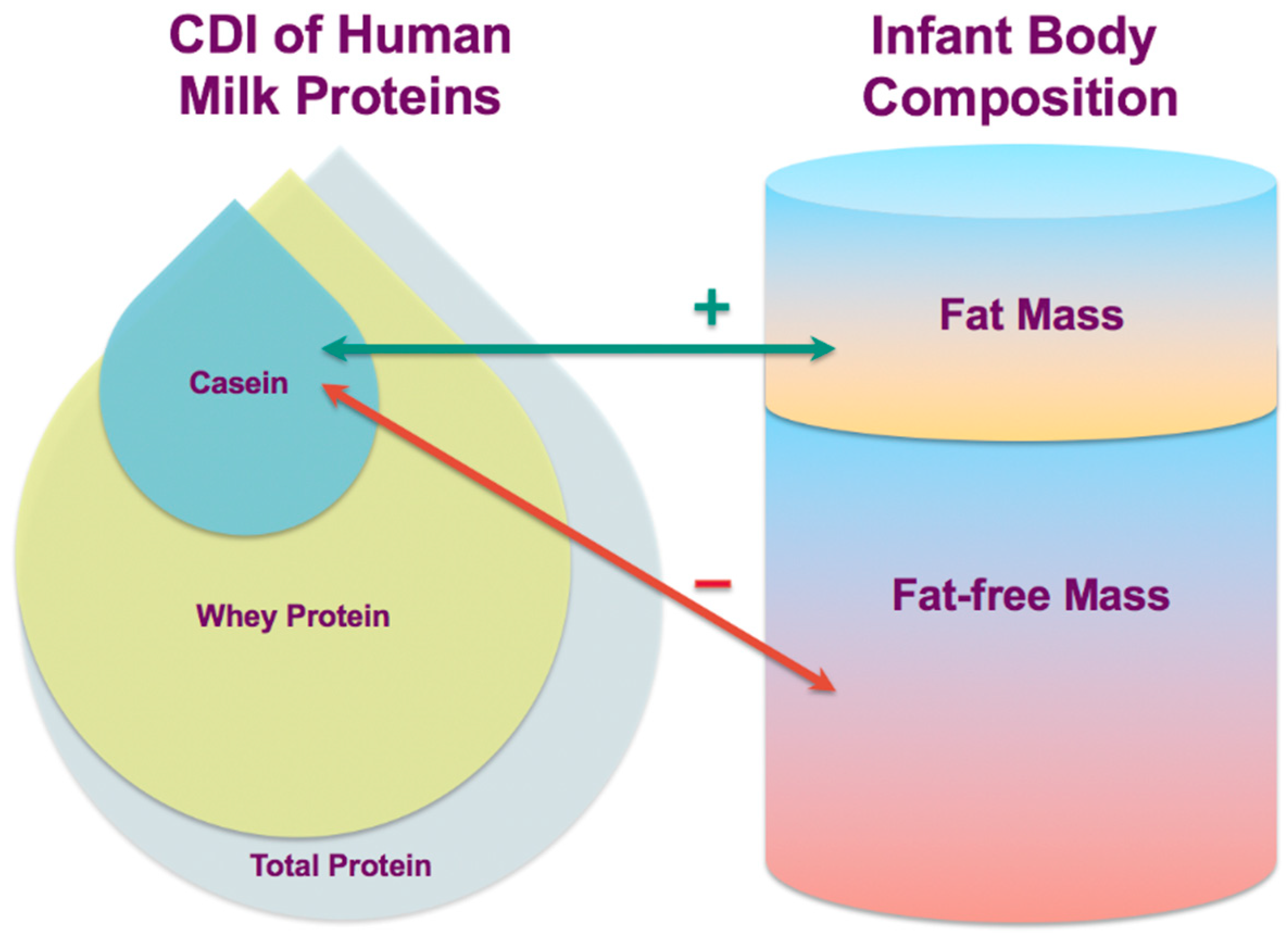
43] and this longitudinal study provides more knowledge on the complex mechanisms by which breastfeeding and HM may influence infant BC and confer some degree of protection from obesity. In this small proof-of-concept study, calculated daily intakes of HM casein have been associated with the development