Keep Calm and Carry on: Parental Opinions on Improving Clinical Dietary Trials for Young Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Focus Groups
2.4. Data Analysis
- Introduction to the proposed dairy study
- ○
- Why it is important
- ○
- Overview of what would be involved
- Brief explanation of assessments planned and what information they provide
- Specifically seeking parental opinions on:
- ○
- Blood measures—fasting
- ■
- Preferred location for sampling—home or hospital/clinic?
- ■
- Analysis for cholesterol and triglycerides planned—would parents be interested in having additional tests done for their own benefit?
- ■
- Concerns around safety or child anxiety, ideas to minimise
- ○
- Body composition
- ■
- Any preference for BOD POD *, DEXA ** or BIA ***
- ■
- Concerns around safety or child anxiety, ideas to minimise
- ○
- Stool samples
- ■
- Thoughts on feasibility of collecting samples
- ■
- Storage preferences—at home, collected by research assistant or stored in mini-freezer provided by study at home
- ○
- Body strength
- ■
- Would a child be capable and willing to do the test procedure (isometric thigh pull)
- ○
- Dietary assessment
- ■
- Thoughts on feasibility of keeping 3-day food records for child
- ○
- Dairy products
- ■
- Delivery methods
- ■
- Information required on labels
- Any further thoughts to improve the study experience for parent or child?
- Would you enrol your child—why or why not?
3. Results
3.1. Participants
3.2. Thematic Analysis of Focus Group Data: Major Parental Concerns
3.2.1. Theme 1: Parent and Child Needle Fear
3.2.2. Theme 2: Child’s Age, Stage, Understanding and Ability to Cope
3.2.3. Theme 3: Study Misunderstandings
3.2.4. Theme 4: Factors Affecting Parental Decisions to Enrol Their Children in the Study
3.3. Proposed Solutions
3.3.1. Theme 1: Parent and Child Needle Fear:
3.3.2. Theme 2: Child’s Age, Stage, Understanding and Ability to Cope:
3.3.3. Theme 3: Study Misunderstandings:
3.3.4. Theme 4: Factors Affecting Parental Decisions to Enrol Their Children in the Study:
4. Discussion
4.1. Parent and Child Anxiety
4.2. Study Misunderstandings and Other Factors Affecting Parental Enrolment
4.3. Child-Centred Research
- 1)
- Use authentic communication (honest explanations about why a test is needed, and what to expect, can empower the child).
- 2)
- Acknowledge emotions (acknowledge the child’s feelings are valid, including fear).
- 3)
- Invite participation (children can practice on a teddy bear or toy before the assessment, or help set up machines or equipment where suitable) [28].
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joseph, P.D.; Jonathan, C.C.; Patrina, H.Y.C. Clinical trials in children. Br. J. Clin. Pharmacol. 2015, 79, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Gillies, K.; Vikki, A.E. Supporting positive experiences and sustained participation in clinical trials: Looking beyond information provision. J. Med. 2012, 38, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Glenton, C.; Oxman, A.D. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: Methodological Study. BMJ 2009, 339, b3496. [Google Scholar] [CrossRef] [PubMed]
- O’Cathain, A.; Thomas, K.J.; Drabble, S.J.; Rudolph, A.; Hewison, J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013, 3, e002889. [Google Scholar] [CrossRef] [PubMed]
