Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
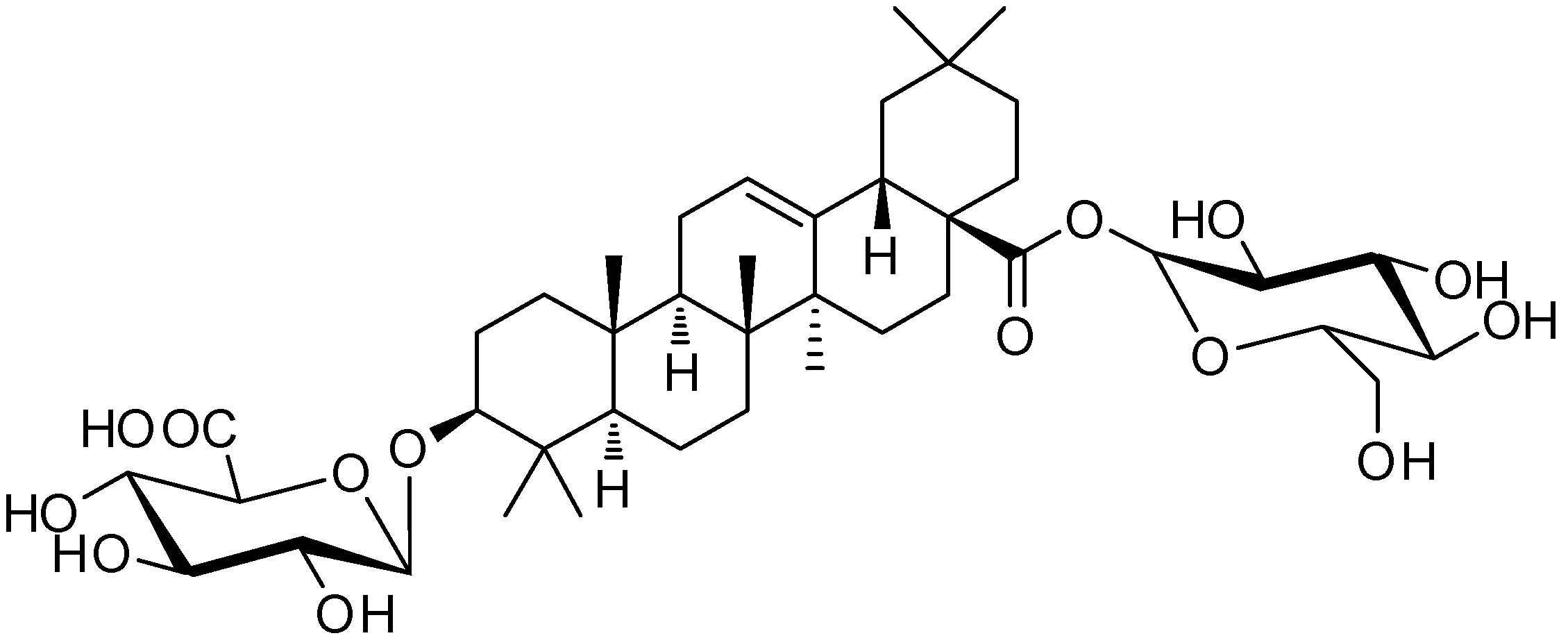
2.2. Extraction and Isolation
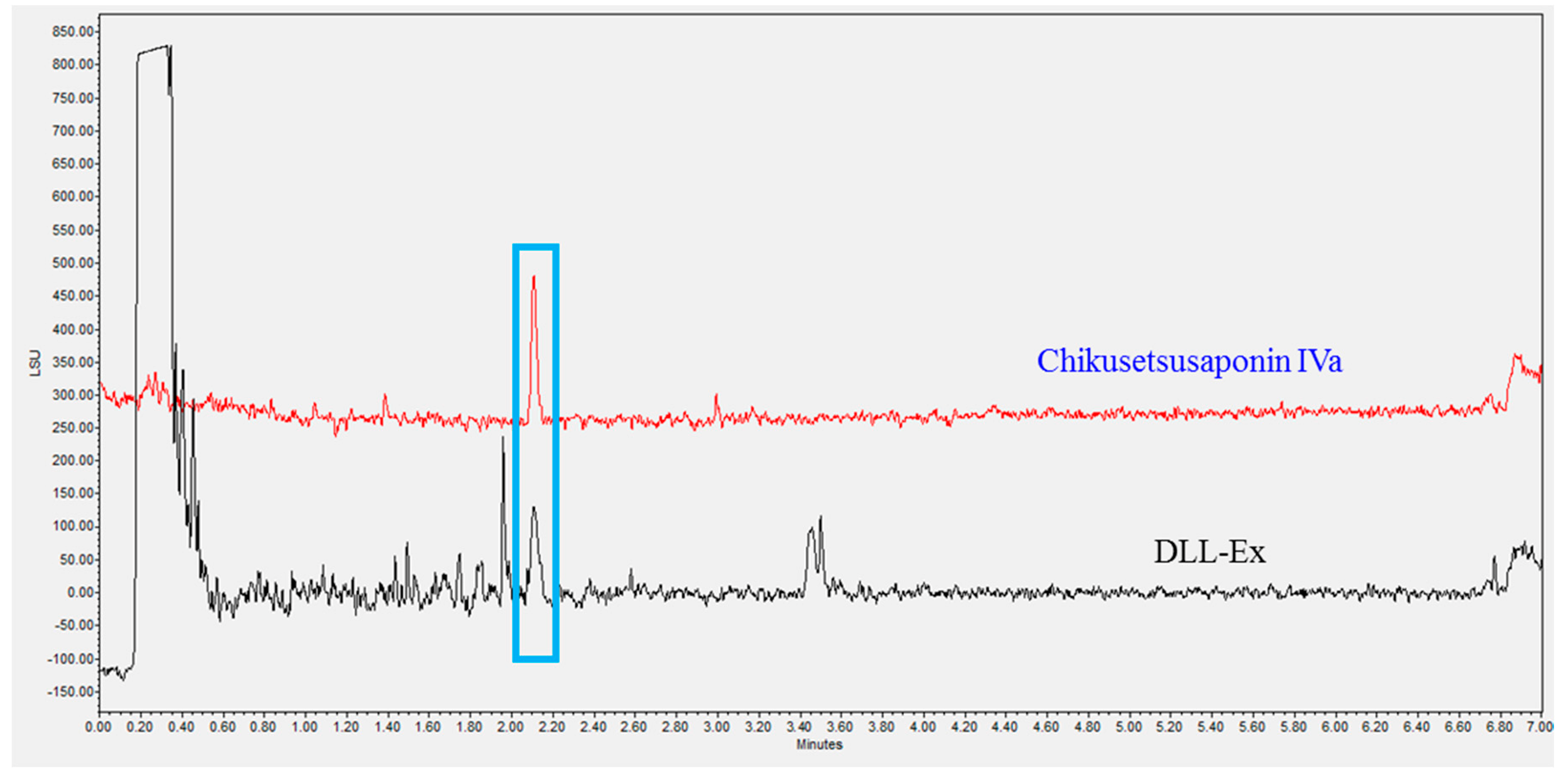
2.3. Ultra-Performance Liquid Chromatography (UPLC) Analysis
2.4. 3T3-L1 Cell Culture and Differentiation
2.5. Cytotoxicity Assay
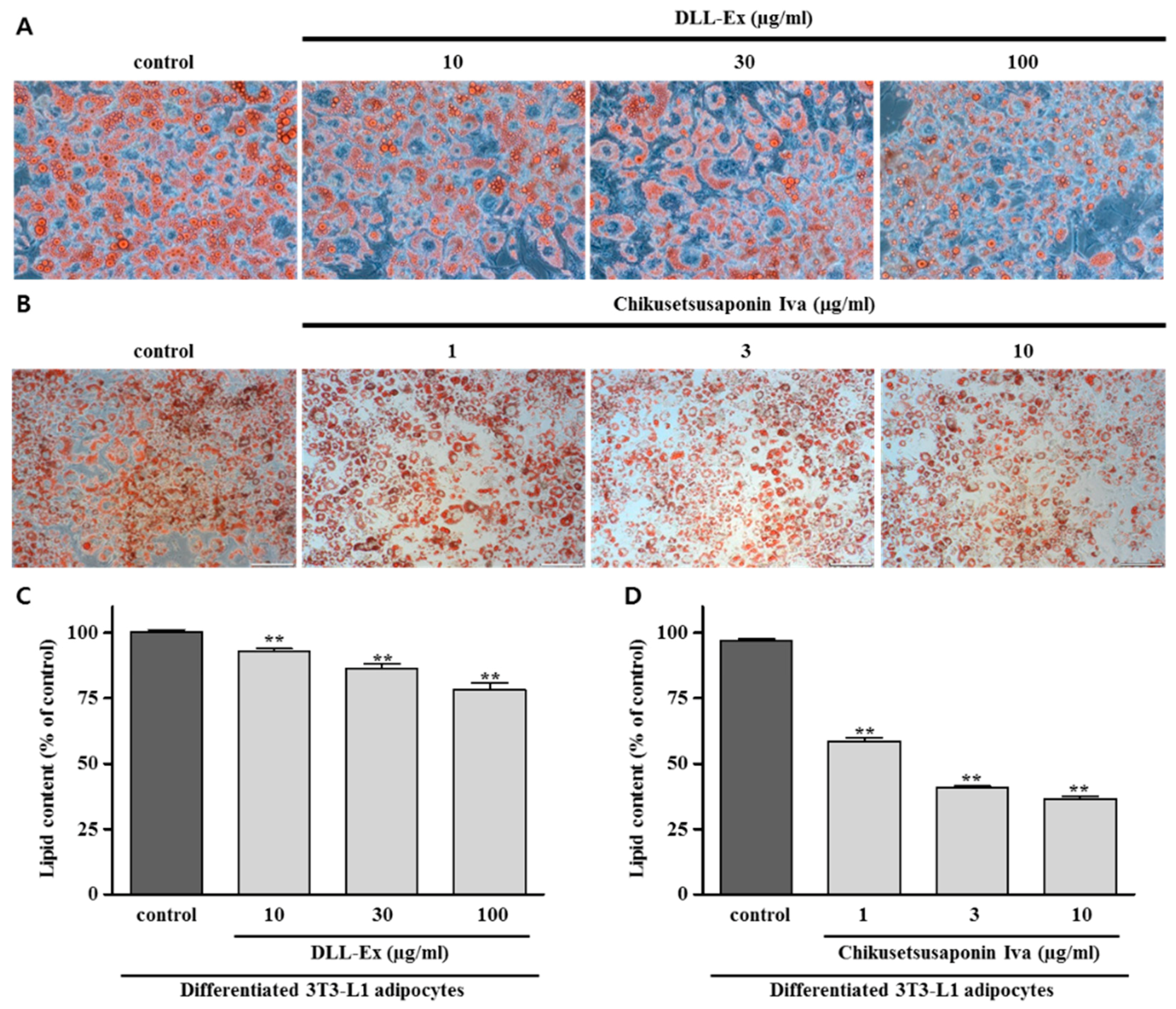
2.6. Oil Red O Staining Assay
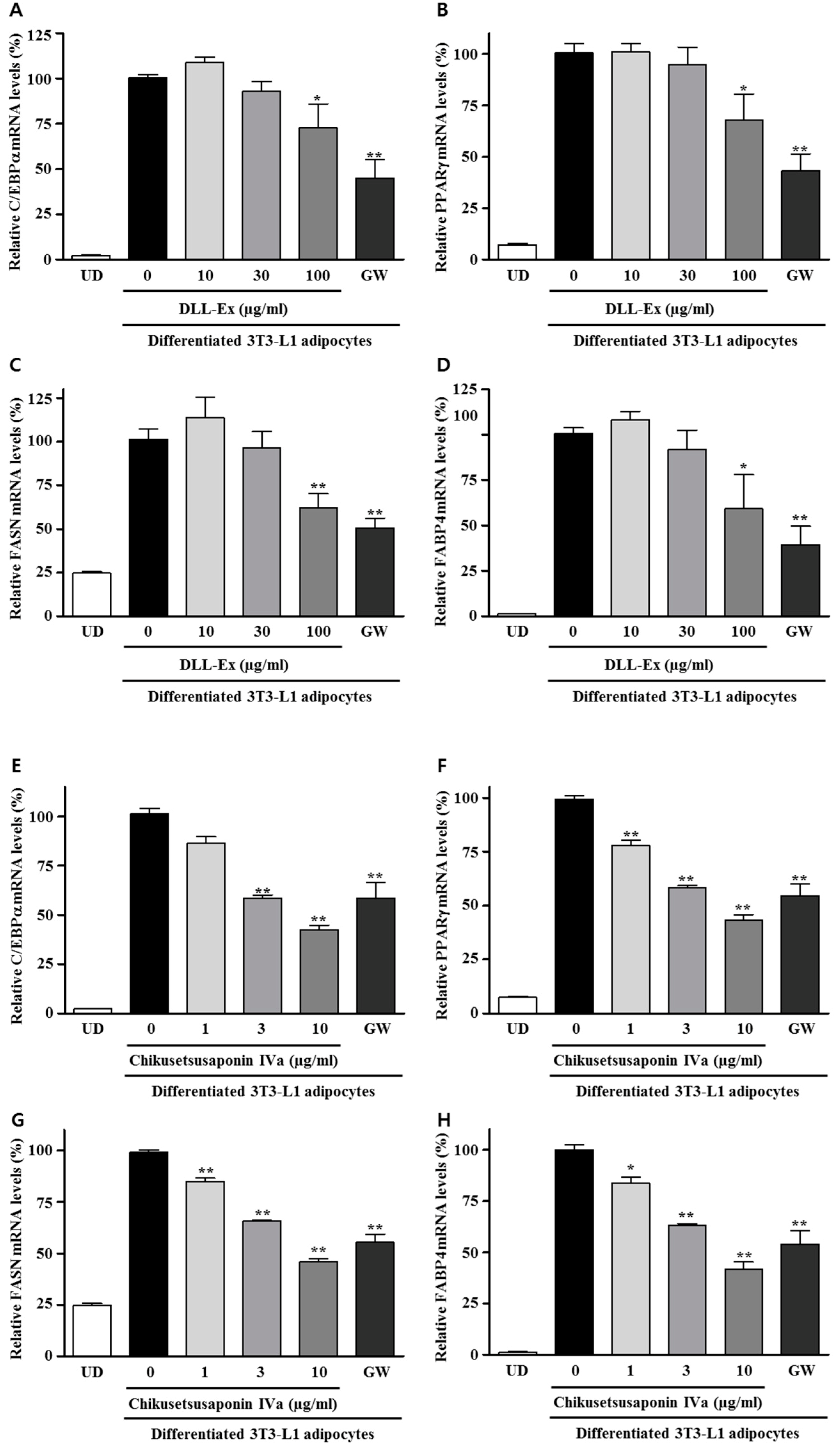
2.7. RNA Isolation and Quantitative Real-Time PCR
2.8. Protein Extraction and Western Blot Analysis
2.9. Statistical Analysis
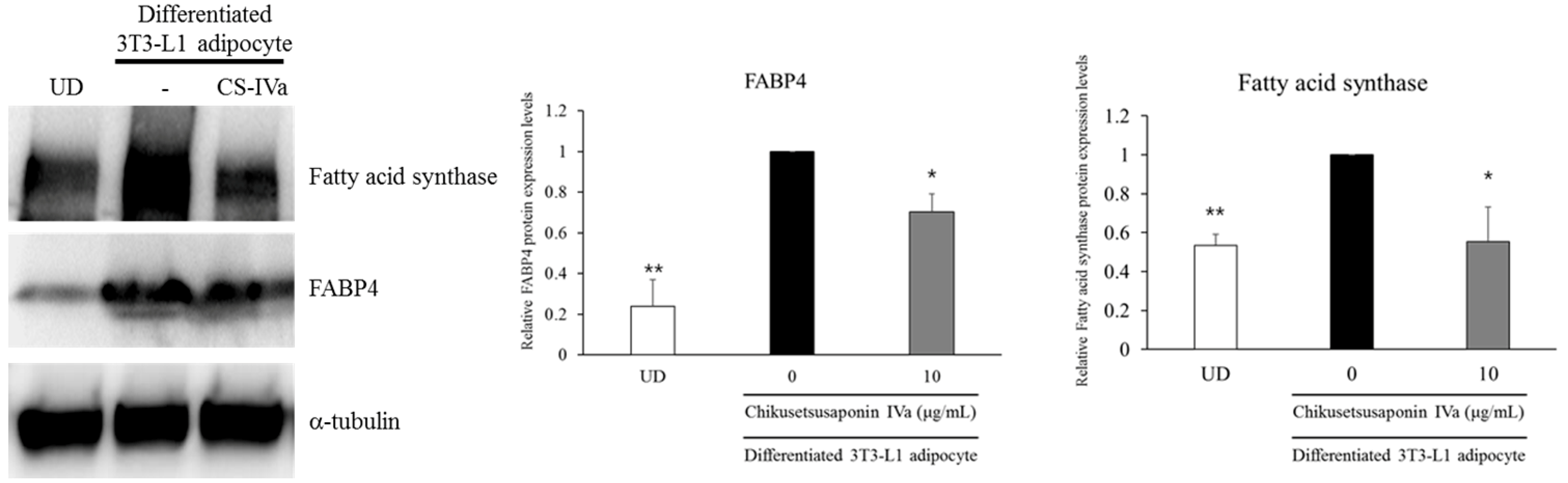