of infant BC and are differentially related to infant FM and FFM (
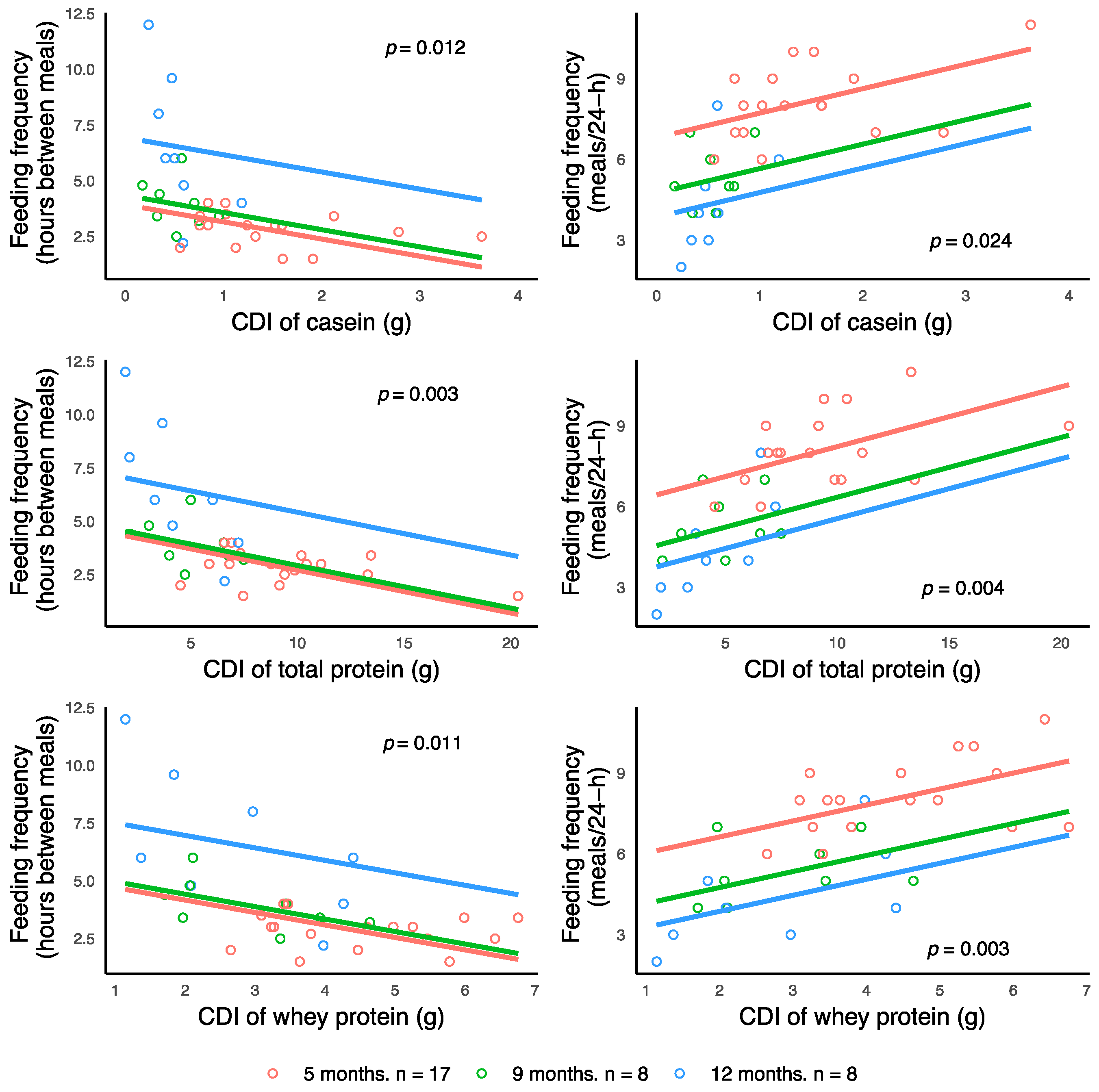
Figure 4). Furthermore, infant FFQ was associated with higher CDI of casein, whey and total protein, emphasizing the critical role of HM and breastfeeding in programming of infant appetite control and growth in the first year of life.