- O’Cathain, A.; Goode, J.; Drabble, S.J.; Thomas, K.J.; Rudolph, A.; Hewison, J. Getting added value from using qualitative research with randomized controlled trials: A qualitative interview study. Trials 2014, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Hoberman, A.; Shaikh, N.; Bhatnagar, S.; Haralam, M.A.; Kearney, D.H.; Colburn, D.K.; Kienholz, M.L.; Wang, L.; Bunker, C.H.; Keren, R.; et al. Factors that influence parental decisions to participate in clinical research: Consenters vs. nonconsenters. JAMA 2013, 167, 561–566. [Google Scholar]
- Fisher, H.R.; McKevitt, C.; Boaz, A. Why do parents enroll their children in research: A narrative synthesis. J. Med. 2011, 37, 544–551. [Google Scholar]
- Tait, A.R.; Terri, V.-L.; Shobha, M. Participation of children in clinical research: Factors that influence a parent’s decision to consent. Anesthesiology 2003, 99, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Tammara, J.; David, W. How do children and parents make decisions about pediatric clinical research? J. Pediatric. Hematol. Oncol. 2008, 30, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Woolfall, K.; Shilling, V.; Hickey, H.; Smyth, R.L.; Sowden, E.; Williamson, P.R.; Young, B. Parents’ agendas in paediatric clinical trial recruitment are different from researchers’ and often remain unvoiced: A qualitative study. PLoS ONE 2013, 8, e67352. [Google Scholar] [CrossRef] [PubMed]
- Shilling, V.; Williamson, P.R.; Hickey, H.; Sowden, E.; Beresford, M.W.; Smyth, R.L.; Young, B. Communication about children’s clinical trials as observed and experienced: qualitative study of parents and practitioners. PLoS ONE 2011, 6, e21604. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, P.H.Y.; Phyllis, N.B.; Jonathan, C.C. Parents’ attitudes to children’s participation in randomized controlled trials. J. Pediatr. 2003, 5, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Coyne, I.; Gallagher, P. Participation in communication and decision-making: Children and young people’s experiences in a hospital setting. J. Clin. Nurs. 2011, 20, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Shier, H. Pathways to participation: Openings, opportunities and obligations. Children Soc. 2001, 15, 107–117. [Google Scholar] [CrossRef]
- Marceau, L.D.; Welch, L.C.; Victoria, L.P.; Gail, D.P. Educating parents about pediatric research: Children and clinical studies website qualitative evaluation. Qual. Health Res. 2015, 26, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Radecki, L.; Olson, L.M.; Frintner, M.P.; Tanner, J.L.; Stein, M.T. What do families want from well-child care? Including parents in the rethinking discussion. Pediatrics 2009, 124, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Cyr, J. The pitfalls and promise of focus groups as a data collection method. Sociol. Meth. Res. 2015, 45, 231–259. [Google Scholar]
- Peterson-Sweeney, K. The use of focus groups in pediatric and adolescent research. J. Pediatr. Health Care 2005, 19, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. Australian Bureau of Statistics [ABS]: Canberra, Australia, 2018. Available online: https://apac01.safelinks.protection.outlook.com/?url=http%3A%2F%2Fwww.webcitation.org%2F71uadHoyg&data=02%7C01%7Ca.nicholl%40ecu.edu.au%7C69eba3e2e302460642e308d609c44a28%7C9bcb323d7fa345e7a36f6d9cfdbcc272%7C1%7C0%7C636707136002582200&sdata=TxEdkB6hL73u7IjgTn91TVwzmgC9%2FJkMeJeF9ZpLnbI%3D&reserved=0 (accessed on 24 August 2018).