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whyte, R.O.; Nilsson-Leissner, G.; Trumble, H.C. Legumes in Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1953. [Google Scholar]
- Stuart, G.A.; Smith, F.P. Chinese Materia Medica; Southern Materials Center: Springfield, MO, USA, 1976. [Google Scholar]
- Revolutionary Health Committee of Hunan Province. A Barefoot Doctors Manual; Running Press: Philadelphia, PA, USA, 1977. [Google Scholar]
- Yeung, H.C. Handbook of Chinese Herbs and Formulas Vol. I; Institute of Chinese Medicine: Hongkong, China, 1985. [Google Scholar]
- Kante, K.; Reddy, C.S. Anti Diabetic Activity of Dolichos lablab (Seeds) in Streptozotocin-Nicotinamide Induced Diabetic Rats. Hygeia J. D Med. 2013, 5, 32–40. [Google Scholar]
- Motalib Momin, M. Anti-Inflammatory, Antioxidant and Cytotoxicity Potential of Methanolic Extract of Two Bangladeshi Bean Lablab purpureus (L.) Sweet White and Purple. Int. J. Pharm. Sci. Res. 2012, 3, 776–781. [Google Scholar]
- Ramakrishna, V.; Jhansi Rani, P.; Ramakrishna Rao, P. Hypocholesterolemic Effect of Diet Supplemented with Indian Bean (Dolichos lablab L. Var lignosus) Seeds. Nutr. Food Sci. 2007, 37, 452–456. [Google Scholar] [CrossRef]