Within the normal developmental context of breastfeeding we found that concentrations of total protein (approximately 11 g/L) were not associated with infant anthropometrics or BC during first 12 months of lactation (
Section 3.4;
Table A2). However, in formula-fed infants high protein formulas (15–19 g/L) are related to accelerated growth trajectory with greater weight gain [
15], while breastfed infants display reduced lean body mass and increased adiposity during the early infancy compared to formula-fed [
18]. Also in contrast to our results, higher HM protein concentrations at four to eight weeks postpartum were related to higher infant BMI at 12 months but not weight gain or BC [
44]. These contrasting results are likely due to the differences in protein composition of formulas and shorter breastfeeding durations. Our study focused on women who breastfed for 12 months and therefore is more reflective of normative development of BC of the breastfed infant.
To account for variation in infant MI we calculated the CDI of total protein and found no association with infant anthropometrics or BC during first 12 months of lactation (
Section 3.5;
Table A3). Whilst volume of HM is known to be related to infant growth rate [
29,
45,
46], it has been shown that infants consuming higher daily intakes of protein at various times during first 12 months have higher weight and FFM [
8,
47,
48]. These studies however included formula-fed infants and concentrated on differences between breastfeeding and formula feeding by calculating combined protein intakes from HM/formula and solids. Our study showed multiple positive associations between CDI of total protein between two and five months and changes in infant BC, but statistical significance did not remain after correction for multiple comparisons (
Table A5). One recent study measured protein intakes from HM during the first three months in two groups of breastfed infants, with higher and lower weight gain [
10] and found protein intakes were not different between the groups. It therefore remains to be elucidated if total protein intake in breastfed infants does impact BC development and larger longitudinal trials are required to do this.
Notwithstanding the absence of infant dietary data, we found that higher CDI of HM casein are associated with both lower infant lean body mass and higher adiposity (
Section 3.5;
Table A3), with associations strengthening at the later months of lactation. Casein is present in HM at a very low concentrations, compared with other species [
49] and is not only a source of amino acids and trace elements (calcium and phosphorus) for the infant [
50] but also breaks down to the bioactive peptides that have an array of functions including antimicrobial, gastrointestinal, immuno-modulating and opioid effects [
51]. Indeed, increased CDI of casein was also related to increased FFQ. Previously we have shown that higher FFQ was related to lower FFM and higher 24-h MI and adiposity [
22]. Furthermore, we have also found higher casein-whey ratios of HM are associated with shorter gastric emptying time in a cross-sectional cohort of exclusively breastfed infants [
52]. Casein-whey ratios also interacted with feed volumes, with higher casein-whey ratios associating with faster gastric emptying of smaller feed volumes and slower gastric emptying of larger feed volumes [
52]. Thus, HM casein may be modulating the development of infant BC via gastric emptying and breastfeeding frequency that in turn influences infant MI.
CDI of HM whey protein produced similar associations with infant BC to CDI of casein; negative with lean mass and positive with adiposity (
Table A3) however, correction for multiple comparisons eliminated significance. We have found previously that higher HM whey protein concentrations are associated with larger post-feed stomach volumes in exclusively breastfed infants [
52], and may therefore have an effect on infant BC, in combination with casein, via modulation of breastfeeding patterns. Studies in animal models indicate that whey from bovine milk may promote the growth of the soft (and adipose) tissue [
53]. Furthermore, infants fed whey-dominant formula had higher weight-for-age and BMI-for-age z-scores at four months compared with breastfed infants despite the protein content being reduced to match HM [
54], although it should be noted that these differences could be explained by the lower feed volumes in the breastfed group. In contrast infants fed a whey- and casein-dominant formula displayed no differences in BC or anthropometrics at four months of age [
55], compared to breastfed infants. Another study has reported greater fat deposition in males and greater gains in lean mass in females fed whey-dominant formula during first three months compared to breastfed infants [
56]. In this study, we did not see any effect of infant sex on relationships of either casein or whey protein concentrations with infant anthropometrics or BC. Larger longitudinal studies that focus on the array of HM whey proteins and sex-specific analysis may resolve these conflicting results.