- Tates, K.; Zwaanswijk, M.; Otten, R.; van Dulmen, S.; Hoogerbrugge, P.M.; Kamps, W.A.; Bensing, J.M. Online focus groups as a tool to collect data in hard-to-include populations: Examples from paediatric oncology. BMC Med. Res. Methodol. 2009, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, L.; Susan, A.; Paul, S. The association between parent’s and healthcare professional’s behavior and children’s coping and distress during venepuncture. J. Pediat. Psychol. 2010, 35, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.; Vohra, J.; Preston, J.; Elliott, C.; Van’t Hoff, W.; Coad, J.; Gibson, F.; Partridge, L.; Brierley, J.; Larcher, V.; et al. Guidance on clinical research involving infants, children and young people: An update for researchers and research ethics committees. Arch. Dis. Child. 2014, 99, 887–891. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.; Meghan, R.P.R.; Anna, T.; Nicole, R.; Gordon, J.G.; Asmundson, M.N.; Christine, T.; Chambers, V.S.; et al. Far from “Just a Poke”: Common painful needle procedures and the development of needle fear. Clin. J. Pain. 2015, 31, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Marechal, C.; Berthiller, J.; Tosetti, S.; Cogniat, B.; Desombres, H.; Bouvet, L.; Kassai, B.; Chassard, D.; de Queiroz Siqueira, M. Children and parental anxiolysis in paediatric ambulatory surgery: A randomized controlled study comparing 0.3 mg kg−1 midazolam to tablet computer based interactive distraction. Br. J. Anaesth. 2017, 118, 247–253. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2018. [Google Scholar]
- Shilling, V.; Young, B. How do parents experience being asked to enter a child in a randomised controlled trial? BMC Med. Ethics 2009, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M. Dear Parent: Caring for Infants with Respect; Weaver, J., Ed.; Resources for Infant Educarers [RIE]: Los Angeles, CA, USA, 1998. [Google Scholar]
- O’Sullivan, T.; Evelegh, K.; Arabait, D.; Russell, K. A respectful approach to dietary research involving children: the importance of communication, acknowledgement and participation. (Conference Abstract). Nutr. Diet. 2016, 73, 51–97. [Google Scholar]
- Pope, N.; Mary, T.; Gavin, L.; Sally, W. Why we need to research with children, not on children. JBI 2017, 15, 1497–1498. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, P.H.Y.; Sharon, B.M.; Phyllis, N.B.; Jonathan, C.C. Clinical trials in children. Lancet 2004, 364, 803–811. [Google Scholar] [CrossRef]
- Howie, S.R. Blood sample volumes in child health research: Review of safe limits. Bull. World Heal. Organ. 2010, 89, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Masty, J.; Celia, F. A goodness-of-fit approach to informed consent for pediatric intervention research. Ethics Behav. 2008, 18, 139–160. [Google Scholar]