- Janarthanan, S.; Sakthivelkumar, S.; Veeramani, V.; Radhika, D.; Muthukrishanan, S. A New Variant of Antimetabolic Protein, Arcelin from an Indian Bean, Lablab purpureus (Linn.) and its Effect on the Stored Product Pest, Callosobruchus maculatus. Food Chem. 2012, 135, 2839–2844. [Google Scholar] [CrossRef] [PubMed]
- Deoda, R.; Pandya, H.; Patel, M.; Yadav, K.; Kadam, P.; Patil, M.J. Antilithiatic Activity of Leaves, Bulb and Stem of Nymphea odorata and Dolichos lablab Beans. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 814–819. [Google Scholar]
- Priya, S.; Jenifer, S. Antibacterial Activity of Leaf and Flower Extract of Lablab purpureus against Clinical Isolates of Staphylococcus aureus. Res. Rev. A J. Drug Des. Discov. 2014, 1, 5–7. [Google Scholar]
- Yoshikawa, M.; Murakami, T.; Komatsu, H.; Matsuda, H. Medicinal Foodstuffs. XII. Saponin Constituents with Adjuvant Activity from Hyacinth Bean, the Seeds of Dolichos lablab L. (1): Structures of Lablabosides A, B, and C. Chem. Pharm. Bull. 1998, 46, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.Y.; Ding, L.S. Chemical Study on the Flower of Dolichos lablab L. J. China Pharm. Univ. 1996, 27, 205–207. [Google Scholar]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2017.