We have examined the casein and total whey proteins of HM however, the HM whey fraction contains proteins that remain soluble in the liquid portion after precipitation of caseins—such as α-lactalbumin, lactoferrin, lysozyme, and secretory IgA [
57]—as well as various hormones, enzymes, and binding proteins [
49]. Many of these proteins have not been studied in relation to infant growth and BC and may either act synergistically or in an antagonistic manner. Administered bovine and HM lysozyme [
58,
59] have been linked to increased weight gain in preterm infants. Furthermore, high levels of lactoferrin in bovine formula was associated with lower weight gain rate in healthy term female infants during first two months after birth compared with control [
60]. Interestingly, α-lactalbumin-enriched formula did not result in any differences in weight gain, weight-for-age and weight-for-length z-scores compared with breastfed infants or infants fed standard formula at four months of age [
61]. This suggests that α-lactalbumin, a rich source of essential amino acids, may play optimal nutritive but not necessarily programming role in infant growth. It is yet to be determined whether the proportions of the most abundant whey proteins impact growth and BC development, in particular lysozyme, which may act via promotion of the colonization of infant gut by the infant-type human-residential bifidobacteria [
62] and via change in gut microbiome composition through both, detrimental microbe reduction and beneficial microbe enrichment as shown in porcine model [
63].
The number of studies showing associations between maternal characteristics and HM components is increasing. We found that higher whey protein concentrations are related to higher maternal weight, BMI, and both fat and lean body mass throughout the first 12 months of lactation (
Section 3.3,
Table A1). This represents a potential pathway by which the protein composition of HM may be altered and potentially improved via optimal maternal BC which may impact infant development. The absence of an association with HM casein concentration suggests it is not modifiable, and reflects the intrinsic synthesis of casein in the breast [
64] as opposed to movement of whey components from the maternal circulation into the milk [
65]. Whilst we did not find any associations between maternal characteristics and total protein concentrations, total protein has previously been positively related to maternal %FM [
23] and BMI [
66,
67,
68,
69]. Other studies have shown no associations [
70] or a negative association [
71]. Larger longitudinal studies would help to clarify these associations.
Breastfeeding frequency is likely a reflection of appetite regulation of the infant [
72] and is highly variable between breastfeeding dyads [
27]. Recently we have shown increased FFQ was associated with increased 24-h MI, and both of these breastfeeding parameters were related to higher infant adiposity [
22]. Both methods that we used to measure infant FFQ have some limitations, especially self-reported FFQ which has been shown to be biased towards reporting higher numbers of feeds in infants that feed more frequently in comparison with 24-h MP FFQ [
22], which is again limited to one measure at the time point of data collection. Nevertheless, positive relationships between FFQ (which were also associated with higher FM accretion) and CDI of HM proteins casein in particular (
Section 3.6;
Table A4) help us to assemble the possible pathways of the mechanisms of infant BC regulation. These results emphasize the importance of including these measures in order to elucidate the mechanisms by which HM components affect infant growth and BC and the degree by which the mother influences HM composition and volumes.
Daily intakes may be a more relevant factor than concentrations of HM components for examining the nutritional physiology of the breastfed infant. In this study, daily intakes were calculated and are considered representative of a typical daily intake due to the absence of significant within-feed [
23], short-term (weekly) [
28], and circadian [
29] variations in HM protein concentrations, including casein and whey [
30], during the established lactation. Furthermore, measurement of 24-h milk intake by test-weighing is an accurate [
73] and reproducible procedure from within two to four days to within one to two weeks between measurements [
45,
74,
75,
76]. Although no dietary data was collected in this study, the evidence is emerging that protein from dairy/milk sources may have different and/or more potent effects on infant growth and adiposity than the vegetable and meat proteins probably due to the bioactive factors present in milks [
77,
78]. The major source of milk protein in this study was HM, as no formula was used by mothers. Furthermore, differences in protein intake between breastfed and formula-fed infants during first 12 months are mainly attributed to a lower intake of protein from HM, not to a difference in protein intake from solid foods [
48].
The strength of this proof-of-concept study is that infants were breastfed on demand up to 12 months and the broad range of adiposity levels among the mothers. The limitations are the small number of participants, the small number of 24-h MP at 9 and 12 months and the absence of infant dietary data between 5 and 12 months of age. Our population was term healthy fully-breastfed singletons from predominantly Caucasian mothers of higher social-economic status therefore, our results may not be applicable to dyads from other backgrounds.