- Lipstein, E.A.; Brinkman, W.B.; Fiks, A.G.; Hendrix, K.S.; Kryworuchko, J.; Miller, V.A.; Prosser, L.A.; Ungar, W.J.; Fox, D. An emerging field of research: Challenges in pediatric decision making. Med. Decis. Mak. 2015, 35, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.D.; Mandy, A.; Lesley, P.; Thane, C. Qualitative evidence in pediatrics. In Handbook of Qualitative Health Research for Evidence-Based Practice; Olson, K., Young, R.A., Schultz, I.Z, Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Crookes, P.A.; Sue, D. Research into Practice, 2nd ed.; Elsevier Ltd.: London, UK, 1998. [Google Scholar]
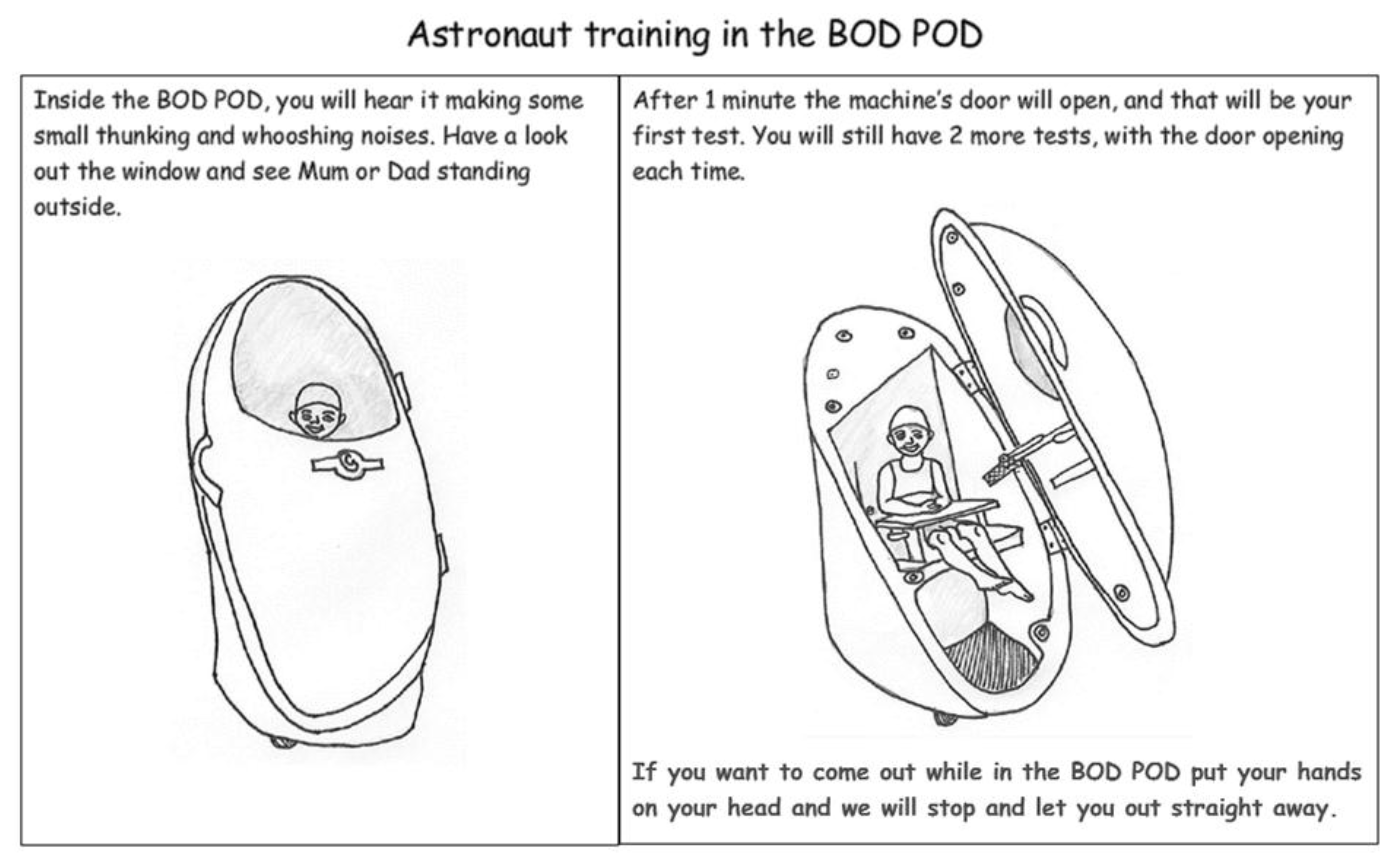

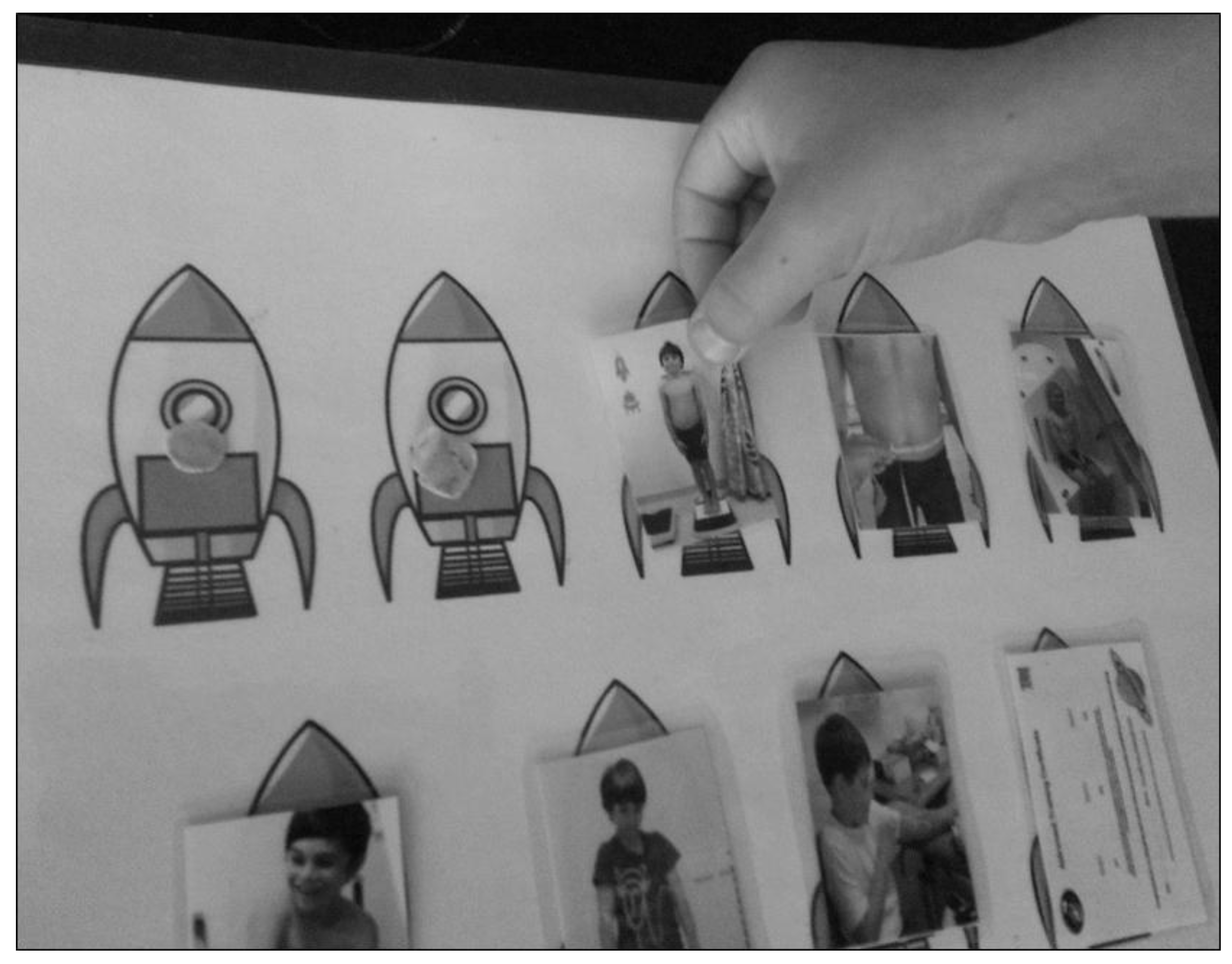
| Issue | Example Parent Quotations (Parent Code (M/F Parent) and Focus Group Reference) |
|---|---|
| Theme 1: Parent and child needle fear | I don’t know. I just don’t, um, see—except if it’s for a medical reason, I suppose ... I hate blood tests at the best of times, so to subject my 3-year-old to it, as well? It’s going to become like World War II ... (FP3, FG2) |
| (Child’s name) and I were part of an egg allergy study last year and he had to have blood taken three times. It was a very challenging experience for me. (Child’s name) cried and cried through the blood taking (FP3, OFG) | |
| Keeping them (the children) calm is the main problem (FP5, FG2) | |
| Theme 2: Child’s age, stage, understanding and ability to cope | Yeah, probably—probably for a 5- or a 6-year-old I might, but I don’t think I’d try and do it for a 2- or a 3- or a 4-year-old ... Just the trauma of it, and they don’t—not sure that they’d quite understand ... Yeah, when you can actually ask them whether they’re willing to participate in it—until then ... (MP1, FG1) |
| Theme 3: Study misunderstandings (i) Lack of awareness of current dietary guidelines promoting low-fat dairy consumption for children over 2 years | I’m like, I just thought it was, like, you always feed your kids fat—it’s not until you are an adult that you ... I didn’t realize that was the official recommendation? (FP2, FG2) |
| (ii) Regular fat dairy is healthier, as reduced fat forms are full of added sugars: | I thought full fat dairy was recommended too. I thought low fat in dairy tends to have high sugar, and sugar is far worse than fat. (FP5, OFG) |
| (ii) Equipoise: randomization to the reduced-fat dairy group will disadvantage the child | See, I don’t, I’m not really that keen on giving them low-fat dairy for 3 months, really, particularly because my girls are, um, already at the bottom of the charts as far as, um, percentiles—particularly (child’s name). She’s, like, already quite a skinny baby? (FP2, FG2) |