- Siersbæk, R.; Nielsen, R.; Mandrup, S. PPARγ in Adipocyte Differentiation and Metabolism–Novel Insights from Genome-Wide Studies. FEBS Lett. 2010, 584, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Auwerx, J.; Martin, G.; Guerre-Millo, M.; Staels, B. Transcription, Adipocyte Differentiation, and Obesity. J. Mol. Med. 1996, 74, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lim, Y.; Kim, E. Therapeutic Phytogenic Compounds for Obesity and Diabetes. Int. J. Mol. Sci. 2014, 15, 21505–21537. [Google Scholar] [CrossRef] [PubMed]
- Im, A.; Kim, Y.H.; Kim, Y.H.; Yang, W.; Kim, S.H.; Song, K.H. Dolichos lablab Protects against Nonalcoholic Fatty Liver Disease in Mice Fed High-Fat Diets. J. Med. Food 2017, 20, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Liu, C.; Wang, T.; He, Y.; Zhou, Z.; Dun, Y.; Zhao, H.; Ren, D.; Wang, J.; Zhang, C.; et al. Chikusetsu Saponin IVa Ameliorates High Fat Diet-Induced Inflammation in Adipose Tissue of Mice through Inhibition of NLRP3 Inflammasome Activation and NF-κB Signaling. Oncotarget 2017, 8, 31023–31040. [Google Scholar] [CrossRef] [PubMed]
- Kohda, H.; Yamaoka, Y.; Morinaga, S.; Ishak, M.; Darise, M. Saponins from Talinum triangulare. Chem. Pharm. Bull. 1992, 40, 2557–2558. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Lane, M.D. Transcriptional Regulation of Gene Expression during Adipocyte Differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Novikoff, A.B.; Novikoff, P.M.; Rosen, O.M.; Rubin, C.S. Organelle Relationships in Cultured 3T3-L1 Preadipocytes. J. Cell Biol. 1980, 87, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.M.; Choy, L.; Hotamisligil, G.S.; Graves, R.A.; Tontonoz, P. Regulation of Adipocyte Gene Expression in Differentiation and Syndromes of Obesity/Diabetes. J. Biol. Chem. 1993, 268, 6823–6826. [Google Scholar] [PubMed]
- Ailhaud, G.; Grimaldi, P.; Negrel, R. Hormonal Regulation of Adipose Differentiation. Trends Endocrinol. Metab. 1994, 5, 132–136. [Google Scholar] [CrossRef]
- Linhart, H.G.; Ishimura-Oka, K.; DeMayo, F.; Kibe, T.; Repka, D.; Poindexter, B.; Bick, R.J.; Darlington, G.J. C/EBPα is Required for Differentiation of White, but Not Brown, Adipose Tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 12532. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Croniger, C.; Lekstrom-Himes, J.; Zhang, P.; Fenyus, M.; Tenen, D.G.; Darlington, G.J.; Hanson, R.W. Metabolic Response of Mice to a Postnatal Ablation of CCAAT/Enhancer-Binding Protein. J. Biol. Chem. 2005, 280, 38689–38699. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Finegold, M.; Bradley, A.; Ou, C.; Abdelsayed, S.; Wilde, M.; Taylor, L.; Wilson, D.; Darlington, G. Impaired Energy Homeostasis in C/EBP Alpha Knockout Mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ is Required for Placental, Cardiac, and Adipose Tissue Development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Duan, S.Z.; Ivashchenko, C.Y.; Whitesall, S.E.; D’Alecy, L.G.; Duquaine, D.C.; Brosius, F.C., III; Gonzalez, F.J.; Vinson, C.; Pierre, M.A.; Milstone, D.S.; et al. Hypotension, Lipodystrophy, and Insulin Resistance in Generalized PPARγ-Deficient Mice Rescued from Embryonic Lethality. J. Clin. Investig. 2007, 117, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Takakuwa, R.; Marchand, S.; Dentz, E.; Bornert, J.; Messaddeq, N.; Wendling, O.; Mark, M.; Desvergne, B.; Wahli, W.; et al. Peroxisome Proliferator-Activated Receptor γ is Required in Mature White and Brown Adipocytes for their Survival in the Mouse. Proc. Natl. Acad. Sci. USA 2004, 101, 4543–4547. [Google Scholar] [CrossRef] [PubMed]
- Weisiger, R.A. Cytosolic Fatty Acid Binding Proteins Catalyze Two Distinct Steps in Intracellular Transport of their Ligands. Mol. Cell. Biochem. 2002, 239, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Coe, N.R.; Bernlohr, D.A. Physiological Properties and Functions of Intracellular Fatty Acid-Binding Proteins. Biochimi. Biophys. Acta. (BBA) Lipids Lipid Metab. 1998, 1391, 287–306. [Google Scholar] [CrossRef]
- Bernlohr, D.A.; Bolanowski, M.A.; Kelly, T.J., Jr.; Lane, M.D. Evidence for an Increase in Transcription of Specific mRNAs during Differentiation of 3T3-L1 Preadipocytes. J. Biol. Chem. 1985, 260, 5563–5567. [Google Scholar] [PubMed]
- Bernlohr, D.A.; Doering, T.L.; Kelly, T.J.; Lane, M.D. Tissue Specific Expression of p422 Protein, a Putative Lipid Carrier, in Mouse Adipocytes. Biochem. Biophys. Res. Commun. 1985, 132, 850–855. [Google Scholar] [CrossRef]
- Smith, P.J.; Wise, L.S.; Berkowitz, R.; Wan, C.; Rubin, C.S. Insulin-Like Growth Factor-I is an Essential Regulator of the Differentiation of 3T3-L1 Adipocytes. J. Biol. Chem. 1988, 263, 9402–9408. [Google Scholar] [PubMed]
- Yang, V.W.; Christy, R.J.; Cook, J.S.; Kelly, T.J.; Lane, M.D. Mechanism of Regulation of the 422(aP2) Gene by cAMP during Preadipocyte Differentiation. Proc. Natl. Acad. Sci. USA 1989, 86, 3629–3633. [Google Scholar] [CrossRef] [PubMed]
- Christy, R.J.; Yang, V.W.; Ntambi, J.M.; Geiman, D.E.; Landschulz, W.H.; Friedman, A.D.; Nakabeppu, Y.; Kelly, T.J.; Lane, M.D. Differentiation-Induced Gene Expression in 3T3-L1 Preadipocytes: CCAAT/Enhancer Binding Protein Interacts with and Activates the Promoters of Two Adipocyte-Specific Genes. Genes Dev. 1989, 3, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Kletzien, R.F.; Foellmi, L.A.; Harris, P.K.; Wyse, B.M.; Clarke, S.D. Adipocyte Fatty Acid-Binding Protein: Regulation of Gene Expression in Vivo and in Vitro by an Insulin-Sensitizing Agent. Mol. Pharmacol. 1992, 42, 558–562. [Google Scholar] [PubMed]
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.M.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Fatty Acid Binding Protein 4 is Increased in Metabolic Syndrome and with Thiazolidinedione Treatment in Diabetic Patients. Atherosclerosis 2007, 195, e150–e158. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Ghosh, S.B.; Bobbu, P.; Anitha, D.; Vijaya, T. Triterpenoid Saponins: A Review on Biosynthesis, Applications and Mechanism of their Action. Int. J. Pharm. Pharm. Sci. 2015, 7, 24–28. [Google Scholar]
- Rao, V.; Gurfinkel, D.M. The Bioactivity of Saponins: Triterpenoid and Steroidal Glycosides. Drug Metab. Drug Interact. 2000, 17, 211–236. [Google Scholar] [CrossRef]
- Garai, S. Triterpenoid Saponins. Nat. Prod. Chem. Res. 2014, 2. [Google Scholar] [CrossRef]
- Kim, J.H.; Hahm, D.H.; Yang, D.C.; Kim, J.H.; Lee, H.J.; Shim, I. Effect of Crude Saponin of Korean Red Ginseng on High-Fat Diet-Induced Obesity in the Rat. J. Pharmacol. Sci. 2005, 97, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, Y.; Gao, Y.; Shi, Z.; Hu, Y.; Ren, G. Suppressive Effects of Saponin-Enriched Extracts from Quinoa on 3T3-L1 Adipocyte Differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Fu, H.; Zhang, Q.; Koike, K. Pancreatic Lipase-Inhibiting Triterpenoid Saponins from Fruits of Acanthopanax senticosus. Chem. Pharm. Bull. 2007, 55, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ogawa, S.; Jisaka, M.; Kimura, Y.; Katsube, T.; Yokota, K. Identification of Novel Saponins from Edible Seeds of Japanese Horse Chestnut (Aesculus turbinata BLUME) After Treatment with Wooden Ashes and their Nutraceutical Activity. J. Pharm. Biomed. Anal. 2006, 41, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kang, M.; Kim, Y.S. Platycodin D Inhibits Lipogenesis through AMPKalpha-PPARgamma2 in 3T3-L1 Cells and Modulates Fat Accumulation in Obese Mice. Planta Med. 2012, 78, 1536–1542. [Google Scholar] [PubMed]