| Theme 4: Factors affecting parental decisions to enrol their children in the study Negative: time commitment; treatment of their child; confronting tests Positive: altruism | Um, to be honest, probably not ... Just because of the commitment that’s required? Um, I’m for it, I’m all for getting it—get the study done, but in terms of—because I’ve got a little one and a whole lot of other commitments, and to be able to manage my time and ... I guess, you know, getting a blood test—I mean, if it’s ... for the better good, I would do it, but I’m - inclined towards not going there - mainly for commitment reasons, then, partly for scare - you know, scaring off my little kids ... Mostly the blood test, yeah, that would be the ... I guess it’s, you know, trying to keep on top of it with all the, um, the food that’s coming in, and tracking it, and, um, the stool, yeah. So that’s quite a lot of things to do ... (FP1, FG1) |
| It’s difficult to know whether to participate. For example, the egg allergy survey: we did all the tests but not the dietary changes because we wanted to introduce eggs earlier (than the study prescribed). (Conflicted) They need people, but the conditions? They treat (children) like guinea pigs. (FP1, FG2) | |
| I don’t mind the dairy (part of the trial). (I’m not so sure about the) poo thing ...? (With the) blood tests, I would want my child to be older–two-and-a-half (years) plus. (FP5, FG2) |
| Issues Identified | Proposed Solution(s) | Example Parent Quotations Quotation, Parent Code (M/F Parent) and Focus Group (FG; OFG = Online Focus Group) | Support from the Literature |
|---|---|---|---|
| Theme 1: Parent and child needle fear | A good experience and a good phlebotomist trump convenience or location | As long as it’s somebody that’s highly experienced with children, because taking (blood) from your child is a completely different thing—they really don’t have any control. It’s that fear, it’s that result. (FP2, FG2) | The healthcare professional may be the best person to manage a child’s distress, in part by helping parents cope [21] |
| My daughter had quite a few blood tests when she was 4, the numbing gel worked well for her. The blood tests she had were taken at (the major children’s hospital in Perth). The staff were fantastic and very good at distraction, using things like bubbles, and showing her colorful pictures etc. Our job was to try to keep her still and as calm as possible, They also had everything in place and ready before taking the blood. She still got upset, but the gel made it less painful and distraction made it a bit easier. (FP3, OFG) | Effective anaesthetic creams and numbing gels are now routinely available [22], and consistent and appropriate pain management should be used during all childhood needle procedures [23] | ||
| I think the numbing gel definitely. I like the idea of the children practicing on the teddy or doll first only cause our kids are a just a little bit older. Not sure how (child’s name) would go cause she’s younger. I guess it would be a case of applying the gel distracting her until the gel kicks in and giving lots of cuddles after (MP1, OFG) | |||
| Additional testing of samples acts as an incentive | So, with the blood, I have a, like, genetic thing. Being Mexican it’s quite common for a large percentage of ... women to be anemic. That’s something that’s worth looking for. So, I know I need an injection of iron or an IV infusion every 6 months or so. I do often wonder about my girls—something about what they need ... (If) they don’t, I will try to give it to them—like meat, specially, and stuff like that ... I find it hard to get kind of enough iron into them—most of the time, so I do wonder—do they always get enough iron in their diet? (FP2, FG2) | The use of incentives in paediatric research trials is controversial, but they should always be appropriate for the age of the child concerned; be acceptable to the relevant research ethics committee; and information and choices should be clear to parents [22] | |
| Theme 2: Child’s age, stage, understanding and ability to cope | Create a themed adventure to reduce child anxiety and make it fun | Yep I think the adult going first (where possible) is a good idea to show the children that it’s not scary etc. The iPad or some sort of game is a great idea. My kids have a Nintendo DS. Love the rocket ship idea. Tell them that they’re going on a trip or something. (MP1, OFG) | iPad games have been shown to be at least as effective as sedatives in reducing anxiety in children and their parents before surgery [24] |
| I think this would be a challenge for my 2 (and-a-) half-yr-old boy, not so much sitting in it, but the fact of keeping still for 2–3 min. Both my 2- and 8-year-old love Star Wars, so probably making it into a game, or definitely dressing it up would help … Yes I think an iPad video would help with them being able to sit still. (FP4, OFG) | |||
| Or, on the day it could be different for (child’s name), like he could just, you know, be inconsolable, or he could think it’s an adventure. But if maybe he had peers around at the same time, that—do you know what I mean?—line up, then, perhaps—yeah, like the adventure-seeker to go first? So you are not going to have a problem, then, once there has been a beautiful start of it. (FP3, FG2) | |||
| Theme 3: Study misunderstandings | Correct parent misunderstandings before they enrol their child | ||
| (i) Lack of awareness of current dietary guidelines promoting low-fat dairy | Australian dietary guidelines for children recommend mostly low fat dairy after two years of age [25] | ||