- Xu, B.J.; Han, L.K.; Zheng, Y.N.; Lee, J.H.; Sung, C.K. In Vitro Inhibitory Effect of Triterpenoidal Saponins from Platycodi Radix on Pancreatic Lipase. Arch. Pharm. Res. 2005, 28, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Zhu, X.M.; Han, L.K.; Saito, M.; Sun, Y.S.; Yoshikawa, M.; Kimura, Y.; Zheng, Y.N. Anti-Obesity Effects of Escins Extracted from the Seeds of Aesculus turbinata BLUME (Hippocastanaceae). Chem. Pharm. Bull. 2008, 56, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Sakamoto, T.; Udagawa, H.; Taniguchi, H.; Kobayashi-Hattori, K.; Ozawa, Y.; Takita, T. Inhibition of Increases in Blood Glucose and Serum Neutral Fat by Momordica charantia Saponin Fraction. Biosci. Biotechnol. Biochem. 2007, 71, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Xu, B.J.; Kimura, Y.; Zheng, Y.; Okuda, H. Platycodi Radix Affects Lipid Metabolism in Mice with High Fat Diet-Induced Obesity. J. Nutr. 2000, 130, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Lee, H.S.; Chung, Y.C.; Jeong, H.G. Saponins from Platycodon grandiflorum Inhibit Hepatic Lipogenesis through Induction of SIRT1 and Activation of AMP-Activated Protein Kinase in High-Glucose-Induced HepG2 Cells. Food Chem. 2013, 140, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, Y.; Wang, Y.; Tang, S.; Pan, F.; Li, Z.; Sung, C. Anti-Obesity Effects of Mogrosides Extracted from the Fruits of Siraitia grosvenorii (Cucurbitaceae). Afr. J. Pharm. Pharm. 2012, 6, 1492–1501. [Google Scholar]
- Fang, X.; Han, Q.; Li, S.; Zhao, Y.; Luo, A. Chikusetsu Saponin IVa Attenuates Isoflurane-Induced Neurotoxicity and Cognitive Deficits Via SIRT1/ERK1/2 in Developmental Rats. Am. J. Transl. Res. 2017, 9, 4288–4299. [Google Scholar] [PubMed]
- Duan, J.; Yin, Y.; Wei, G.; Cui, J.; Zhang, E.; Guan, Y.; Yan, J.; Guo, C.; Zhu, Y.; Mu, F.; et al. Chikusetsu Saponin IVa Confers Cardioprotection via SIRT1/ERK1/2 and Homer1a Pathway. Sci. Rep. 2015, 5, 18123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Cui, J.; Jia, N.; Wu, Y.; Xi, M.; Wen, A. Chikusetsu Saponin IVa Regulates Glucose Uptake and Fatty Acid Oxidation: Implications in Antihyperglycemic and Hypolipidemic Effects. J. Pharm. Pharmacol. 2015, 67, 997–1007. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Seo, C.-S.; Hwang, I.H.; Lee, M.W.; Song, K.H. Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds. Nutrients 2018, 10, 1221. https://doi.org/10.3390/nu10091221
Yin J, Seo C-S, Hwang IH, Lee MW, Song KH. Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds. Nutrients. 2018; 10(9):1221. https://doi.org/10.3390/nu10091221
Chicago/Turabian StyleYin, Jun, Chang-Seob Seo, In Hyeok Hwang, Min Won Lee, and Kwang Hoon Song. 2018. "Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds" Nutrients 10, no. 9: 1221. https://doi.org/10.3390/nu10091221
APA StyleYin, J., Seo, C.-S., Hwang, I. H., Lee, M. W., & Song, K. H. (2018). Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds. Nutrients, 10(9), 1221. https://doi.org/10.3390/nu10091221