| (ii) Regular fat dairy is healthier as reduced fat forms are full of added sugars | Provide nutrition information on all dairy products to parents in advance, to show that reduced fat products have similar sugar content to the regular fat versions | ||
| (iii) Equipoise: randomization to the reduced fat dairy group will disadvantage the child | Provide clear information on randomization, equipoise and that no child can knowingly be disadvantaged in interviews, parent information leaflets and informed consent forms; allow time for questions before parents sign consent [10,11,26] | ||
| Theme 4: Factors affecting parental decisions to enrol their children in the study time commitment; treatment of their child; confronting tests; altruism | Use child-centred research in study design to help address concerns and keep participants engaged throughout | Parents have reported valuing practitioners and researchers who consider the needs and preferences of their children [11] Shier’s Pathways to Participation is a child-centred model that recommends introducing five increasing levels of participation: children are listened to; supported when expressing their views and opinions; have their views actively considered; are involved in the process of making decisions; and share the responsibility of making decisions that affect them [14] | |
| Magda Gerber’s Resources for Infant Educarers (RIE) model promotes respectful treatment of very young children. Three RIE principles, (1) use of authentic communication, (2) acknowledging emotions and (3) inviting participation [27,28] appear particularly applicable to paediatric clinical trials involving young children. | |||
| Research involving children needs appropriate and ethical information provided for recruitment and consent, and should be tailored towards educating the child as well as the family [12]. An example could be to provide pictorial child information leaflets, along with parent information leaflets. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholl, A.; O’Sullivan, T.A. Keep Calm and Carry on: Parental Opinions on Improving Clinical Dietary Trials for Young Children. Nutrients 2018, 10, 1166. https://doi.org/10.3390/nu10091166
Nicholl A, O’Sullivan TA. Keep Calm and Carry on: Parental Opinions on Improving Clinical Dietary Trials for Young Children. Nutrients. 2018; 10(9):1166. https://doi.org/10.3390/nu10091166
Chicago/Turabian StyleNicholl, Analise, and Therese A. O’Sullivan. 2018. "Keep Calm and Carry on: Parental Opinions on Improving Clinical Dietary Trials for Young Children" Nutrients 10, no. 9: 1166. https://doi.org/10.3390/nu10091166
APA StyleNicholl, A., & O’Sullivan, T. A. (2018). Keep Calm and Carry on: Parental Opinions on Improving Clinical Dietary Trials for Young Children. Nutrients, 10(9), 1166. https://doi.org/10.3390/nu10091166






